There is growing interest in the glycaemic properties of foods and the possible contribution of glycaemic properties to nutrition and health(Reference Howlett and Ashwell1). Over the last decades, a growing body of research has shown that diets based on low glycaemic and low insulinaemic foods reduce the risk of developing diabetes, obesity and CVD, improve blood glucose control in people with diabetes, may influence blood lipids and can be useful for weight management(Reference Dickinson and Brand-Miller2–Reference Livesey, Taylor and Hulshof4). Furthermore, low glycaemic and low insulinaemic foods are considered as favourable as they could contribute to a prolonged feeling of satiety and a sustained energy release with further implications for physical and mental performance(Reference Benton and Nabb5, Reference Siu and Wong6).
Conventional carbohydrate ingredients for the manufacturing of composite foods, like processed starch, glucose as such, glucose syrups, maltodextrins but also sucrose (suc) are predominantly more rapidly digested carbohydrates that induce a relatively high glycaemic and insulinaemic response. Even so, food manufacturers have the possibility to modify the glycaemic impact of foods by substitution of these more rapidly digested and thus high glycaemic carbohydrates by low glycaemic carbohydrate alternatives. Isomaltulose (iso; Palatinose™) is a recent example of such an alternative carbohydrate ingredient.
Iso is a reducing disaccharide comprised of glucose and fructose joined by an α-1,6-glycosidic bond. iso is naturally present in honey and sugarcane juice(Reference Lina, Jonker and Kozianowski7). Commercial iso (Palatinose™) is manufactured from food-grade suc by enzymatic rearrangement of the glycosidic linkage followed by crystallisation(Reference Mauch and Schmidt-Berg-Lorenz8–Reference Schiweck, Munir and Rapp10). Iso has been used as sugar in Japan and other Asian countries for more than two decades. In the European Union, it was approved as Novel Food in 2005(11) and is marketed as generally recognised as safe in the US(12).
Taste and appearance of iso are similar to suc, and the sweetness is about half of that of suc. In contrast to suc, iso is hardly utilised by oral plaque bacteria and is thus non-cariogenic(Reference Jonker, Lina and Kozianowski13). Due to its more stable α-1,6-glycosidic bond iso is slowly, though completely, hydrolysed by small intestinal disaccharidases. In studies utilising human small intestinal mucosa homogenates as enzyme source, iso was hydrolysed with Vmax of 26–45 % compared with suc(Reference Lina, Jonker and Kozianowski7). The released monosaccharides glucose and fructose are absorbed and metabolised. Due to the slower release and absorption of the monosaccharides, blood glucose and insulin response after oral administration were found to rise slower and reach lower maxima than after suc administration. The glucose supply is thus sustained over a longer period of time(Reference Lina, Jonker and Kozianowski7). With a calculated glycaemic index (GI) of 32, iso is a low glycaemic carbohydrate(Reference Atkinson, Foster-Powell and Brand-Miller14).
The digestibility characteristics of iso have been particularly derived from earlier in vitro and animal studies. Up to present, no human data were available whether iso, when incorporated in complex food and beverages, is indeed a slowly digestible, yet fully available carbohydrate. The studies carried out with iso so far are mainly single-meal trials in healthy human subjects. Thus, health effects of regular and longer-term consumption of iso are hardly known particularly in subjects with increased risk for non-communicable diseases.
The objectives of the present study were therefore: (a) to investigate the actual availability, i.e. digestibility and absorption of iso from human small intestine after consumption of iso-rich meals; (b) to examine glucose and insulin response to iso intake in healthy subjects; (c) to study possible health effects of a regular consumption of iso in volunteers with hyperlipidaemia.
Subjects and methods
A total of three human intervention trials were performed. The small intestinal digestion and absorption of iso incorporated in different foods were examined in a sequential, not blinded trial in healthy ileostomates. Trials 2 and 3 were performed in a double-blind, randomised, controlled cross-over design. Blood glucose and insulin profiles were determined over 3 h after the intake of iso in healthy subjects. Trial 3 investigated the effects of regular consumption of iso in overweight hyperlipidaemic subjects.
Trial 1 – ileostomy study on the digestion and absorption of isomaltulose
Ten healthy subjects with an end ileostomy without ileal resection participated in the trial (eight women and two men; mean age of 47 years, range 33–67 years). They had undergone colectomy between 1 and 28 years (mean 10 years) before the study; the underlying conditions were Crohn's disease (n 6), ulcerative colitis (n 3) or familial adenomatous polyposis (n 1).
On two separate days, the participants consumed a test meal including 50 g iso for breakfast after overnight fasting. The two study days were separated by at least 6 d. All the test meal products were prepared and supplied by Suedzucker AG (Mannheim/Ochsenfurt, Germany). Test meal 1 was a beverage (500 ml); meal 2 consisted of a beverage (250 ml) and biscuits (140 g) each containing 25 g iso. The 140 g biscuits provided 2·3 MJ, 79·2 g carbohydrate, 9·8 g protein, 44·8 g fat. Both the test meals were consumed within 5–7 min. After 5 h, the subjects were served a standardised lunch (400 g lasagne: 2·8 MJ, 56 g carbohydrate, 24 g protein, 40 g fat). The ileostomy bags were collected immediately before and hourly within the next 8 h after the test meal intake. The ileostomy effluents were weighed, homogenised, and aliquots were frozen at − 20°C until analysis. To avoid bacterial degradation of the effluent, antibiotic solutions (5 ml ampicillin and 5 ml metronidazole) were added to each new applied bag. The suitability was confirmed in pre-tests with spiked ileostomy samples.
The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the local Ethics Committee of the Faculty of Medicine, University of Würzburg, Germany. Written informed consent was obtained from all the subjects.
Trial 1 was carried out at University of Würzburg, Department of Medicine II, Division of Gastroenterology, Grombühlstr. 12, D-97 080 Würzburg, Germany.
Analytic procedures
Ileostomy fluid homogenate samples were analysed for iso, as well as glucose and fructose as hydrolysis products using GC (Agilent 6890) and a capillary fused silica column CP-Sil 8CB (15 m × 150 μm × 0·15 μm) with FID. Carrier gas was helium. The temperature program was 80°C for 1 min, 30°C/min to 170°C, 10°C/min to 350°C (3 min). The method was validated beforehand. Linearity of the GC method was confirmed by analysis of standard solutions of different iso concentrations with a (linear) coefficient of correlation of 0·99 993. In ileostomy samples free of any additions, iso co-eluted only with negligible amounts ( < 0·1 %) of unknown compounds. Repeatability was calculated from ten replicate determinations of a sample (in each case completely separate sample preparation) with a coefficient of variation of 2·4 % for the iso content and 0·06 % for the retention time was obtained. Recovery determined by samples spiked at three concentration levels (10, 1 and 0·1 % iso) was 96, 100 and 111 %.
Sample preparation and derivatisation was carried out as follows. Approximately, 1 g of sample and 0·05 g Phenyl-β-d-glucopyranoside (internal standard) were weighed with an accuracy of ± 0·1 mg. After addition of 1 ml ultra-pure water and 7·95 ml pyridine, sample was stirred for 30 min. To 0·2 ml of this sample, 0·8 ml oxime reagent (10 g hydroxyl ammonium hydrochloride in 90 ml pyridine) was added and warmed for 30 min to 60°C. After cooling to room temperature, 0·4 ml pyridine and 0·5 ml N-methyl-N-trimethylsilyltrifluoracetamide (MSTFA) were added to 0·1 ml of this solution. After warming to 60°C for another 30 min and cooling down, the sample was directly injected into the GC. Concentrations of saccharides were calculated based on peak area ratios of the sample compared to standard chromatograms.
Calculations
The apparent digestibility of iso was calculated as intake of iso (50 g) minus excretion of iso (g/8 h) as a percentage of intake. Furthermore, the absorption was calculated as intake of carbohydrate amount (50 g iso) minus excretion of carbohydrate amount (g/8 h; sum of iso and of glucose and fructose as resulting monosaccharides from the intestinal hydrolysis of iso) as a percentage of intake.
Trial 2 – blood glucose and insulin response study
A total of ten healthy subjects (nine female, one male, age: 30·9 (sd 8·5) years; BMI: 23·3 (sd 2·8) kg/m2) were studied on two study days with respect to their blood glucose and insulin response over 3 h following ingestion of either 50 g of iso (Palatinose™) or suc dissolved in 0·5 litre water. Subjects came to the institute having fasted and refrained from smoking for 12 h. To obtain baseline values, capillary blood glucose was measured (from the fingertip), and venous blood for insulin determination was taken via indwelling cannula inserted in a suitable forearm vein. Thereafter, the subjects received one portion of the iso or suc drink that was at room temperature and was ingested within 5 min.
Measurements of postprandial capillary blood glucose concentrations and blood sampling for determination of insulin in plasma were performed over 3 h at 15, 30, 45, 60, 90, 120, 150 and 180 min after ingestion of the test food.
Capillary blood glucose measurements were performed using a Super GL analyzer (Hitado, Delecke-Möhnesee, Germany), utilising the glucose oxidase technique. Measurements were performed on the whole blood haemolysate in a flow chamber using membrane-bound glucose oxidase. Insulin was analysed using an electrochemiluminescence immunoassay (Elecsys® 1010/2010/MODULAR ANALYTICS E170, Roche Diagnostics GmbH, Mannheim, Germany).
For each test food and individual subject, incremental area under the curves above baseline of blood glucose and plasma insulin concentrations was calculated.
The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the local Ethics Committee of the Ärztekammer Nordrhein, Düsseldorf, Germany. Written informed consent was obtained from all the subjects.
Trial 2 was carried out at the Profil Institut für Stoffwechselforschung GmbH, Hellersbergstr. 9, D-41 460 Neuss, Germany.
Trial 3 – effects of regular consumption of isomaltulose in hyperlipidaemic subjects
Seventy-four volunteers were screened to recruit twenty subjects fulfilling all inclusion and none of exclusion criteria. All twenty volunteers recruited for the trial completed the study (twelve women and eight men). Before entry, the subjects were screened for their blood lipid status and were considered hyperlipidaemic with baseline cholesterol level>6·5 mmol/l and/or TAG level>2·3 mmol/l, i.e., levels usually associated with a higher risk for CVD(Reference Graham, Atar and Borch-Johnsen15). Exclusion criteria were: age < 18 years; BMI < 25 or>40 kg/m2; history of severe chronic medical disease, including gastrointestinal diseases and diabetes; glucocorticoid therapy; pregnancy; unusual dietary habits.
The study was designed as a randomised, double-blind, controlled, cross-over trial with two 4-week test periods separated by a 4-week washout phase. During the two 4-week study periods, the participants consumed a controlled diet. The diets were isoenergetic and composed as a typical Western diet (low-fibre and high-fat), containing the same basic foods and test products, except that the test products provided either 50 g/d iso or 50 g/d suc. Comprising 50 g/d iso or suc, the diets finally provided 47 % of energy as carbohydrates, 39 % of energy as fat, 14 % of energy as protein and 1·4 g/MJ dietary fibre (Table 1). Iso and suc were included in sweet foods (pudding, biscuits, toffees) and beverages (milk drinks, soft drinks, tea beverages). All the test products were prepared and supplied by Suedzucker AG. As shown in Table 2, a 7 d rotating menu including ready-to-eat foods (Eismann Tiefkühl-Heimservice, Mettmann, Germany; Hofmann Menü-Manufaktur, Boxberg-Schweigern, Germany) was used. Meals were weighed and packaged in the metabolic unit of the clinic. All meals were given to the subjects to be eaten at home. Additional food was not allowed, the participants were instructed to completely consume the provided portions. Water, tea and coffee were allowed ad libitum. Alcohol consumption was restricted to 500 ml beer or 250 ml wine per week. The macronutrient composition of the diet was calculated with PRODI expert 4.5 software (Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart, Germany). Food was given to the subjects every other day in the metabolic unit by a study nutritionist. This regular contact was maintained to ensure volunteers' compliance. In addition, the subjects were advised to return all blank packages of the test food at the following visit. Body weight was measured at entry and then at 2 d intervals in the first week and thereafter at weekly intervals in both phases.
Table 1 Cross-over study: composition of the study diet
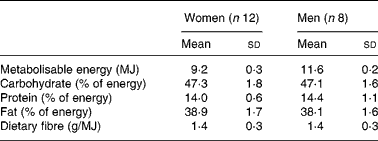
The energy, macronutrient and dietary fibre intake were calculated with PRODI expert 4.5 software (Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart, Germany). In case of ready-to-eat foods (Eismann Tiefkühl-Heimservice, Mettmann, Germany; Hofmann Menü-Manufaktur, Boxberg-Schweigern, Germany), the data were based on manufacturer information.
Table 2 Cross-over study: 7 d rotating menu containing basic diet and test products for men

The calculated daily energy was 11·6 MJ for men. The menu for women was lower in energy (9·2 MJ) but containing the same foods.
Each ready-to-eat menu contained meat and side dishes. Total weight was 480–600 g.
* Drink/food containing isomaltulose or sucrose.
† Ready-to eat food by Eismann Tiefkühl-Heimservice, Mettmann, Germany.
‡ Ready-to-eat food by Hofmann Menü-Manufaktur, Boxberg-Schweigern, Germany.
The subjects kept a daily record of subjective gastrointestinal parameters, stool frequency and stool consistency during the two 4-week study periods. Abdominal distension, flatulence, abdominal pain and nausea were scored on semi-quantitative scales from 0 (absent) to 3 (severe). Stool consistency was also scored from 1 (hard) to 4 (watery).
Twenty-four-hour urine collections were obtained before and after each test period and analysed for electrolytes and albumin. At the same time venous blood was taken in the morning after 12 h overnight fasting period and analysed for general safety measurements as well as metabolic and risk measurements. Routine biochemical measurements and blood lipid status were analysed in the Central Laboratory of the University Hospital. In addition, tests for the assessment of renal and liver function, blood glucose, HbA1c, C-reactive protein, uric acid, electrolytes, blood coagulation tests and a complete blood cell count were performed. Serum and plasma samples for the determination of fructosamine, proinsulin, insulin, C-peptide, adiponectin, leptin, NEFA, oxidised LDL were stored at − 80°C until further analysis.
The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the local Ethics Committee of the Faculty of Medicine, University of Würzburg, Germany. Written informed consent was obtained from all the subjects.
Trial 3 was carried out at University of Würzburg, Department of Medicine II, Division of Gastroenterology, Grombühlstr. 12, D-97 080 Würzburg, Germany.
Mean transit time and stool weight
Mean transit time was measured from days 21–23 of each study period using the single stool method of Cummings & Wiggins(Reference Cummings and Wiggings16) and radiopaque markers (P & A Mauch, Münchenstein, Switzerland) as described previously by Gostner et al. (Reference Gostner, Schäffer and Theis17). From days 24–26 of each study period, stools were collected for quantitative assessment. Each stool was collected separately, frozen and stored at − 25°C. After completion of the study, stool samples were thawed, and stool wet weight and faecal pH value were recorded. Three-day stool collections were pooled using a grinder. Faecal dry weight was measured after lyophilisation (Gamma IA apparatus; Christ, Osterode, Germany) of an aliquot to a constant weight.
Biochemical analyses
Cholesterol, TAG, HDL cholesterol and LDL cholesterol in serum were analysed using colorimetric enzyme kits (Roche Diagnostics). apo A1 and apo B100 were measured immuno-turbidimetrically (Roche Diagnostics). HbA1c measurement was performed by HPLC, and fructosamine was determined using an enzymatic colorimetric assay kit (Boehringer, Mannheim, Germany). Venous blood glucose samples were analysed by hexokinase method (Roche Diagnostics). Proinsulin concentration was determined using a RIA kit (Linco Research, St Charles, MO, USA). Serum insulin and C-peptide were measured by RIA (Schering GmbH, Berlin, Germany). The homeostasis model assessment for insulin resistance (HOMA-IR) was determined for each individual according to Matthews et al. (Reference Matthews, Hosker and Rudenski18). Oxidised LDL was determined in plasma samples using an ELISA kit (Mercodia, Uppsala, Sweden). NEFA level was determined by enzymatic colorimetric assay kit (Wako Chemicals GmbH, Neuss, Germany). Adiponectin concentration was measured using a RIA kit (Linco Research). Leptin was analysed in serum probes with a RIA kit (Mediagnost, Reutlingen, Germany).
Statistical analysis
Statistical analyses were carried out using SPSS for Windows version 14.0.1. Values are given as mean values and standard deviations. The non-parametric Wilcoxon rank sum test for paired data was used to determine the specific differences between iso and suc (trials 2 and 3) and between the two test meals (trial 1). Differences were considered to be significant at P < 0·05.
In addition, for blood lipids, measurements of carbohydrate metabolism, adipocytokines and risk factors (trial 3), Wilcoxon test was performed to test for significant differences between baseline and 4-week measurements within each treatment.
Results
Trial 1 – ileostomy study
The amount of iso excreted after intake of both the test meals was very low, corresponding to 4·5 % (test meal 1) and 1·2 % (test meal 2) of intake, indicating a high apparent digestibility and absorption, unaffected by the nature of the meal (liquid v. solid). After consumption of 500 ml beverage including 50 g iso (meal 1), the determined apparent digestibility was 95·5 %, ranging from 75·6 to 100 % (median: 99·1 %). The apparent absorption rate of ingested iso from test meal 1 was estimated to be 93·6 % (range: 69·3–99·8 %; median: 98·1 %). Similar results were found after consumption of a combination of 250 ml beverage and 140 g biscuits including 50 g iso. The determined apparent digestibility was 98·8 % (range: 95·3–100 %; median: 99·5 %) and apparent absorption was 96·1 % (range: 88·6–99·6 %; median: 97·7 %). Digestibility and absorption of iso were almost complete in both the tests. Thus, in this assay, the nature of the meal (liquid or solid) did not significantly influence the digestibility and absorption of iso. Data are shown in Fig. 1.

Fig. 1 Ileostomy study: apparent digestibility, absorption and excretion of isomaltulose from different meals.1, Test meal 1 was a beverage (500 ml) containing 50 g isomaltulose; 2, test meal 2 was a beverage (250 ml) and biscuits (140 g) together containing 50 g isomaltulose. ▧, Digestibility; ![]() , absorption; ■, isomaltulose excretion.
, absorption; ■, isomaltulose excretion.
Trial 2 – blood glucose and insulin response study
The mean 3 h blood glucose and insulin response curves for the 50 g portions of suc and iso are shown in Fig. 2. Iso produced the lowest blood glucose response curve (incremental area under the curves: 118 (sd 54) v. 184 (sd 70) min × mmol/l; P = 0·037), increasing gradually and only moderately. A plateau rather than a peak was obtained of about 5·8 mmol/l approximately 15–30 min later than the peak with suc. After the maximum, blood glucose slowly declined and remained above baseline until the end of testing. After intersection of blood glucose profiles of suc and iso at about 60–90 min, blood glucose with iso remained higher for more than 90 min.

Fig. 2 Blood glucose and insulin response study. (a) Blood glucose profiles of 50 g isomaltulose (![]() ) and sucrose (●) over 3 h. (b) Insulin profiles of 50 g isomaltulose and sucrose over 3 h. Mean values were significantly different: *P < 0·05, **P < 0·01 by Wilcoxon test for paired data.
) and sucrose (●) over 3 h. (b) Insulin profiles of 50 g isomaltulose and sucrose over 3 h. Mean values were significantly different: *P < 0·05, **P < 0·01 by Wilcoxon test for paired data.
The insulin response for the saccharides was directly proportional to their glycaemic response, both in the magnitude and shape of the response curve (incremental area under the curves: 15 208 (sd 8639) v. 23 347 (sd 14 451) min × pmol/l; P = 0·005). Iso evoked the lowest insulin response with a maximum of 227·8 pmol/l, which is more than 50 % lower compared with suc (470·1 pmol/l).
Trial 3 – effects of regular consumption of isomaltulose in hyperlipidaemic subjects
Subjects and compliance
Twenty volunteers, aged 21–61 years (mean 48·2 years) completed the study (twelve women and eight men; no drop-out). The two 4-week study periods had the same number of subjects. The mean BMI of the women was 31·9 (sd 3·1) kg/m2 and of the men 33·4 (sd 2·4) kg/m2. Mean BMI and mean body weight did not differ between the treatments. There was a slight and similar reduction in body weight with both the treatments ( − 0·9 (sd 1·0) kg with iso and − 1·1 (sd 0·7) kg with suc). Subjective intestinal characteristics such as distension were usually rated as low or unchanged and did not significantly differ between iso and suc. With each treatment, pain and nausea were scored as absent. In conclusion, the daily consumption of 50 g iso was well accepted and tolerated by the subjects.
The compliance of volunteers was maintained throughout the study as also indicated by the virtually complete return (97 %) of blank packages of test products.
Routine blood measurements
Routine biochemical and standard clinical measurements for the assessment of renal and liver function, clotting (prothrombin time and partial thromboplastin time) and blood cell counts were not significantly different between both the treatments. The values remained in the normal range with both interventions (data not shown).
Urine and stool measurements
Routine urine measurements, including Ca, K, Na, phosphate and albumin excretion were comparable with both the treatments and remained in the normal range (data not shown).
Mean values of stool frequency and stool consistency were not significantly different between iso and suc intervention (data not shown). Furthermore, mean transit time (iso 47·3 (sd 13·4); suc 42·8 (sd 11·8) h) as well as wet weight (iso 151·1 (sd 55·8); suc 162·7 (sd 70·5) g/d), dry weight (iso 29·4 (sd 13·3); suc 32·2 (sd 14·8) g/d), faecal water content (iso 121·7 (sd 51·7); suc 130·6 (sd 59·8) g/d) and pH (iso 7·3 (sd 0·3); suc 7·5 (sd 0·4) g/d) were not influenced by iso intake and did not differ significantly between the treatments.
Blood lipids
No significant treatment difference was obtained for total cholesterol (baseline: iso 6·2 (sd 1·0) v. suc 6·2 (sd 1·0) mmol/l; week 4: iso 6·1 (sd 0·9) v. suc 6·2 (sd 0·9) mmol/l), HDL cholesterol (1·3 (sd 0·4) v. 1·3 (sd 0·4) mmol/l; 1·3 (sd 0·4) v. 1·4 (sd 0·3) mmol/l), LDL cholesterol (3·9 (sd 1·0) v. 3·8 (sd 0·9) mmol/l; 3·8 (sd 0·9) v. 3·8 (sd 1·0) mmol/l), TAG (2·2 (sd 1·2) v. 2·5 (sd 1·9) mmol/l; 2·4 (sd 1·4) v. 2·3 (sd 1·6) mmol/l), apo A1 and apo B100 as well as LDL/HDL ratio and Apo B100/Apo A1 ratio.
Measurements of carbohydrate metabolism
The mean fasting levels of blood glucose, insulin, C-peptide, proinsulin, HbA1c, fructosamine and insulin resistance (HOMA-IR) at baseline were not significantly different, except values of blood glucose. Mean fasting levels at all time points were similar in the higher normal range or in the case of proinsulin slightly higher. There was no significant treatment difference. With both the interventions, there was a slight but statistically significant decrease in concentrations of C-peptide (baseline: iso 1·1 (sd 0·4) v. suc 1·1 (sd 0·4) nmol/l; week4: iso 1·0 (sd 0·4) v. suc 1·0 (sd 0·3) nmol/l) and proinsulin (17·2 (sd 11·4) v. 18·6 (sd 12·3) pmol/l; 15·1 (sd 12·8) v. 14·6 (sd 10·0) pmol/l). Significant lower fasting blood glucose levels (5·3 (sd 0·5) v. 5·0 (sd 0·6) mmol/l; 4·9 (sd 0·4) v. 4·9 (sd 0·3) mmol/l; P = 0·002) together with reduced HOMA-IR (4·6 (sd 2·7) v. 4·8 (sd 2·6); 3·8 (sd 2·1) v. 3·9 (sd 1·7); P = 0·036) were noted after the 4-week iso intervention compared with baseline (Fig. 3), however, no significant differences in fasting blood glucose levels and HOMA-IR were observed at 4 weeks between iso and suc.
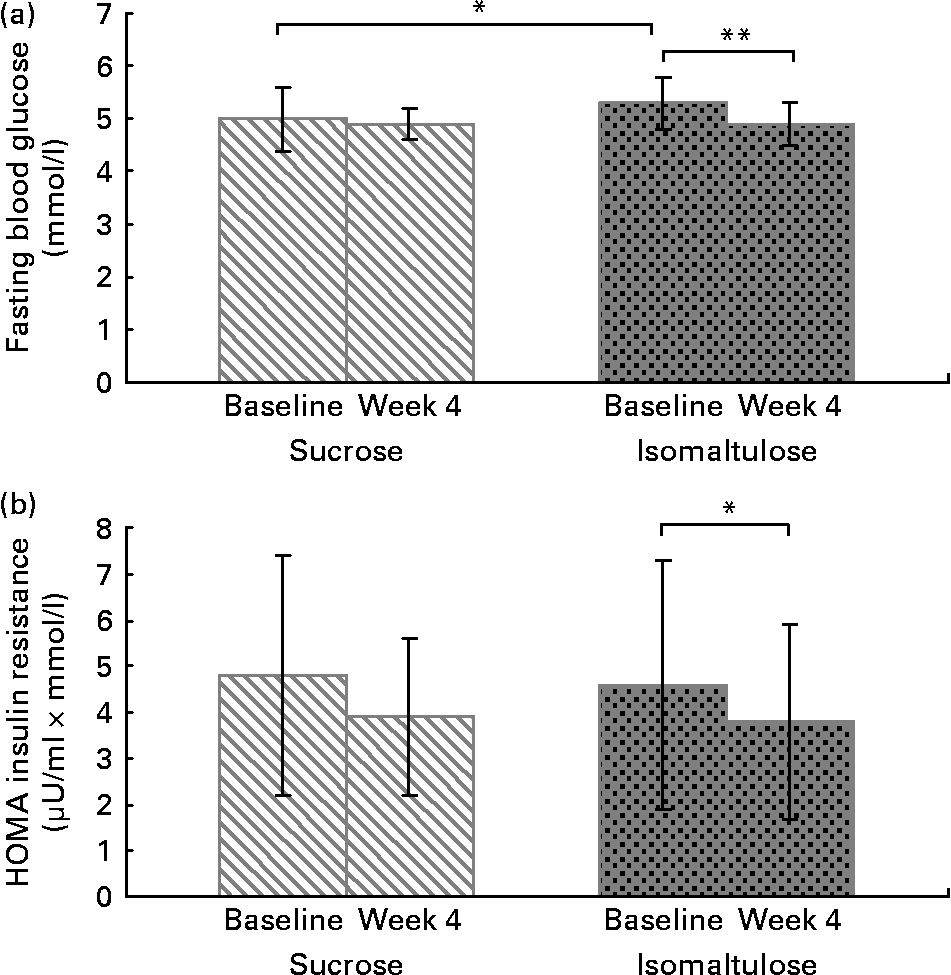
Fig. 3 Cross-over study. (a) Fasting blood glucose levels of twenty hyperlipidaemic subjects following consumption of either 50 g isomaltulose/d or 50 g sucrose/d for 4 weeks. (b) HOMA insulin resistance of twenty hyperlipidaemic subjects following consumption of either 50 g isomaltulose/d or 50 g sucrose/d for 4 weeks. Mean values were significantly different: *P < 0·05, **P < 0·01 by Wilcoxon test for paired data.
Adipocytokines and risk factors
Adiponectin levels were comparable with both the treatments. There was a slight and comparable reduction in leptin concentrations with iso and suc intervention. The cardiovascular risk factors oxidised LDL and NEFA were stable with iso consumption and did not differ significantly in comparison with suc (data not shown).
Discussion
To our knowledge, this is the first study investigating the digestibility and absorption of iso in vivo in human subjects. The ileostomy model applied here is a suitable method for evaluating absorption processes and was extensively used in many studies for determination of small intestinal digestion of carbohydrates(Reference Sandberg, Ahderinne and Andersson19–Reference Normén, Laerke and Jensen24). Results from our ileostomy study with iso confirmed findings from earlier in vitro and animal studies(Reference Lina, Jonker and Kozianowski7) that iso is virtually completely digested and absorbed from the human small intestine, irrespective of the food matrix and the food consistency. Together, with the findings from the postprandial blood glucose and insulin determination after iso consumption, these results confirm that iso is completely, but very slowly hydrolysed and absorbed in the small intestine, leading to a prolonged delivery of blood glucose for metabolism. Thus, the present study confirms that iso is a completely available low glycaemic and low insulinaemic carbohydrate.
In the 4-week cross-over trial on the effects of regular iso consumption, iso was given within a controlled and typical Western diet (low-fibre and high-fat) in the form of a variety of foods like pudding, biscuits, toffees and beverages (Table 2). Iso and its products used in the present study were well accepted and 50 g/d over the 4-week test period was well tolerated by the participants.
The 4-week cross-over study was aimed to examine the effects of regular iso consumption instead of higher glycaemic ingredients in hyperlipidaemic subjects. To our knowledge, this is the first human intervention study looking at regular consumption of iso over a longer period. In the present study, blood lipids (cholesterol, LDL, HDL, TAG and apo) were not affected by low glycaemic carbohydrate iso. Oxidised LDL and NEFA levels stayed constant both with suc and iso. Relationships between dietary GI and blood lipid fractions have been assessed in several prospective observational studies. An inverse association between HDL cholesterol and dietary GI was found in many studies(Reference Liu, Manson and Stampfer25–Reference Slyper, Jurva and Pleuss27), although one study detected no association(Reference Murakami, Sasaki and Takahashi28). Furthermore, observational studies showed that TAG levels tend to rise when GI or glycaemic load (GL) of the habitual diet increases. However, results from the intervention trials differed from those of observational studies. Opperman et al. (Reference Opperman, Venter and Oosthuizen29) have carried out a meta-analysis of fourteen randomised, controlled clinical trials to determine if low GI diets, compared with conventional or high GI diets, have beneficial effects on markers for lipid metabolism. In the studies reviewed, low GI diets caused a statistically significant improvement in total cholesterol concentrations but not in TAG, HDL and LDL cholesterol. Another meta-analysis by Livesey et al. (Reference Livesey, Taylor and Hulshof4) demonstrated no clear evidence for a difference in TAG following lower GI/GL intervention, although TAG levels were reduced in those groups with the highest levels. In hyperlipidaemic subjects without diabetes, Jenkins et al. (Reference Jenkins, Wolever and Kalmusky30) showed that 4 weeks on low GI diet could reduce total cholesterol and LDL cholesterol by about 10 % and TAG by 20 % compared with high GI diet. This indicates that influence of the overall dietary GI or GL on markers of lipid metabolism varies between studies. Moreover, it would be conceivable that more pronounced favourable effects of lower GI/GL intervention may particularly present in more symptomatic hyperlipidaemics.
With regard to carbohydrate metabolism, significant lower fasting blood glucose levels and insulin resistance (HOMA-IR) were noted after 4-week iso compared with baseline, whereas these differences were non-significant with suc treatment. However, there were no significant differences between iso and suc at 4 weeks in any measurement of carbohydrate metabolism. Both the interventions slightly improved proinsulin and C-peptide levels. Most intervention trials looking at effects of GI/GL on carbohydrate metabolism were carried out in type 2 or type 1 diabetics. Meta-analyses demonstrated that studies in patients with type 2 diabetes showed beneficial effects of low GI/GL diets reflected in decreased HbA1c, fructosamine(Reference Opperman, Venter and Oosthuizen29, Reference Brand-Miller, Hayne and Petocz31) as well as HOMA-IR, fasting blood glucose and insulin levels(Reference Livesey, Taylor and Hulshof4). Data for subjects with unimpaired carbohydrate metabolism, however, are rare. Brand-Miller et al. (Reference Brand-Miller, Hayne and Petocz31) reported a non-significant decrease in fructosamine in healthy subjects by low GI diets. In addition, Livesey et al. (Reference Livesey, Taylor and Hulshof4) observed that for improvement in fasting blood glucose levels and glycated proteins, there is evidence that the effects are greatest among those with poorest glycaemic control. For reduction in fasting blood glucose, the threshold is about 5 mmol/l. With reference to insulin levels, no treatment effects were determined for concentrations < 100 pmol/l(Reference Livesey, Taylor and Hulshof4). Hence, our data are consistent with the literature. In subjects with more obvious disturbances in blood glucose control, such as people with impaired glucose tolerance or manifest diabetes, presumably more pronounced beneficial longer term effects may be possible as cause of the lower and prolonged glycaemic response and the lower insulinaemic burden associated with iso.
In conclusion, the present study confirms that iso is completely, but very slowly hydrolysed and absorbed from the human small intestine, irrespective of food matrix and consistency, leading to a prolonged delivery of blood glucose. Furthermore, the present study confirms that regular consumption of iso over longer terms is well tolerated also in subjects with increased risk for vascular diseases.
Acknowledgements
We thank K. Backhaus, D. Dorbath, E. Kelber, H. Lichtlein and A. Volk for excellent technical assistance; Margit Arenz and Tillmann Doerr of Suedzucker AG for development and preparation of the test products; Dierk Martin and Willi Kundel of Suedzucker AG, CRDS for analysis of ileostomy samples; the central laboratory of the University of Würzburg, Germany for routine blood and urine analyses. The present study was funded by Beneo-Palatinit, Mannheim, Germany, a member of the Südzucker Group. The study results and data contained in the publication have been developed by and/or for Beneo. Beneo reserves the exclusive right to use the results and data for possible health claim requests. The authors' responsibilities were as follows: I. H., A. G., S. T. and W. S. conceived and designed the study; I. H., A. G., T. K. were responsible for implementation of trials 1 and 3; L. N. was responsible for implementation of trial 2; I. H. was responsible for data analysis and interpretation and writing of the manuscript; A. G., S. T. and W. S. were responsible for the critical revision of the manuscript and its important intellectual content; R. M. and W. S. were responsible for study supervision. None of the authors had a conflict of interest. (S. T. is employed by Suedzucker AG.)







