Vitamin B12 (B12) is an essential nutrient for DNA synthesis, methylation reactions and neural myelination making it critical for fetal and neonatal growth and development(Reference Finkelstein, Layden and Stover1). Lower maternal B12 status has been associated with adverse birth outcomes including small for gestational age(Reference Muthayya, Kurpad and Duggan2,Reference Dwarkanath, Barzilay and Thomas3) , low birth weight(Reference Rogne, Tielemans and Chong4) and preterm birth(Reference Rogne, Tielemans and Chong4). These adverse birth outcomes have been associated with perinatal morbidity and mortality(Reference Saigal and Doyle5,Reference Sharma, Farahbakhsh and Shastri6) , as well as an increased risk of chronic disease and mortality as an adult(Reference Risnes, Vatten and Baker7,Reference Barker8) . Furthermore, a pooled analysis of case studies showed that infants born to B12-deficient mothers had impaired neurodevelopment and motor deficits, such as developmental delay and hypotonia(Reference Dror and Allen9). Studies suggest a possible association between lower maternal B12 status and birth head circumference(Reference Gadgil, Joshi and Pandit10,Reference Jiang, Cao and Chen11) , which has been suggested as an indicator of fetal brain growth(Reference Cooke, Lucas and Yudkin12).
Due to increased B12 requirements to support the growth and development of the fetus, pregnant women are at greater risk of B12 inadequacy. As well, establishing sufficient B12 stores in the fetus is dependent on maternal B12 status(Reference Allen13). Because pregnancy-specific reference values for optimal B12 concentration have not been established, reference values for non-pregnant individuals are often used. However, the use of these reference values in pregnant women may not be appropriate, due to physiological changes during pregnancy, such as hemodilution, that affect the biochemical indicators of B12 status(Reference Murphy, Molloy and Ueland14). In Canada, approximately 17–25 % of pregnant women had a total B12 concentration <148 pmol/l (i.e. the cut-off for B12 deficiency in non-pregnant adults) in early pregnancy(Reference Visentin, Masih and Plumptre15-Reference Schroder, Sinclair and Mattman17). While maternal B12 status has been found to be associated with neural tube defects in Canadian pregnant women(Reference Thompson, Cole and Ray18), there is limited research on the association between maternal B12 status and other birth outcomes, such as newborn anthropometrics and gestational age at birth, in Canada. Thus, the objectives of this study were to describe the change in maternal B12 biomarker concentrations during early pregnancy and to determine the association of first- and second-trimester maternal B12 status with birth outcomes (birth weight z-score, head circumference z-score and gestational age at birth) in a cohort of Canadian mother–newborn dyads.
Methods
Study design
This study is a secondary analysis of data collected in a retrospective cohort study investigating the B12 status of pregnant women who underwent prenatal genetic screening between November 2014 and May 2016 as part of the British Columbia (BC) Prenatal Genetic Screening Program(19). Additional maternal and neonatal health data were obtained from the BC Perinatal Data Registry (BCPDR)(20). The sample consists of a cohort of healthy pregnant women aged 19–44 years who are of South Asian (n 357) or European (n 352) ethnicity (self-reported) and residing in BC, as well as their newborns (n 709) born in the study catchment area of BC. The sample size (Fig. 1) was determined based on the primary objective of the original study, which was to compare the first- and second-trimester total B12 concentrations between pregnant women of South Asian and European descent(Reference Schroder, Sinclair and Mattman17). A waiver of individual consent for the secondary use of deidentified clinical samples from the BC Prenatal Genetic Screening Program was approved by the University of British Columbia Children’s and Women’s Research Ethics Board (institutional approval no.: H15-00820).

Fig. 1 Flow diagram for the derivation of mother–newborn pairs from the original study sample. Sequence of biochemical analyses refers to the prioritisation of maternal biomarker analyses, which was dependent on serum volume availability and successful biomarker quantitation. B12, vitamin B12; holoTC, holotranscobalamin; MMA, methylmalonic acid; tHcy, total homocysteine.
Exclusion criteria
Women with multiple gestation, who were smoking during the pregnancy, HIV positive, diagnosed with diabetes mellitus Type I or II, who conceived through in vitro fertilisation or taking intravenous or oral steroid medication during pregnancy were excluded. Women who were identified to be at increased risk of trisomy 21 (Down Syndrome), 18, 13 or open neural tube defects according to the BC Prenatal Genetic Screening Program (Perinatal Services BC) were also excluded from the study. In addition, women with impaired renal function were excluded, as renal impairment has been associated with elevated methylmalonic acid (MMA) and total homocysteine (tHcy) concentrations. MMA and 2-methylcitric acid concentrations were used to identify renal insufficiency. Specifically, women were excluded if 2-methylcitric acid concentration exceeded MMA concentration(Reference Allen, Stabler and Savage21) and MMA concentration was >210 nmol/l.
Maternal biomarker data
The biochemical analyses of the maternal B12 biomarkers were previously described(Reference Schroder, Sinclair and Mattman17). In brief, data on maternal B12 biomarkers were obtained from the analysis of non-fasting maternal serum samples from the BC Prenatal Genetic Screening Program. Non-fasting samples were used, since B12 biomarkers are not impacted by fed or fasting state(Reference Refsum, Johnston and Guttormsen22,Reference Allen, Miller and Groot23) . Maternal blood samples were collected at 8–14 gestational weeks (first trimester) and 15–21 gestational weeks (second trimester). Maternal serum total B12 and holotranscobalamin (holoTC) concentrations, which are the direct indicators of B12 status, were quantified using fully automated immunoassays (Access 2 Immunoassay System by Beckman Coulter and Architect Immunoassay Analyzer by Abbott Technologies, respectively). Total B12 concentrations > 1107 pmol/l (first trimester: n 1; second trimester: n 3) and holoTC concentrations > 128 pmol/l (first trimester: n 125/636, 20 %; second trimester: n 83/615, 13 %) were not quantified as they were outside the assay range. Maternal serum MMA and tHcy, which are the functional indicators of B12 status, were quantified by stable isotope dilution-LC-tandem MS (LC-MS/MS). In addition, information on maternal age, self-reported ethnicity and weeks of gestation at blood collection, obtained during the prenatal genetic screening visit for blood collection, were abstracted from medical charts.
British Columbia Perinatal Data Registry health data collection
Data on obstetric history, current pregnancy and birth outcomes were retrieved from the BCPDR. The BCPDR contains data abstracted from obstetrical and neonatal medical records on nearly 100 % of births in the province of BC from over sixty acute care facilities, as well as births occurring at home attended by BC registered midwives, including women who had pregnancies ending in a live or still birth of at least 20 gestational weeks or 500 g birth weight(24). Gestational age at birth in completed weeks was calculated using a hierarchical algorithm based on information on last menstrual period, first ultrasound in early pregnancy, newborn clinical examination and maternal chart (online Supplementary Table S1).
Sociodemographic data collection
The forward sortation area indicating the first three characters of each mother’s resident postal code was retrieved from the BCPDR and linked to aggregated socio-economic data at the forward sortation area level from the 2016 Canadian census using the Canadian Census Analyzer (University of Toronto, 2018). Forward sortation area-level (or neighbourhood-level) indicators of socio-economic status obtained from the census include median family income (including lone-parent families and couples with children), which was adjusted for the average family size in each forward sortation area (i.e. divided by the square root of average family size). The adjusted median income for all forward sortation areas in BC was categorised into quintiles. The respective quintiles were then assigned to each of the women. In addition, the proportion of individuals aged 25–64 years who did not have a high school diploma or equivalency certificate in each mother’s resident forward sortation area was used to calculate forward sortation area-level education. The models were adjusted for both neighbourhood income and education, as previous research suggested that these measures capture different facets of socio-economic status(Reference Braveman, Cubbin and Egerter25).
Standardisation of birth weight and head circumference
The newborn anthropometric outcomes, birth weight and head circumference, were standardised for gestational age at birth and sex using newborn anthropometric charts. The models were not adjusted for gestational age at birth, as gestational age at birth may be a possible mediating variable in the association between maternal B12 status and newborn anthropometrics and adjusting for gestational age at birth may lead to bias. Furthermore, the use of anthropometric charts accounts for the non-linear association between birth weight and gestational age at birth, which are closely related, and standardised measurements are more appropriate for outcomes associated with an ongoing risk throughout pregnancy(Reference Caughey, Stotland and Washington26), such as lower birth weight and birth head circumference.
Neonatal birth weight and head circumference were standardised for gestational age at birth and sex based on the Kramer(Reference Kramer, Platt and Wen27) and Barbier(Reference Barbier, Boivin and Yoon28) neonatal charts, respectively. Fetal charts, such as the WHO Fetal Growth Charts(Reference Kiserud, Piaggio and Carroli29), which are considered a more accurate representation of optimal growth for preterm newborns, were not used as they were a poor fit for the sample of newborns in this study. Given the low proportion (<10 %) of preterm births in this cohort, the Kramer and Barbier neonatal charts were used to standardise birth weight and head circumference, as they were based on Canadian populations. Although the Kramer and Barbier charts were not derived using the same neonatal data, the birth weights of newborns in the Barbier study were shown to be comparable to that of the Kramer study(Reference Barbier, Boivin and Yoon28). Since neonatal anthropometric charts were used in lieu of fetal anthropometric charts, sensitivity analyses excluding preterm births were conducted.
Data analysis
Descriptive statistics
The characteristics of the study sample were summarised using descriptive statistics (absolute value, percentage, median and interquartile range). Normality of individual numerical variables was assessed visually using histograms, as well as quantile–quantile plots.
Change in maternal vitamin B12 biomarker concentrations
The association between gestational weeks at maternal serum sample collection and maternal serum total B12, MMA and tHcy concentrations was determined using linear mixed effects modelling; the association between gestational weeks at maternal sample collection and maternal holoTC was estimated using multilevel Tobit regression because 20 % and 13 % of first- and second-trimester holoTC concentrations, respectively, were higher than the upper limit of the assay range, that is, >128 pmol/l. Sample ID was included as a random effect to account for multiple measures within individuals.
Association between maternal vitamin B12 status and birth outcomes
Univariable linear regression, as well as multivariable linear regression, models adjusted for confounding variables were used to determine the association between maternal B12 status, as indicated by first- and second-trimester serum total B12, holoTC, MMA and tHcy and birth outcomes (birth weight z-score, head circumference z-score and gestational age at birth). Given a sample size of about 600, we had 95 % power to detect R 2 = 0·04 with α = 0·05 and ten independent predictors in the regression models for the association between maternal B12 biomarkers and birth outcomes. All multivariable models were adjusted for maternal ethnicity, maternal age, parity, pre-pregnancy BMI, gestational weight gain, gestational diabetes mellitus, hypertensive disorder of pregnancy and neighbourhood-level income and education. The models with gestational age at birth as the outcome were additionally adjusted for newborn sex. These confounding variables were determined a priori based on a literature review to identify factors associated with maternal B12 status and birth outcomes, as well as using directed acyclic graphs . The maternal B12 biomarkers and trimesters were analysed separately. Total B12 concentrations > 1107 pmol/l were excluded from analyses, as only a small proportion of total B12 samples were above the assay range. HoloTC concentrations > 128 pmol/l were included in the regression analyses, since a large proportion of the holoTC samples exceeded the assay range. Discontinuous regression, in which a dummy variable (holoTC value > 128 pmol/l = ‘1’; non-censored holoTC value = ‘0’) was added to the regression model, was applied for all models that included holoTC as an independent variable. A sensitivity analysis for all regression models with holoTC was conducted comparing the discontinuous regression models to models that assigned a concentration of 129 pmol/l for holoTC concentrations >128 pmol/l. All linear regression assumptions were tested for each model. Linearity was visually assessed using augmented component-plus-residual plots, and homoscedasticity was visually assessed using residual plots. Non-linear associations were explored by log-transforming the B12 biomarker and with polynomial regression (i.e. addition of quadratic term). Regression models with restricted cubic splines were used to further explore if there were any non-linear associations between each maternal B12 biomarker and birth outcome. The restricted cubic spline models included five knots, which according to Harrell(Reference Harrell30) is reasonable with larger sample sizes (e.g. n ≥ 100). The locations of the knots were determined using equally spaced quantiles suggested by Harrell(Reference Harrell30) (i.e. 5th, 27·5th, 50th, 72·5th and 95th percentiles). Non-linearity in each model was tested using Wald’s statistics (null hypothesis: beta coefficient for 2nd, 3rd and 4th splines equals zero; P-value <0·05 considered significant), as well as visually, using graphs of the adjusted predictions and marginal effects. Additional sensitivity analyses excluding influential outliers, which were identified visually using augmented component-plus-residual plots, were conducted.
A significance level (α) of 0·05 for two-tailed tests was used. All analyses were conducted in Stata 15 (StataCorp. 2017. Stata Statistical Software: Release 15. StataCorp LLC).
Handling missing data
Several methods for handling missing data were used for all analyses that included pre-pregnancy BMI and gestational weight gain due to a high proportion of missing data (21 % and 39 %, respectively); this includes the missing indicator method, complete case analysis and multiple imputation(Reference Pedersen, Mikkelsen and Cronin-Fenton31). The missing indicator method was used as the primary analysis. In this method, pre-pregnancy BMI and gestational weight gain were converted to categorical variables using WHO(32) and Institute of Medicine(33) guidelines, respectively, including a category for missing data. Complete case analysis, which involves the exclusion of individuals with any missing data, and multiple imputation by chained equations were also conducted as a sensitivity analysis. A description of the methods for the multiple imputation model is included in the Supplementary Material.
Results
Maternal and newborn characteristics
The study included 709 mother–newborn dyads. One mother was excluded due to suspected renal insufficiency. The maternal characteristics of the entire sample are summarised in Table 1. Approximately 20 % (n 134/656) and 51 % (n 332/656) of women in the first trimester and 25 % (n 180/706) and 58 % (n 408/706) of women in the second trimester had a total B12 concentration <148 pmol/l (cut-off for B12 deficiency in non-pregnant adults) and <221 pmol/l (cut-off for low B12 in non-pregnant adults), respectively. Newborn characteristics are summarised in Table 2. Nine women had a stillbirth, defined as fetal death after at least 20 weeks of pregnancy or fetal weight of at least 500 grams.
Table 1. Maternal characteristics*
(Median and interquartile ranges (IQR); absolute numbers and percentages, n 709)
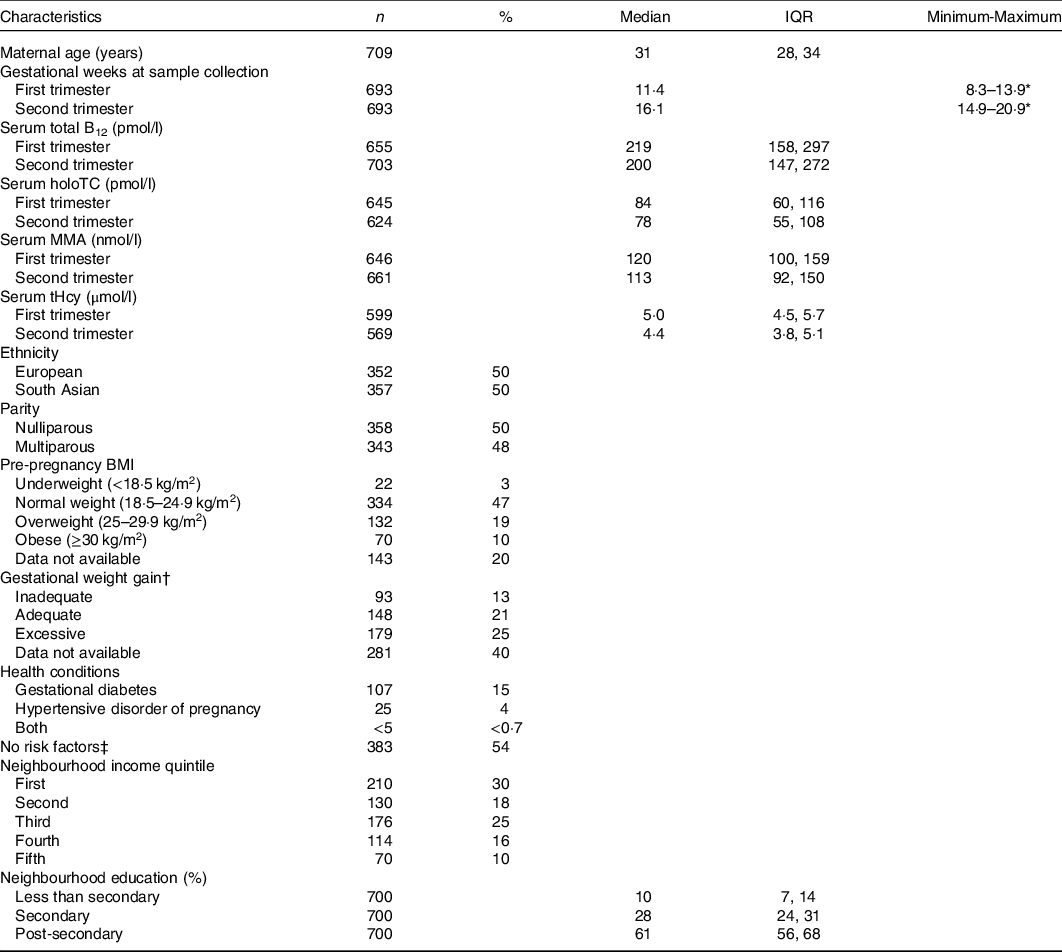
B12, vitamin B12; holoTC, holotranscobalamin; MMA, methylmalonic acid; tHcy, total homocysteine.
* Percentages may not add up to 100% due to missing data.
† Gestational weight gain based on Institute of Medicine guidelines. Adequate weight gain defined according to maternal pre-pregnancy BMI: BMI < 18·5 kg/m2, 12·5–18 kg; BMI 18·5–24·9 kg/m2, 11·5–16 kg; BMI 25–29·9 kg/m2, 7–11·5 kg; BMI ≥ 30 kg/m2, 5–9 kg.
‡ No specific risk factors in the current or past pregnancy or mother’s medical history.
Table 2. Newborn characteristics*
(Medians and interquartile ranges (IQR); absolute numbers and percentages, n 709)
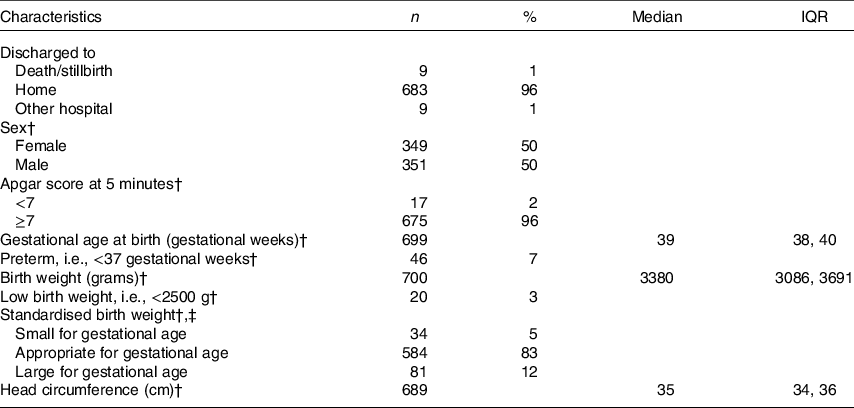
* Percentages may not add up to 100 % due to missing data.
† Excluding stillbirths (n 9).
‡ Based on Kramer birth weight charts compared with newborns of the same sex and gestational age at birth. Small for gestational age: <10th percentile; appropriate for gestational age: 10th–90th percentile; large for gestational age: >90th percentile.
Gestational weeks and Biomarker concentrations
Each of the maternal B12 biomarkers, total B12, holoTC, MMA and tHcy, was inversely associated with gestational weeks. The mean (95 % CI) decrease in maternal serum total B12 and holoTC was −3·64 (95 % CI −4·64, −2·65) pmol/l (n 693; P < 0·001) and −1·04 (95 % CI −1·42, −0·660) pmol/l (n 648; P < 0·001) with each gestational week, respectively. For the functional biomarkers, MMA and tHcy, the mean (95 % CI) decrease with each gestational week was −1·44 (95 % CI −2·30, −0·587) nmol/l (n 689; P = 0·001) and −0·104 (95 % CI −0·121, −0·086) μmol/l (n 674; P < 0·001), respectively.
Maternal vitamin B12 status and birth outcomes
None of the maternal B12 biomarkers in either trimester was linearly associated with birth weight z-score, head circumference z-score and gestational age at birth (β-coefficient P > 0·05; Tables 3–5). Each model met linear regression assumptions. Non-linear associations were not observed using restricted cubic spline models. For the sensitivity analysis, excluding preterm births did not significantly affect the β-coefficient estimates in each of the models (data not shown). Furthermore, the estimates did not differ among the methods used for handling missing data, including the missing indicator method (results presented in Tables 3–5), complete case analysis (data not shown) and multiple imputation (online Supplementary Tables S3–S5). The results remained not significant following the removal of influential outliers.
Table 3. Association between maternal vitamin B12 (B12) status and birth weight z-score in Canadian mother–newborn dyads*
(β-coefficients and 95 % confidence intervals)
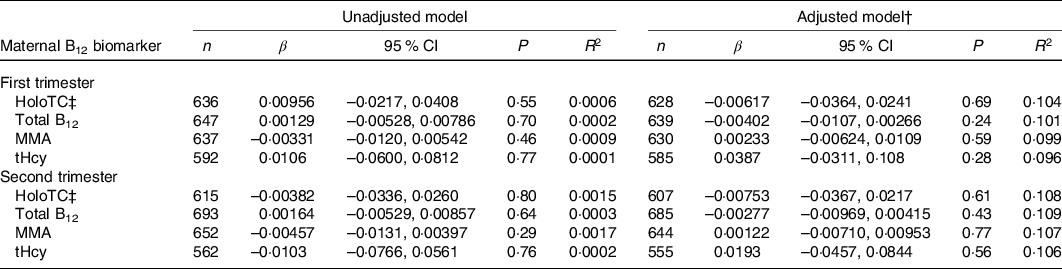
holoTC, holotranscobalamin; MMA, methylmalonic acid; tHcy, total homocysteine.
* Unadjusted R 2 reported for univariable model and adjusted R 2 reported for multivariable models. β-coefficients represent change in birth weight z-score with each 10-unit increase in maternal serum holoTC, total B12 and MMA concentration and 1-unit increase in tHcy.
† All multivariable models were adjusted for maternal age, maternal ethnicity, parity, GWG, pre-pregnancy BMI, hypertensive disorder of pregnancy, gestational diabetes, neighbourhood income and neighbourhood education.
‡ Discontinuous regression.
Table 4. Association between maternal vitamin B12 (B12) status and head circumference z-score in Canadian mother–newborn dyads*
(β-coefficients and 95 % confidence intervals)
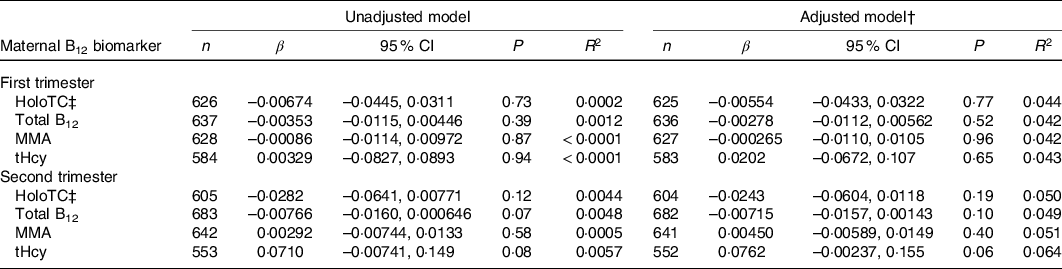
holoTC, holotranscobalamin; MMA, methylmalonic acid; tHcy, total homocysteine.
* Unadjusted R 2 reported for univariable model and adjusted R 2 reported for multivariable models. β-coefficients represent change in head circumference z-score with each 10-unit increase in maternal serum holoTC, total B12 and MMA concentration and 1-unit increase in tHcy.
† All multivariable models were adjusted for maternal age, maternal ethnicity, parity, GWG, pre-pregnancy BMI, hypertensive disorder of pregnancy, gestational diabetes, neighbourhood income and neighbourhood education.
‡ Discontinuous regression.
Table 5. Association between first trimester maternal vitamin B12 (B12) status and gestational age at birth in weeks in Canadian mother–newborn dyads*
(β-coefficients and 95 % confidence intervals)

holoTC, holotranscobalamin; MMA, methylmalonic acid; tHcy, total homocysteine.
* Unadjusted R 2 reported for univariable model and adjusted R 2 reported for multivariable models. β-coefficients represent change in gestational age at birth (weeks) with each 10-unit increase in maternal serum holoTC, total B12 and MMA concentration and 1-unit increase in tHcy.
† All multivariable models were adjusted for maternal age, maternal ethnicity, parity, GWG, pre-pregnancy BMI, hypertensive disorder of pregnancy, gestational diabetes, neighbourhood income, neighbourhood education and newborn sex.
‡ Discontinuous regression.
Discussion
Previous studies investigating the association between maternal B12 status, primarily as total B12 and tHcy concentrations, and birth outcomes have shown inconsistent findings. Despite a small but statistically significant decrease in maternal B12 status with each gestational week, the current study did not observe an association between each maternal B12 biomarker concentration (total B12, holoTC, MMA and tHcy) in the first and second trimesters and the birth outcomes, birth weight z-score, head circumference z-score and gestational age at birth.
Maternal vitamin B12 status and birth outcomes
The lack of linear association between maternal total B12 concentration and birth weight z-score in the current study is consistent with a recent meta-analysis of eighteen studies by Rogne et al. (Reference Rogne, Tielemans and Chong4). However, a subgroup analysis of low- and middle-income countries consisting predominantly of studies in India found a mean (95 % CI) increase in birth weight standard deviation score of 0·08 (95 % CI 0·03, 0·14) with each 1 sd increase in maternal total B12 concentration(Reference Rogne, Tielemans and Chong4). Similarly, a meta-analysis of nineteen studies by Hogeveen, Blom and Heijer(Reference Hogeveen and Blom34) also observed a 0·062 (95 % CI 0·025, 0·10) decrease in birth weight standard deviation score for each 1 sd increase in maternal tHcy concentration. These findings, although statistically significant, may not be clinically significant due to the small effect sizes, which were similarly observed in the current study. The lack of association between first- and second-trimester maternal tHcy and birth weight z-score in the current study may be due to the low concentrations of serum tHcy (i.e. median of 5·0 and 4·4 μmol/l in first and second trimester, respectively) compared with those described in the meta-analysis by Hogeveen, Blom and Heijer(Reference Hogeveen and Blom34). The low tHcy concentrations in these pregnant women are likely the result of their high folate concentrations(Reference Schroder, Sinclair and Mattman17), with folate being the main determinant of circulating tHcy(Reference Clarke and Armitage35). The high folate concentrations may be attributed to the high prevalence of folic acid containing prenatal supplement use in Canada(Reference Chalmers, Dzakpasu and Heaman36) – with most supplements providing 1 mg/d of folic acid – and to a lesser extent, mandatory folic acid fortification of flour products. The comparable tHcy concentration of the pregnant women in this study to that of other studies of healthy pregnant women in Canada(Reference Visentin, Masih and Plumptre15,Reference Wu, Innis and Mulder16) supports this hypothesis.
In contrast to the current study, the Generation R Study, a prospective cohort study of 5890 pregnant women in the Netherlands, observed a 1·6 cm (95 % CI 0·1, 3·1) lower birth head circumference in the newborns of mothers with an early pregnancy tHcy concentration in the highest quintile of tHcy (≥8·31 μmol/l) compared with the lowest quintile (≤5·80 μmol/l)(Reference Bergen, Schalekamp-Timmermans and Jaddoe37). The inverse association between maternal tHcy concentration and birth head circumference may be due to the higher concentration and greater variability in maternal tHcy concentration (median (90 % range): 6·9 (4·9, 10·5) μmol/l)(Reference Bergen, Schalekamp-Timmermans and Jaddoe37), which may be attributed to the 19 % of women who reported no folic acid supplement use and the absence of food fortification with folic acid in the Netherlands. The tHcy concentrations were lower in this Canadian cohort compared with the Generation R Study, which possibly contributed to the null findings between maternal tHcy and birth head circumference.
The meta-analysis by Rogne et al. (Reference Rogne, Tielemans and Chong4) did not observe a linear association between maternal total B12 concentration and gestational age at birth; however, they found an 11 % (95 % CI 3, 18) decreased risk of preterm birth with each standard deviation increase in maternal total B12 concentration after adjusting for maternal age, parity and BMI or weight. The inverse association between maternal B12 status and preterm birth in the meta-analysis by Rogne et al. (Reference Rogne, Tielemans and Chong4) suggests that lower maternal B12 status is a risk factor for preterm birth. Preterm birth and other adverse birth outcomes were not explored as outcomes in the current cohort due to their low prevalence.
Maternal biomarker concentrations
The small decrease in maternal total B12, holoTC and tHcy concentrations between the first and second trimester is consistent with previous longitudinal studies of maternal B12 status in early pregnancy(Reference Koebnick, Heins and Dagnelie38,Reference Solé-Navais, Salat-Batlle and Cavallé-Busquets39) . Circulating maternal MMA concentration in early pregnancy has been described to remain largely unchanged(Reference Murphy, Molloy and Ueland14,Reference Greibe, Andreasena and Lildballe40) . In contrast, the current study observed a decrease in maternal MMA concentration with each gestational week, albeit this change was small. The decrease in maternal B12 status may be attributed to normal physiological changes that occur during pregnancy, such as hemodilution, hormonal changes and active maternal–fetal transport of B12 (Reference Murphy, Molloy and Ueland14,Reference Carlin and Alfirevic41) . Mørkbak et al. (Reference Mørkbak, Hvas and Milman42) found that the 50 % decrease in maternal total B12 concentration from mid to late pregnancy was primarily due to a decrease in haptocorrin, and not holoTC, concentration. In comparison with the Mørkbak et al. (Reference Mørkbak, Hvas and Milman42) study, the serum samples in the current study were collected earlier in pregnancy between 8 and 21 gestational weeks, which may explain the less marked decrease in total B12 concentration.
The proportion of women with a total B12 concentration < 148 pmol/l (20 and 25 % in the first and second trimester, respectively) was similar to the prevalence of B12 insufficiency reported in a systematic review and meta-analysis by Sukumar et al. (Reference Sukumar, Rafnsson and Kandala43) (first trimester 21 %; second trimester 19 %). This suggests that maternal total B12 concentration in this cohort is comparable to those of other studies. Although 20–25 % of the pregnant women had a total B12 concentration < 148 pmol/l, the lack of association between maternal B12 status and birth outcomes suggests the women were likely B12 replete. Studies from India(Reference Muthayya, Kurpad and Duggan2,Reference Dwarkanath, Barzilay and Thomas3) and the meta-analysis by Rogne et al. (Reference Rogne, Tielemans and Chong4) observed an association between maternal total B12 concentration, categorised using tertiles and cut-offs and birth weight. Due to the lack of pregnancy-specific cut-offs for all B12 biomarkers, maternal B12 biomarker concentrations were only assessed as continuous variables in the current study.
Maternal nutrient status
The potential influence of other micronutrients, such as Fe, cannot be fully ruled out due to the lack of data on diet and supplement use, as well as nutrient status indicators. Considering the co-enzymatic role of B12, it may not be as influential as other nutrients, such as folate, which serves as a methyl donor in one-carbon metabolism. This is supported by the small effect sizes observed for the association between maternal B12 biomarkers and each of the birth outcomes. Other pregnancy cohort studies have reported a high proportion (93–97 %) of prenatal multivitamin supplement use in early pregnancy(Reference Gómez, Field and Olstad44,Reference Masih, Plumptre and Ly45) . If this is the case in the current study, the women are less likely to be deficient with respect to their overall micronutrient status.
Study strengths and limitations
Strengths of this study include the large sample size and the assessment of both direct and functional B12 biomarkers in early pregnancy. The longitudinal nature of the study and the narrow range in gestational weeks at sample collection also allowed for the analysis of first and second-trimester-specific maternal B12 status. As well, additional data were obtained from the BCPDR, which enabled adjustment for confounding in the regression models. Last, there was no consent bias, since consent for access to the bio-banked serum samples was waived. Due to the selection for healthy women, the findings may not be generalisable to the general Canadian population. Furthermore, we were unable to perform analyses for adverse birth outcomes (i.e. low birth weight, small for gestational age and preterm birth) as a result of the limited number of cases in the current study.
Conclusion
In apparently healthy pregnant women, maternal B12 biomarker concentrations in early pregnancy were not associated with birth weight z-score, head circumference z-score and gestational age at birth. The findings of this study contribute to the body of research on the role of maternal B12 status in fetal growth and development, specifically in Canadian pregnant women. Studies of the association between maternal B12 biomarkers and birth outcomes are needed in women at higher risk of adverse birth outcomes in Canada. Furthermore, research is needed in infant outcomes after birth that have been associated with maternal B12 status, such as cognitive function.
Acknowledgements
The authors would like to thank Dr Theresa Schroder for her contribution to the primary study; Dr Jennifer Hutcheon for her guidance in regard to the perinatal data collection and the statistical analysis plan; Dr Maria Fernanda Mujica-Coopman for her contribution to data management; the staff at the BC Newborn Screening laboratory for their support in sample collection; Matthew Saunders, Ori Nevares, Pablo Elizondo and Benny Chan from UBC for their support in sample processing and biomarker analyses; Dr Benjamin Jung for supervising the serum total vitamin B12 analyses; and Dr Arianne Albert from the Women’s Health Research Institute for statistical support.
This study was supported by the Canadian Institutes for Health Research/Canada Research Chair Program, and a grant of no charge materials from Abbott Laboratories. A. T. was supported by the Canadian Institutes of Health Research Canada Graduate Scholarship Master’s Award. The funding sources had no influence on the design of the study, data analysis or writing of the manuscript.
Y. L. and A. T. formulated the research question; Y. L. and A. T. designed and conducted the study; A. T. analysed the data; G. S., A. M. and H. D. V. provided input on study design, execution and data interpretation; A. T. and Y. L. interpreted the findings and wrote the article. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
All inferences, opinions and conclusions drawn in this publication are those of the authors and do not reflect the opinions or policies of Perinatal Services BC.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114521000581









