The number of people with type 2 diabetes (T2D) may reach 552 million within the next 25 years( Reference Lorenzo, Wagenknecht and D'Agostino 1 , Reference DeFronzo 2 ). No simple or unique cure has been documented for this major health problem and the risk factors related to this disease. Therefore, there is an urgent need for preventative measures and effective approaches to overcome this serious health issue.
This may require a combination of several interventions. Currently, the strategy for treating T2D is primarily focused on changing lifestyle, e.g. increasing physical activity and making changes to the diet, which is identical to the strategy for treating insulin resistance, impaired glucose tolerance and obesity. Furthermore, a variety of medicines are used.
The regulation of blood glucose concentrations is the major focus of T2D research as even a moderate increase in postprandial glucose responses increases the risk of developing T2D and is an independent risk factor for the development of at least macrovascular complications in patients with diabetes mellitus( 3 – Reference Ceriello, Colagiuri and Gerich 5 ). One of the ways to regulate blood glucose and insulin secretion is to control the intake of food components that have a large effect on blood glucose concentrations. This especially includes decreasing the intake of starch and sugar, which can be a challenge in industrialised countries such as Denmark, where the overall intake of starch and sugar is high( Reference Pedersen, Fagt and Groth 6 ).
One of the ways to decrease postprandial hyperglycaemia is to modulate the absorption of glucose from digested carbohydrates. Increasingly, research has focused on substances inhibiting carbohydrate digestive enzymes( Reference Tucci, Boyland and Halford 7 – Reference Seri, Sanai and Matsuo 9 ) and thereby delaying absorption. Pentoses such as l-arabinose have been of interest as disaccharidase inhibitors, and several studies in rats and pigs have demonstrated a reduction in postprandial glucose( Reference Seri, Sanai and Matsuo 9 ) and insulin responses( Reference Seri, Sanai and Matsuo 9 , Reference Osaki, Kimura and Sugimoto 10 ) following ingestion of sucrose in combination with different concentrations of l-arabinose. A study using sucrose-rich diets supplemented with l-arabinose carried out in both healthy subjects and T2D patients has demonstrated a decreased glucose response after consumption of diets supplemented with 3 and 4 % l-arabinose( Reference Inoue, Sanai and Seri 11 ). Another study has demonstrated that l-arabinose added to a sucrose-rich drink has a lowering effect on postprandial blood glucose, insulin and C-peptide concentrations and that the mechanism underlying this effect is probably non-competitive inhibition of the brush border enzyme sucrase( Reference Krog-Mikkelsen, Hels and Tetens 12 ). The use of higher dietary doses of sucrose, however, would be unfeasible in terms of palatability and would have limited clinical applicability as very few people consume sucrose at quantities of 75 g in one drink, as has been used in the above-mentioned study. Of greater importance would be the combination of l-arabinose with starch, as starch is the main glucose-providing carbohydrate in the diet.
A recently performed in vitro study using l-arabinose and d-xylose has demonstrated an inhibiting effect on maltase (I Krog-Mikkelsen, S Petersen, K Halschou-Jensen, O Hels, I Tetens, JJ Holst, JR Andersen and K Bukhave, unpublished results). Maltase is a disaccharidase that is important for the breakdown of starch and is present in the brush border. The data indicated that l-arabinose might have a lowering effect on blood glucose and insulin concentrations after the intake of not only sucrose but also starch.
For public health purposes, lowering effects on blood glucose and insulin concentrations would be expected if blood glucose-lowering components could be incorporated in the habitual and/or recommended diet for the majority of the population.
We hypothesised that responses similar to the positive glucose and insulin responses in human subjects following ingestion of sucrose drinks supplemented with l-arabinose would occur in subjects who consume a mixed-meal diet containing sucrose and/or starch supplemented with l-arabinose. This hypothesis was tested in the present study. Furthermore, the possible inhibiting effects of l-arabinose on maltase were investigated separately.
Experimental methods
Subjects
Study 1
A total of seventeen healthy males with normal fasting blood glucose concentrations were recruited for this study.
The inclusion criteria were as follows: healthy male; age between 18 and 30 years; normal weight (BMI 18–25 kg/m2); waist circumference < 94 cm (>94 cm defined as abnormal for European men); no use of dietary supplements or regular use of medicine; not elite athlete; no changes in physical activity during the study period; no blood donation 3 months before study entry, no smoking; no alcohol intake >21 units of alcohol/week. The baseline characteristics of the subjects were as follows: age 22·5 (sd 2·6) years; BMI 22 (sd 1·22) kg/m2; blood pressure 131/71 (sd 12/8); pulse 68 (sd 12).
Study 2
A total of six healthy males were recruited for this study. The inclusion criteria were exactly the same as those of study 1. The baseline characteristics of the subjects were as follows: age 23·3 (sd 2·9) years; BMI 22·4 (sd 1·4) kg/m2; blood pressure 127/70 (sd 12/4); pulse 60 (sd 6).
Design
Study 1
This study was designed as a double-blind, controlled six-way cross-over design intervention trial and consisted of six test days of approximately 5 h duration. The subjects were served two different meals supplemented with three different concentrations of l-arabinose. Each test day was separated by a 1-week washout period. The subjects were instructed to refrain from consuming alcohol and performing intense physical activity 24 h before each test day and to fast from 20.00 hours the evening before the test day and allowed to drink only 0·5 litres of water during the fasting period. The subjects were instructed to avoid physical stress on the morning of each test day and to maintain their habitual diet and other lifestyle habits throughout the study period.
Study 2
To explore possible reasons for the findings from study 1, a smaller study was designed with focus on starch and the possible inhibition of maltase in the intestine. This study was designed as a double-blind, controlled six-way cross-over intervention trial and consisted of six test days of approximately 5 h duration. Each test day was separated by a minimum washout period of 48 h. To assess variations in gastric emptying, the subjects were served three different meals varying from liquid to solid with 0 or 20 % of l-arabinose. In addition, paracetamol was chosen as an indirect marker to assess gastric emptying. Paracetamol is poorly absorbed in the stomach, but rapidly absorbed in the small intestine and quantified in the blood( Reference Willems, Quartero and Numans 13 ). The subjects were instructed to behave exactly as in study 1 during the study period.
Both studies were conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethics Committee of the Region of Copenhagen. Written informed consent was obtained from all subjects. The protocols for the postprandial study were registered at ClinicalTrials.gov (ID: NCT00302302). The studies were carried out at the Department of Nutrition, Exercise and Sports, University of Copenhagen.
Meal designs
Study 1
Test meal A
Meal A contained 2 MJ in total, divided into 8·4 % of energy as protein, 34·2 % of energy as fat and 57·4 % of energy as carbohydrate. This meal consisted of a bun and a muffin containing starch and sucrose from wheat flour supplemented with 0, 5 or 10 % l-arabinose by weight, 10 g of raspberry jam supplemented with 0, 5 or 10 % arabinose, 10 g of butter, tea of universal herbs and 300 ml of water. The amount of l-arabinose in this meal corresponded to 0, 2·9 or 5·9 g.
Test meal B
Meal B contained 2·1 MJ in total, divided into 13·5 % of energy as protein, 29·1 % of energy as fat and 57·4 % of energy as carbohydrate. This meal consisted of two buns containing starch from wheat flour supplemented with 0, 5 or 10 % l-arabinose by weight, 20 g of cheese, 10 g of butter, tea of universal herbs and 300 ml of water. The amount of l-arabinose in this meal corresponded to 0, 2·5 or 4·9 g.
Study 2
Solid test meal
The solid meal contained 2·1 MJ in total, divided into 13·5 % of energy as protein, 29·1 % of energy as fat and 57·4 % of energy as carbohydrate. This meal consisted of two buns containing starch from wheat flour supplemented with 0 or 20 % l-arabinose by weight, 20 g of cheese, 10 g of butter, 100 ml of tea of universal herbs and 300 ml of water. The amount of arabinose in this meal corresponded to 0 or 10·2 g.
Semi-solid test meal
The semi-solid meal contained 2·1 MJ in total, divided into 13·5 % of energy as protein, 29·1 % of energy as fat and 57·4 % of energy as carbohydrate. This meal consisted of two buns containing starch from wheat flour supplemented with 0 or 20 % l-arabinose by weight, 20 g of cheese, 10 g of butter, 100 ml of tea of universal herbs and 300 ml of water.
The solids were blended with 150 ml of water and served as porridge. The remaining 50 ml of water was served as a drink together with the tea. The amount of arabinose in this meal corresponded to 0 or 10·2 g.
Liquid test meal
The liquid meal contained 75 g of maltose in 300 ml of water with and without 20 % l-arabinose (corresponding to 15 g of l-arabinose in the meal).
Test products
Buns and muffins with 0, 5, 10 and 20 % arabinose were baked at the bakery Nordic Sugar A/S in Arløv, Sweden. Arabinose was added on a weight % basis in relation to starch and sucrose contents. l-Arabinose was purchased from Danisco A/S.
The ingredients of buns and muffins are listed in Tables 1 and 2, respectively.
Table 1 Ingredients of buns used in meals A and B
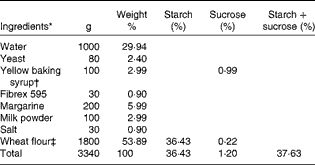
* Weight 70 g/bun.
† 33 % sucrose.
‡ 67·6 % starch, 0·4 % sucrose and 2·5 % fibre.
Table 2 Ingredients of muffins used in meal A
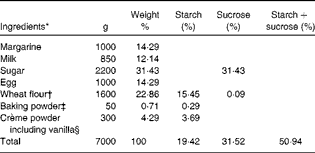
* Weight 50 g/muffin.
† 67·6 % starch, 0·4 % sucrose and 2·5 % fibre.
‡ 40 % starch.
§ 86 % starch.
To determine the amount of arabinose in the test meals, the buns and muffins were analysed after baking to determine the arabinose content. The analyses were carried out at the Department of Animal Science, University of Aarhus, Denmark.
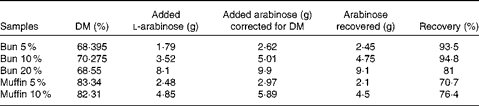
Arabinose content in the buns and muffins was determined using two different methods. Arabinose content in the buns was determined by direct hydrolysis – homogenisation with 12 m-H2SO4 followed by hydrolysis with 2 m-H2SO4 ( Reference Knudsen 14 ). Arabinose content in the muffins was determined by direct hydrolysis after removal of lipids by extraction with diethyl ether. Arabinose in the extracts or hydrolysates was reduced with potassium borohydride to the corresponding sugar alcohol, which was acetylated with acetic anhydride using n-methyl imidazole as a catalyst( Reference Connors and Pandit 15 ) before gas chromatographic analysis. The DM content in the dough, buns and muffins was determined by freeze-drying. The recovery of l-arabinose that was added was estimated to be 89 (sem 7·6) % for the buns and 73 (sem 4·0) % for the muffins. l-arabinose recovery data are given in Table 3.
Table 3 Recovery of l-arabinose in the buns and muffins used in meals A and B
Measurements
Study 1
On each test day, the subjects were weighed to the nearest 0·05 kg on a digital scale (Lindeltronic 8000; Lindells). A venflon catheter was inserted into the antecubital vein and the subjects were asked to rest for 10 min before collecting the baseline blood samples to measure glucose, insulin and C-peptide concentrations. Fasting blood samples were collected at − 15 min and again at 0 min to ensure proper baseline values. The test meal was served immediately after collecting the blood sample at 0 min and was consumed within 10 min by the subjects. Further blood samples were drawn at 15, 30, 45, 60, 75, 90, 105, 120 and 180 min after consumption of the test meal. The subjects were instructed to rest and were not allowed to sleep during the postprandial period.
Study 2
The same programme followed in study 1 was repeated in study 2. In addition, the subjects were served 1·5 g of paracetamol dissolved in 50 ml of water together with the test meal immediately after collecting the blood sample at 0 min and asked to consume the meal within 10 min. They were instructed to mix the meal and the dissolved paracetamol. The following parameters were used for determining the rate of paracetamol absorption: peak plasma concentration (C max); the time to reach C max (T max); the AUC; the slope for the three different meals.
Post-study measurements
On each test day, the following gastrointestinal side effects were recorded for the following 24 h with questionnaires using a five-level scale: heartburn; distension; nausea; vomiting; stomach ache and rumbling in the gut; flatulence; diarrhoea.
Laboratory analysis
Blood glucose concentrations were determined using an enzymatic colorimetric method on an ABX Pentra analyser (HORIBA ABX). Insulin and C-peptide concentrations were measured using a chemiluminescent immunometric assay (Immulite 1000; Diagnostic Products). Paracetamol concentrations were determined using an enzyme-specific reaction method on a Horiba ABX Pentra 400 analyser (HORIBA ABX).
Statistical analyses and calculations
The incremental AUC (iAUC) or incremental area above the curve (iAOC), ignoring the area beneath (or above in the case of iAOC), for fasting concentrations was calculated geometrically by applying the trapezoidal rule. The iAUC or the iAOC was calculated for the entire test period for 0–180 min. Postprandial response curves were evaluated by comparing C max values and iAUC values using ANCOVA with fasting values as covariates and T max values using ANOVA. A repeated-measures ANOVA was used to examine the effect of meal and time on the postprandial response curves. Data were controlled for homogeneity of variance, which was verified by residual plots, and assumption of normal distribution was investigated using normal probability plots and histogram plots. Significant results were analysed using the Tukey–Kramer test for post hoc analysis. All data are presented as means with their standard errors, unless otherwise indicated, and the statistical significance level was defined as P< 0·05. All statistical analyses and calculations were performed using the Statistical Analysis System software package, version 9.2 (SAS Institute, Inc.).
Power calculations were based on a standard formula using variance data from a previous study with l-arabinose added to a sugar drink( Reference Krog-Mikkelsen, Hels and Tetens 12 ).
A sample size of twenty-seven subjects was considered to be sufficient to detect a difference of 60 pmol/l in the concentration of C-peptide, with a power of 80 % and α = 0·05. As the study had a paired design, the sample size was reduced to seventeen subjects.
Results and discussion
The inhibition of small-intestinal carbohydrate digestive enzymes is a feasible means of suppressing abnormal postprandial changes in blood glucose concentrations and minimising the progression of T2D. This has been done using acarbose, which is a α-glucosidase inhibitor( Reference Najjar, Parsons and Duncan 16 ).
l-Arabinose has been suggested to be a potent sucrase inhibitor and, recently, a maltase inhibitor, as an in vitro study using l-arabinose demonstrated a non-competitive inhibition of sucrase( Reference Krog-Mikkelsen, Hels and Tetens 12 ) and a yet-to-be-published study (I Krog-Mikkelsen, S Petersen, K Halschou-Jensen, O Hels, I Tetens, JJ Holst, JR Andersen and K Bukhave, unpublished results) has demonstrated a similar inhibition of maltase. The inhibition of maltase is not as pronounced as that of sucrose, but the intake of starch is relatively higher than that of sucrose, which leads to a potentially new perspective regarding l-arabinose in relation to lowering postprandial blood glucose concentrations and insulin secretion.
Studies 1 and 2 were performed in healthy young men in accordance with ethical guidelines on the use of patients as subjects in trials without a documented effect in healthy human subjects.
The test meals were designed to resemble a normal breakfast meal with bread, muffin, butter, jam and tea and to contain both sucrose and starch from wheat flour or only starch from wheat flour to investigate the effects of l-arabinose on both sucrase and maltase.
Juntunen et al. ( Reference Juntunen, Niskanen and Liukkonen 17 ) showed that a meal containing wheat bread with 50 g of available carbohydrate increases plasma glucose concentrations up to 7 mmol/l and then restores them to basal levels within 3 h. This generates desirable conditions for measuring postprandial blood responses and the meals contained a minimum of 50 g of carbohydrate from sugars and wheat flour. Serum C peptide was used as a measure of β-cell function and thereby insulin secretion( Reference Horwitz, Starr and Mako 18 ).
l-Arabinose was added on a weight % basis in relation to sucrose and starch contents and the concentrations – 5 and 10 % l-arabinose – were based on a previous study with l-arabinose (maximum 8 %) added to a sucrose drink( Reference Krog-Mikkelsen, Hels and Tetens 12 ).
To determine the amount of l-arabinose included in the meals, the buns and muffins were analysed for l-arabinose content after baking. This analysis showed 90 and 70 % recovery of l-arabinose in the buns and muffins, respectively. A 100 % recovery was expected and further analyses were carried out. Analysis of the dough and extraction of fat revealed a similar recovery. It was assumed that all l-arabinose that was added was available in the meals and that the low recovery of l-arabinose was due to the method used for measuring the l-arabinose content.
The main findings from study 1 were that there were no differences in the C max or T max values of plasma glucose, serum insulin and serum C-peptide after consumption of meal A or meal B (Fig. 1)

Fig. 1 Mean plasma and serum concentrations of (a) glucose, (b) insulin and (c) C-peptide in seventeen normal men after consumption of meal A containing sucrose and starch from wheat flour supplemented with 0, 5 and 10 % l-arabinose by weight, butter, raspberry jam supplemented with arabinose and tea and meal B consisting of two buns containing starch from wheat flour supplemented with 0, 5 and 10 % l-arabinose by weight, butter, cheese and tea. No significant changes were observed in peak plasma concentrations, time to reach peak plasma concentrations or incremental AUC (iAUC) after consumption of meal A. No significant changes were observed in peak plasma concentrations and time to reach peak plasma concentrations after consumption of meal B. The iAUC for meal B with 10 % arabinose was 8 % greater than that for meal B with 0 % arabinose (P= 0·022). Values are means, with their standard errors represented by vertical bars. Meal A: ![]() , control;
, control; ![]() , 5 %;
, 5 %; ![]() , 10 %. Meal B:
, 10 %. Meal B: ![]() , control;
, control; ![]() , 5 %;
, 5 %; ![]() , 10 %.
, 10 %.
A few significant findings were recorded after consumption of test meal B, i.e. the iAUC values of insulin and C-peptide were higher after consumption of meal B with 0 % l-arabinose than after consumption of meal B with 10 % l-arabinose (P= 0·022). These results conflicted with our expectation.
This could be due to delayed gastric emptying after consumption of the solid food. This could affect the presentation of l-arabinose, disaccharides and starch to the epithelium of the small intestine. In addition, the concentration of sucrose in meal A could provide an explanation, as the amount of sucrose used in the drinking study was three times greater than that in meal A.
To investigate some of the limitations mentioned herein, an indirect measurement of gastric emptying was performed in study 2.
In study 2, starch from wheat flour was used in the mixed meals. Although the inhibiting effect on maltase seems to be less pronounced than that on sucrase, it led us to investigate the interaction of l-arabinose with meal B containing starch, as starch is the main glucose-providing carbohydrate.
In addition, no liquid version of starch was tested, and based on the questions about gastric emptying rate (GER) and the probability of dilution of the gastric volume, the dose of l-arabinose added to starch was increased to 20 % by weight. Meal B was designed in three different textures: a liquid maltose drink; a semi-solid meal B; a solid meal B, all supplemented with 20 % l-arabinose.
The main findings from study 2 were that there were no differences in the C max, T max or iAUC values of plasma glucose, serum insulin and C-peptide after consumption of the solid meal, semi-solid meal or liquid meal containing 0 and 20 % arabinose (Fig. 2).

Fig. 2 (a) Mean plasma and serum concentrations of glucose, insulin and C-peptide in six normal men after consumption of solid meal B consisting of two buns containing starch from wheat flour supplemented with 0 or 20 % l-arabinose by weight, butter, cheese and tea. No significant changes were observed in peak plasma concentrations, time to reach peak plasma concentrations or incremental AUC (iAUC). (b) Mean plasma and serum concentrations of glucose, insulin and C-peptide in six normal men after consumption of semi-solid meal B consisting of two buns with starch supplemented with 0 and 20 % l-arabinose by weight, butter, cheese and tea. No significant changes were observed in peak plasma concentrations, time to reach peak plasma concentrations or iAUC. (c) Mean plasma and serum concentrations of glucose, insulin and C-peptide in six normal men after ingestion of a maltose drink with 75 g of maltose in 300 ml water with 0 and 20 % l-arabinose by weight. No significant changes were observed in peak plasma concentrations, time to reach peak plasma concentrations or iAUC. Values are means, with their standard errors represented by vertical bars. ![]() , Solid 0 %;
, Solid 0 %; ![]() , solid 20 %;
, solid 20 %; ![]() , semi-solid 0 %;
, semi-solid 0 %; ![]() , semi-solid 20 %;
, semi-solid 20 %; ![]() , liquid 0 %;
, liquid 0 %; ![]() , liquid 20 %.
, liquid 20 %.
As has been mentioned above, the GER is an important factor in digestion and studies have shown that the GER is affected by the composition of food and the transition time for solid foods is greater than that for a liquid meal( Reference Maes, Hiele and Geypens 19 ) and, accordingly, absorption is slower.
There were no differences in the AUC or C max values of paracetamol in the plasma after consumption of the solid, semi-solid or liquid meal.
However, the T max was different (P< 0·0001) for the three meals. The T max for the solid meal was 35 min compared with 88 min for the semi-solid meal (P= 0·08) and 118 min for the liquid meal (P< 0·0001). Furthermore, the slope, which describes the GER, was similar for the liquid and semi-solid meals (0·008 and 0·0046), whereas the slope for the solid meal was negative ( − 0·006). These findings were rather contradictory in the sense that the observed difference in the GER was due to the solid meal having a greater T max value and a negative GER compared with the semi-solid and liquid meals, both of which had lower T max values. These contradictory findings could be due to the method used to measure paracetamol concentrations.
The results of serum paracetamol assay performed in study 2 indicate that the GER is greater for the liquid meal than for the semi-liquid meal, which confirms the assumption that the GER may be a factor that affects the results recorded after consumption of the sucrose meal in study 1.
At present, various methods are available to assess gastric emptying, of which scintigraphy is the best validated method and considered the gold standard( Reference Parkman, Harris and Krevsky 20 , Reference Smout, Horowitz and Armstrong 21 ). However, scintigraphic measurements require medical equipment and radiation exposure. Therefore, a more simple and easy-to-perform method is of interest for gastric emptying studies. Willems et al. ( Reference Willems, Quartero and Numans 13 ) found that the paracetamol method generally correlates well with scintigraphy of liquid-phase gastric emptying. Orally administrated paracetamol is poorly absorbed in the stomach, but rapidly absorbed from the small intestine. As gastric emptying is a rate-limiting step, it is believed that the rate of appearance of paracetamol in the blood reflects the GER( Reference Heading, Nimmo and Prescott 22 ).
One of the most important conditions for the measurement of gastric emptying is that the marker should be emptied at the same rate as the compound in question. Whether this is true for the solid and semi-solid meals used in the present study is questionable. The subjects were instructed to mix paracetamol dissolved in water with the test meal during consumption. Whether paracetamol was mixed efficiently with the meal is questionable and may be one of the reasons for the findings. In other studies using this method, paracetamol was baked into the test products( Reference Najjar, Parsons and Duncan 16 , Reference Juntunen, Niskanen and Liukkonen 17 ). The results of study 2 raise questions about the method and its feasibility when using a solid meal as the test meal.
In conclusion, the results of studies 1 and 2 showed that l-arabinose had the greatest sucrase-inhibiting effect and the effect was most promising when it was mixed with sucrose-containing drinks than with starch-containing drinks. Furthermore, the inhibiting effects were not observed when l-arabinose was added to solid mixed meals containing sucrose and starch from wheat flour. It could have been interesting to have recruited subjects with insulin resistance, as they have a much higher risk of developing T2D.
Acknowledgements
The authors thank Mona Pedersen, Elin Skytte and Kristina Møller for expert technical assistance and the study subjects for their willing participation.
Nordic Sugar (Copenhagen, Denmark) funded the study, but had no influence on the protocols, design, calculations or decision to publish the manuscript. Nordic Sugar A/S (Copenhagen, Denmark) supported PhD student K. H.-J., but had no influence on the protocols, design, calculations or decision to publish the manuscript.
The authors’ contributions are as follows: K. H.-J., J. R. A., K. B., S. N. and K. E. B. K. contributed to the study design and protocol; K. H.-J. collected the data; K. H.-J., J. R. A., K. B. and K. E. B. K. analysed the data; K. H.-J., J. R. A., K. B., S. N. and K. E. B. K. wrote the manuscript; K. H.-J., J. R. A., K. B., S. N. and K. E. B. K. approved the final version of the manuscript.
None of the authors has any conflicts of interest to declare.








