Frailty is a state of high vulnerability for adverse health outcomes, including disability, dependency, falls, need for long-term care and mortality(Reference Fried, Ferrucci and Darer1). Korea is one of the most rapidly ageing countries worldwide(Reference He, Goodkind and Kowal2), and the prevalence of frailty defined by the Korean version of the Fatigue, Resistance, Ambulation, Illnesses, and Loss of weight (K-FRAIL) index has been reported to be 12·4 % in Koreans aged 70 years or older(Reference Kim, Shin and Choi3).
One of the important factors for frailty is weight loss since the decline in body weight leads to frequently observed underweight in the older adults aged 70 years or older(Reference Morley4,Reference Reinders, Visser and Schaap5) . Epidemiological studies consistently reported that the prevalence of frailty was higher in the older adults who are underweight (BMI < 20 kg/m2)(Reference Sanchez-Garcia, Sanchez-Arenas and Garcia-Pena6–Reference Rietman, Van Oostrom and Picavet10) and lower in those who are overweight (BMI 25–29·9 kg/m2)(Reference Hubbard, Lang and Llewellyn8–Reference Rietman, Van Oostrom and Picavet10). However, the association between obesity and frailty is controversial. The previous studies showed that obesity defined by BMI ≥ 30 kg/m2 was positively associated with the prevalence and incidence of frailty in the older adults(Reference Hubbard, Lang and Llewellyn8,Reference Rietman, Van Oostrom and Picavet10–Reference Garcia-Esquinas, Jose Garcia-Garcia and Leon-Munoz16) , but a few studies showed no significant association between obesity and the risk of frailty(Reference Lee, Kim and Han7,Reference Ferriolli, Pessanha and Moreira9,Reference Garcia-Esquinas, Jose Garcia-Garcia and Leon-Munoz16) .
Garcia-Esquinas et al. (Reference Garcia-Esquinas, Jose Garcia-Garcia and Leon-Munoz16) reported that obesity was positively associated with the risk of frailty in the older adults, but the association was not significant after adjusting for waist circumference (WC), suggesting that the association between BMI and frailty could be mediated by abdominal obesity. A few previous studies reported that abdominal obesity was associated with higher prevalence and incidence of frailty in the older adults(Reference Hubbard, Lang and Llewellyn8,Reference Ferriolli, Pessanha and Moreira9,Reference Garcia-Esquinas, Jose Garcia-Garcia and Leon-Munoz16,Reference Liao, Zheng and Xiu17) . Increased WC has been suggested to be associated with a marker of oxidative stress and inflammation, independent of BMI(Reference Weinbrenner, Schröder and Escurriol18), which may be the major mechanism leading to frailty(Reference Serviddio, Romano and Greco19).
Abdominal obesity is commonly defined by WC, and waist:height ratio (WHtR) has been suggested to be a better predictor of whole-body fat percentage and visceral adipose tissue mass as well as increased health risk compared with WC(Reference Swainson, Batterham and Tsakirides20). Partezani-Rodrigues et al. (Reference Rodrigues, Fhon and Huayta21) reported that in the older adults the risk of frailty was significantly associated with WHtR but not with WC. However, no study investigated the mediating effect of WHtR on the association between BMI and the risk of frailty. Therefore, the present study aimed to confirm the hypothesis that the risk of frailty was positively associated with obesity, but the association was mediated by WHtR in the older women and men.
Methods
Participants
The present study used the data from the Korean Frailty and Aging Cohort Study (KFACS), a multicentre, longitudinal study of community-dwelling older adults(Reference Won, Lee and Choi22). Between June 2016 and November 2017, the participants of the KFACS were recruited from ten participating centres across the urban and rural areas of Korea on the basis of age- and sex-specific strata. The following participants were included in the study: those aged 70–84 years, those living independently at home, those with no plans to move out in the next 2 years and those having no serious problems with communication. Among the 3014 participants who completed the initial assessment, 2997 participants with non-missing data on frailty assessments were included in the present study. The study was conducted according to the guidelines in the Declaration of Helsinki(Reference Dale and Salo23) and was approved by the institutional review board of Kyung Hee University (KHUH-2015-12-103-044) and the institutional review board of Hanyang University (HYI-18-167-1). All participants signed a written informed consent form.
Data collection
Data were obtained from the KFACS(Reference Won, Lee and Choi22) and included age, sex, height, weight, WC, educational level (less than middle school or more than high school), job (currently working, previously working or non-working), smoking (current, former or never), alcohol drinking (once or more times in a month) and living alone. Polypharmacy was defined as the regular use of five or more prescription medications. Cognitive impairment was defined as a Korean Mini-Mental State Examination score of less than 24(Reference Kang, Na and Hahn24). Co-morbidity was defined as the number of self-reported diseases diagnosed by a physician (hypertension, dyslipidaemia, CVD, peripheral arterial disease, cerebrovascular disease, osteoarthritis, osteoporosis, respiratory disease, lung disease, thyroid disease, dementia, kidney disease and cancer). Disabilities were defined as dependence in at least one item in the Korean activities of daily living(Reference Won, Rho and Kim25) and Korean instrumental activities of daily living(Reference Won, Rho and Duk26).
Weight was measured to the nearest 0·1 kg using a portable digital scale, and height was measured to the nearest 0·1 cm using a measuring tape. The BMI was calculated as weight divided by square of the height. According to the criteria of the World Health Organization Asia-Pacific Region(27), BMI was categorised as <18·5 kg/m2, 18·5–<23 kg/m2, 23–<25 kg/m2, 25–<30 kg/m2 and ≥30 kg/m2. The WC was measured at the middle point between the rib margin and iliac crests in a horizontal plane using an inelastic measuring tape. The WHtR (cm/cm) was calculated as WC divided by height with the median value of 0·557 as the cut-off point in the study participants. Dual-energy X-ray absorptiometry was used to obtain appendicular skeletal muscle mass (ASM) of the four limbs, and ASM index was calculated as the sum of muscle mass in the four limbs (kg) divided by the squared height (m2).
Frailty indices
Frailty was defined using the K-FRAIL index(Reference Jung, Yoo and Park28), a modified version of the published FRAIL scale(Reference Morley, Malmstrom and Miller29). The FRAIL scale exhibited the strongest predictive validity for disability and mortality compared with the Cardiovascular Health Study (CHS) index(Reference Malmstrom, Miller and Morley30). Since the K-FRAIL index is easier to measure compared with CHS because of interview-based test(Reference Woo, Leung and Morley31), the K-FRAIL index was measured in all participants in the study. The K-FRAIL index assigns 1 point to each of the following five components. Fatigue was assessed by asking the participants for how much time during the preceding 4 weeks they felt tired, with responses of ‘all of the time’ or ‘most of the time’ scored as 1 point. Resistance was defined as difficulty in walking up to ten stair steps alone without resting and without aid, and ambulation was assessed by asking whether they had any difficulty in walking 300 m alone and without aid. Illness was defined as having five or more conditions among the following eleven conditions: hypertension, diabetes mellitus, chronic obstructive pulmonary disease, angina, myocardial infarction, heart failure, asthma, arthritis, stroke, renal disease and cancer. Loss of weight was recorded as loss of at least 5 % of the body weight within the preceding year. Participants with a total score of 3 or higher are classified as frail.
Statistical analysis
Independent two-sample t tests for continuous variables and χ 2 tests for categorical variables were used to compare the differences in the characteristics and risk factors for frailty between older women and men. Continuous variables were presented as means and standard deviations, and categorical variables were expressed as the number of participants (percentage distribution). Pearson’s correlation analysis was performed to determine the bivariate association between the main variables. To assess whether the association between BMI and risk of frailty weakened or became non-significant after adjusting for WHtR, binary logistic regression analysis was performed after adjusting for confounding factors. ANCOVA with least significant difference post hoc test was used in comparing the marginal means of lean mass by adjusting the confounding variables.
Moderation, mediation and moderated mediation analyses were performed using the Hayes PROCESS macro (models 1, 4 and 14)(Reference Hayes32). For these analyses, independent, dependent and mediator variables or categorical covariates were designated as numerical variables or dummy variables. Additionally, moderator variables were recoded using indicator coding, with men as the reference category(Reference Hayes and Preacher33). This macro used a bootstrapping strategy to test the validity of the indirect effects and calculate the 95 % bias-corrected CI from 5000 bootstrap samples(Reference Hayes34). These analyses were considered significant if the CI excluded zero. In the multivariable models, the covariates showing a significance level below 0·2 were included in the final model(Reference Greenland and Pearce35). All statistical tests were two sided according to a 0·05 significance level and performed using SPSS (version 25.0; SPSS Inc.).
Results
Characteristics of older women and men
The prevalence of frailty was 12·3 % and was higher in women than in men (Table 1). Women were younger; and had lower educational level, proportion of currently working, smoking, alcohol drinking, polypharmacy and instrumental activities of daily living disability. However, women had higher BMI, WHtR, proportion of living alone, cognitive impairment, co-morbidity and activities of daily living disability compared with men.
Table 1. Characteristics of women and men*
(Mean values and standard deviations; percentages)

WC, waist circumference; WHtR, waist:height ratio; ADL, activities of daily living; IADL, instrumental activities of daily living.
* Number of participants was discordant; education (n 2861), at work (n 2860), smoking (n 2861), alcohol drinking (n 2852), polypharmacy (n 2848) and IADL (n 2857).
† P values were analysed using the independent t test for continuous variables and χ 2 test for categorical variables.
Association between frailty and body composition
Multivariable-adjusted regression analysis revealed that the risk of frailty was higher in all older individuals and in older men with BMI < 18·5 kg/m2 before and after adjusting for WHtR (Table 2). Furthermore, the risk of frailty was higher in all older individuals and in older women with BMI ≥ 30 kg/m2 before adjusting for WHtR, but the risk disappeared after adjusting for WHtR. On the other hand, the risk of frailty was lower in men with BMI 25–<30 kg/m2 before adjusting for WHtR but lower in all older individuals and older women with BMI 25–<30 kg/m2 after adjusting for WHtR.
Table 2. Association between BMI and risk of frailty before and after adjusting for waist:height ratio (WHtR)*
(Odds ratios and 95 % confidence intervals)
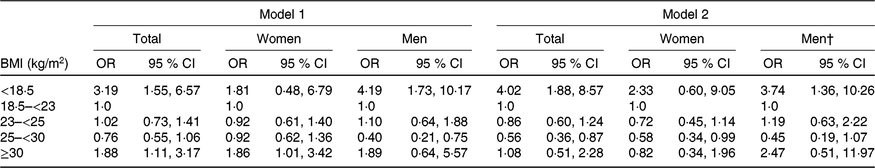
ADL, activities of daily living; IADL, instrumental activities of daily living.
* For logistic regression model 1, it was adjusted for age, sex, education, alcohol drinking, polypharmacy, cognitive impairment, co-morbidity, ADL and IADL in total older individuals (n 2830); age, education, polypharmacy, cognitive impairment, co-morbidity, ADL and IADL in women (n 1465); age, education, alcohol drinking, living alone, polypharmacy, co-morbidity and IADL in men (n 1365). For logistic regression model 2, model 1 adjusted additionally for WHtR in total older adults, women and men.
† As WHtR was a NS covariate variable (P = 0·643), the Hosmer–Lemeshow test was significant (χ 2 = 16·689, df = 8, P = 0·034) for men after adjusting for WHtR in model 2.
When participants were divided into low and high WHtR groups according to the median value of 0·557 as cut-off point, the risk of frailty was higher in all older individuals and in older men with BMI < 18·5 kg/m2 and low WHtR compared with those with BMI 18·5–<23 kg/m2 and low WHtR (Table 3). Moreover, the risk of frailty was higher in all older individuals and women with BMI ≥ 30 kg/m2 and high WHtR than those with BMI 18·5–<23 kg/m2 and low WHtR. On the other hand, the risk of frailty was lower in men with BMI 25–<30 kg/m2 and high WHtR compared with those with BMI 18·5–<23 kg/m2 and low WHtR.
Table 3. Subgroup analysis of waist:height ratio (WHtR) on the association between BMI and risk of frailty*†
(Odds ratios and 95 % confidence intervals)
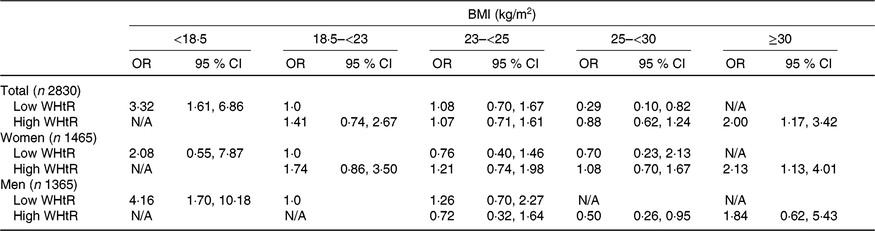
N/A, not applicable; ADL, activities of daily living; IADL, instrumental activities of daily living.
* N/A was used if there were no frail or older adult participants.
† Confounding factors were age, sex, education, alcohol drinking, polypharmacy, cognitive impairment, co-morbidity, ADL and IADL in total older adults; age, education, polypharmacy, cognitive impairment, co-morbidity, ADL and IADL in women; age, education, alcohol drinking, living alone, polypharmacy, co-morbidity, ADL and IADL in men.
Mediating effects of waist:height ratio on the association between BMI and frailty
Bivariate correlations revealed that BMI was positively correlated with WHtR (r 0·82, P < 0·001) and frailty (r 0·04, P = 0·024), and WHtR was positively correlated with frailty (r 0·18, P < 0·001) (online Supplementary Table S1). In the mediation analysis, the negative direct effect and the positive indirect effect of BMI on the score of frailty were significant in all older individuals, in older women and in older men (Fig. 1). On the other hand, the total effect of BMI on the score of frailty was positive in women but negative in men. Additionally, the mediating effect of WHtR on the score of frailty was positive in both women and men, but the score of frailty was associated with BMI positively in women but negatively in men, suggesting that the association was affected by sex (online Supplementary Fig. S1).

Fig. 1. Mediating effects of waist:height ratio (WHtR) on the association between BMI and frailty score in total older adults (A), women (B) and men (C). Unstandardised coefficients and 95 % confidence intervals are presented: ‘a’ is the linear regression coefficient of the BMI–WHtR association and ‘b’ is that of the WHtR–frailty association. Adjusted confounding factors were age, sex, education, alcohol drinking, polypharmacy, cognitive impairment, activities of daily living (ADL) and instrumental activities of daily living (IADL) in total older adults; age, education, polypharmacy, cognitive impairment, ADL and IADL in women; age, education, alcohol drinking, living alone, polypharmacy, ADL and IADL in men. * P < 0·05, ** P < 0·001.
Discussion
The present study showed that the risk of frailty was higher in the older individuals with BMI ≥ 30 kg/m2 than those with BMI 18–<23 kg/m2 before adjusting for WHtR but not after adjusting for WHtR in total older individuals and women but not in men. The association between BMI and the risk of frailty was mediated by WHtR in the older individuals, particularly in women.
Consistent with the present study, the majority of previous studies reported that obesity (BMI ≥ 30 kg/m2) was associated with a higher prevalence or incidence of frailty in the older adults without adjusting for abdominal obesity(Reference Hubbard, Lang and Llewellyn8,Reference Rietman, Van Oostrom and Picavet10–Reference Garcia-Esquinas, Jose Garcia-Garcia and Leon-Munoz16) . However, a few studies showed that the risk of frailty was not significantly higher in obese older adults(Reference Lee, Kim and Han7,Reference Ferriolli, Pessanha and Moreira9,Reference Garcia-Esquinas, Jose Garcia-Garcia and Leon-Munoz16) . This inconsistency could be partly because of the proportion of frailty among the female study participants. Older women tended to be more prone to obesity(Reference Ogden, Yanovski and Carroll36) and had a higher prevalence of frailty compared with older men(Reference Collard, Boter and Schoevers37). In previous studies reporting the absence of an association between obesity and frailty, the proportion of frailty in women (8–9 %) was lower than that in the present study (17 %)(Reference Lee, Kim and Han7,Reference Ferriolli, Pessanha and Moreira9) . Oestrogen levels declined after menopause, leading to a decrease in bone density, muscle mass and muscle strength but an increase in visceral adiposity(Reference Messier, Rabasa-Lhoret and Barbat-Artigas38). Consistent with the findings of the present study, the prevalence of obesity was higher in women than in men(Reference Hong, Bang and Kang39). Additionally, previous studies did not include abdominal obesity as a confounding factor to confirm the association between frailty and obesity(Reference Lee, Kim and Han7–Reference Blaum, Xue and Michelon15). In the present study, obesity was also associated with a higher risk of frailty before adjusting for WHtR, but it was not associated with the risk of frailty after adjusting for WHtR. Previous epidemiological studies consistently reported that abdominal obesity defined by WC(Reference Hubbard, Lang and Llewellyn8,Reference Ferriolli, Pessanha and Moreira9,Reference Garcia-Esquinas, Jose Garcia-Garcia and Leon-Munoz16,Reference Liao, Zheng and Xiu17,Reference Liu, Lee and Chen40) and WHtR(Reference Rodrigues, Fhon and Huayta21) was associated with a higher prevalence and incidence of frailty in the older adults. Older adults with high WC had high levels of oxidative stress markers, independent of BMI, suggesting a possible involvement of oxidative stress in the genesis of frailty(Reference Weinbrenner, Schröder and Escurriol18,Reference Serviddio, Romano and Greco19) . Garcia-Esquinas et al. (Reference Garcia-Esquinas, Jose Garcia-Garcia and Leon-Munoz16) reported that the association between obesity and the risk of frailty was not significant in the older adults after adjusting for WC, suggesting that the association could be mediated by abdominal obesity. Moreover, Ferriolli et al. (Reference Ferriolli, Pessanha and Moreira9) suggested that the WC may be one of the main factors that associate obesity with frailty in the older adults. However, Rodrigues et al. (Reference Rodrigues, Fhon and Huayta21) reported that the risk of frailty was positively associated with WHtR ≥ 0·50 but not with WC, suggesting that WHtR could be a better marker of abdominal obesity. Hirani et al. (Reference Hirani, Naganathan and Blyth41) also reported that the risk of frailty was significantly higher in the older adults with sarcopenic obesity than in those with non-sarcopenic normal weight, but it was not significantly different from those with non-sarcopenic obesity. It is well known that sarcopenia, defined as an abnormal loss of muscle mass and strength, is one of the major causes for an increase in the risk of frailty(Reference Yu, Ruan, D’Onofrio and Dionyssiotis42). Falsarella et al. (Reference Falsarella, Gasparotto and Barcelos43) reported that frail older individuals had a lower muscle mass and higher percentage of body fat compared with non-frail older individuals. In the present study, the obese older individuals had a higher lean mass compared with the older individuals with normal weight (online Supplementary Table S2).
In the present study, obesity was not associated with risk of frailty in men before and after adjusting for WHtR. The mediating effect of WHtR on the association between BMI and risk of frailty was shown only in women but not in men, suggesting that the mediating effect was moderated by sex (online Supplementary Fig. S1). In the present study, it could be impossible to detect the association between BMI and the risk of frailty in men, because there were only five frail men with obesity. Furthermore, according to the previous studies, men had a higher muscle mass compared with women(Reference Bredella and Jarvis44), and high muscle mass has been shown to be associated with lower risk of frailty in the older adults(Reference Meng, Shi and Shi45). The present study also showed that men had more muscle mass compared with women (online Supplementary Table S2). Bigaard et al. (Reference Bigaard, Tjønneland and Thomsen46) reported that WC reflected visceral fat, whereas BMI reflected fat-free mass or fat deposit elsewhere, suggesting that BMI in men could not be associated with the risk of frailty because of the body composition effect. In the present study, the effect of BMI on frailty score was positive in women, but negative in men, suggesting that high muscle mass in men could attenuate the adverse effect of obesity through WHtR on the risk of frailty (online Supplementary Fig. S1).
The previous studies consistently suggested that underweight was associated with higher prevalence and incidence of frailty(Reference Sanchez-Garcia, Sanchez-Arenas and Garcia-Pena6–Reference Rietman, Van Oostrom and Picavet10, Reference Lopez, Montero and Carmenate12–Reference Blaum, Xue and Michelon15). Underweight could have resulted from malnutrition, cachexia and sarcopenia, which were also associated with increased risk of frailty and represent low reserve capacity and weight loss, a key component of frailty(Reference Rolland, Van Kan and Gillette Guyonnet47). In the present study, underweight was associated with higher risk of frailty in the older men before and after adjusting for WHtR, suggesting that WHtR was not associated with the risk of frailty in men who are underweight. However, in the present study, the risk of frailty was not higher in underweight women because of a small proportion of underweight women with frailty (n 4).
Previous studies reported that overweight defined by BMI 25–29·9 kg/m2 was associated with lower prevalence and incidence of frailty in the older adults(Reference Hubbard, Lang and Llewellyn8–Reference Rietman, Van Oostrom and Picavet10,Reference Liao, Zheng and Xiu17) . Overweight was also associated with lower risk of mortality in the older adults, suggesting that overweight could represent a higher reserve capacity than those who are underweight or even normal weight(Reference Lee, Kim and Han7). Oreopoulos et al. (Reference Oreopoulos, Kalantar-Zadeh and Sharma48) reported that a higher BMI in advanced age can actually be considered a protective factor against oxidative stress, inflammation, malnutrition, fractures and cognitive decline. Additionally, the negative association between BMI and mortality suggested a paradoxical association of overweight or obesity with reduced CVD-related mortality in the older adults(Reference Kim49). In the present study, BMI 25–29·9 kg/m2 was associated with lower risk of frailty in all older individuals and in women after adjusting for WHtR.
The present study has a few limitations. First, the present study was composed of only a small proportion of frail older individuals. Second, the cross-sectional study design was not able to identify the causal association between BMI and WHtR with the risk of frailty. Third, although adjustments for confounders were made, unmeasured factors might affect the results of the present study.
In conclusion, the present study showed that the risk of frailty was higher in obese individuals, which was mediated by WHtR in older individuals, particularly older women, suggesting that abdominal obesity could increase the risk of frailty in women but not in men. A large population-based prospective observational study is needed to confirm whether the reduction in WHtR in obese older adults reduces the risk of frailty.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT) (NRF-2018R1A2B6002486) and a grant of the Korea Health Technology R&D Project through the Korean Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, the Republic of Korea (grant number: HI15C3153).
The authors’ contributions are as follows: M. K. performed the statistical analyses and prepared the first draft, Y. L. interpreted the results and edited the manuscript, E. K. assisted with statistical analyses and interpretation of results and edited the manuscript and Y. P. designed the study and finalised the manuscript. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114519002058







