Introduction
Docosahexaenoic acid (DHA) and arachidonic acid (AA) are important to foetal and infant growth and development. Observational and intervention studies provide consistent evidence that maternal dietary and circulating DHA is an important determinant of foetal blood concentrations of DHA(Reference Innis1–Reference Koletzko, Larque and Demmelmair6). Although linoleic acid (LA) and α-linolenic acid (ALA), and preformed long chain polyunsaturated fatty acids (LCPUFAs), can be transported through the placenta, there is a preferential transfer of the latter forms(Reference Koletzko, Larque and Demmelmair6, Reference Larque, Krauss-Etschmann and Campoy7). Several studies have assessed the effect of prenatal LCPUFA dietary supplementation on pregnancy outcome, aiming to improve it—i.e., preventing preeclampsia, prolonging gestation, preventing preterm birth, and improving foetal growth(Reference Elias and Innis3, Reference Olsen, Hansen and Sorensen8–Reference Olsen9).
On the other hand, the importance of DHA in central nervous system (CNS) development is one of the most intensely studied areas (Reference Heird and Lapillonne10–Reference Hibbeln, Ferguson and Blasbalg14). DHA functions in neurogenesis, neurotransmission and protection against oxidative stress(Reference Innis15). AA is also important for infant growth and development. n-3 and n-6 LCPUFA are critical for infant and child brain development; they are involved in numerous neuronal processes, ranging from effects on membrane fluidity to gene expression regulation(Reference Schuchardt, Huss and Stauss-Grabo16). Brain accumulation of DHA starts in utero, with quantitatively marked deposition in the second half of gestation(Reference Clandinin, Chappell and Leong17–Reference Innis19), coinciding with the growth spurt in the grey matter(Reference Innis19). Deficiencies and imbalances of LCPUFAs are associated with impairments in cognitive and behavioural performance(Reference Innis19–Reference Williams, Birch and Emmett20).
Fish intake during pregnancy and a higher n-3 LCPUFA status at birth were associated with a better visual development in infants born at term(Reference Williams, Birch and Emmett20–Reference Hibbeln, Davis and Steer22). However, levels of DHA and eicosapentaenoic acid (EPA) are often low in the Western diet(Reference Koletzko, Lien and Agostoni23–Reference Gibson, Muhlhausler and Makrides24). It remains controversial whether LCPUFA supplementation to pregnant and breastfeeding mothers is beneficial for the development of their infants(Reference Delgado-Noguera, Calvache and Bonfill25–Reference Dziechciarz, Horvath and Szajewska26), optimal doses for efficacy and long-term effects at different developmental ages remain to be determined. Today, there are in excess of 40 perinatal randomized controlled trials involving LCPUFA interventions assessing different aspects of early childhood development and growth(Reference Birch, Birch and Hoffman29).
The earliest publications in human infants from the early 1990s showed that preterm infants fed a formula supplemented with n-3 LCPUFA, mainly as DHA, had improved retinal sensitivity and visual acuity compared with preterm infants fed the standard un-supplemented formulas of the day, which were low in n-3 PUFA (most were lacking alpha-linolenic acid) and were rich in n-6 PUFA(Reference Uauy, Birch and Birch28–Reference Carlson, Werkman and Rhodes30). Other intervention studies has also provide evidence that dietary DHA improves visual, mental, and motor skill development in some preterm and term infants fed supplemented formula(Reference Makrides, Simmer and Goggin31–Reference Hoffman, Birch, Birch and Wheaton34). In a non-randomised observational study, term infants fed breast milk have been found to have more mature visual acuities and correlated to higher erythrocyte DHA levels than those receiving formula(Reference Makrides, Simmer and Goggin31). Evidence to suggest that breast-fed infants have a long term IQ advantage over those who have been fed formula has been evident in the literature for many years(Reference Morrow-Tlucak, Haude and Ernhart35–Reference Uauy37). Moreover, we realize that the majority of comparisons between breast fed and formula-fed infants are confounded by genetic polymorphisms that affect LCP metabolism and socioeconomic factors which affect the outcomes of most studies(Reference Caspi, Williams and Kim-Cohen38–Reference Anderson, Maude and Alvarez40).
The present review was undertaken to systematically assess the evidence of short and long-term effects of n-3 LCPUFA supplementation during pregnancy and/or postnatal life on the visual acuity, psychomotor development, mental performance and growth of children.
Methods
Criteria for considering studies for this review (Table 1)
Types of interventions: inclusion criteria
All relevant RCTs with LCPUFA intervention in healthy pregnant women, lactating mothers and healthy full-term infants ( ≥ 37 weeks gestation at birth) were elegible for inclusion.From the randomised control trials RCTs or quasi-RCTs found in literature, only the ones designed to study the effects of n-3 LCPUFA supplementation on any standardized measures of growth, psychomotor development, mental performance and visual acuity in the offspring were selected. A trial was defined as quasi-random if the method used to allocate study pregnant women or infants to the study group was either not statistically random or was not clearly stated.
Interventions during pregnancy, lactation and with infant formula for full-term infants with DHA plus AA or DHA alone were included to be compared to those un-supplemented or receiving placebo during pregnancy or postnatally.
LCPUFA supplements could be from any source including fish oil, egg triglycerides or fungal oils. Trials in which precursor essential FAs (α-linolenic and linoleic acids) were used in the intervention group were not included, because intake of the precursors is far less effective with respect to LCPUFA deposition in fetal brain.
Studies involving prenatal and lactating mothers supplementation were assessed independently from studies with postnatal supplementation of infant formula.
The selection of interventions with infant formula in full-term infants had the following criteria: 1) Study formula was commenced within two weeks after birth; 2) Study formula was the only source of milk from the time of randomisation until at least 8 weeks of age; 3) A minimum of three months follow-up data on clinical outcomes of interest was available.
Table 1 Inclusion and exclusion criteria established for the systematic review

RCTs: Randomized clinical trials; DHA: Docosahexaenoic acid; AA: Arachidonic Acid; FAs: Fatty acids.
Types of interventions: exclusion criteria
Trials in women with high-risk pregnancies were not included, with high-risk pregnancy being defined as one in which a condition places the mother, the developing foetus, or both at higher-than-normal risk for complications during or after pregnancy and birth (e.g., a preterm delivery during an earlier pregnancy, intrauterine growth retardation, pregnancy-induced hypertension, or multiparity).
We excluded studies if the title and abstract were not relevant; however, we obtained articles for all potentially relevant studies if the abstract contained insufficient information to warrant exclusion. All areas of disagreement were discussed by the reviewers to achieve a consensus, and taken also into account the quality assessment.
Trials which used breast milk in addition to study formula during the first 8 weeks of life were not included to address the effect of early intervention with LCPUFA in formula-fed term infants. Trials reporting on only biochemical outcomes were also excluded.
Types of outcome measures
Visual acuity: measured either using Teller cards, visual evoked potentials (VEP), electroretinography (ERG), electroencephalography (EEG) and steroacuity. Neurodevelopmental outcomes: General Quotient, Intelligent Quotient, Psychomotor Scores, Behavior and other measures of Cognitive Functions. Growth: weight, length, head circumference and/or BMI.
Search methods for identification of studies
The search strategy included the use of a validated filter for identifying RCTs(Reference Robinson and Dickersin41) which was combined with a topic specific strategy using the following PubMed's MeSH terms: (FA OR omega-6 OR omega-3 OR n-6 OR n-3 OR eicosapentaenoic acid OR EPA or docosahexaenoic acid OR DHA OR arachidonic acid OR AA OR LCPUFA OR long-chain FA OR essential FA OR fish oil) AND (mother supplementation OR pregnancy OR lactation OR breastfeeding OR infant formula) AND (Infant OR Newborn OR Neonate) AND (growth OR weight OR length OR height OR head circumference) AND (cognitive function OR development OR neurodevelopment OR psychomotor development OR memory OR attention OR language OR intelligence OR cognition OR visual function OR visual acuity OR electroretinogram OR visual evoked potentials OR VEP OR behaviour OR neurobehaviour) AND (Clinical Trial OR Randomised Controlled Trial OR Review). The reference lists of identified studies and key review articles, including previously published reviews, as well as, major epidemiological studies and position papers, were also searched for all studies that assessed the effects of n-3 LCPUFA supplementation on child growth, neurodevelopment and/or visual function. We performed a computerized literature search of MEDLINE (from 1966 to April 2011), EMBASE (from 1980 to April 2011), LILACS (from 1980 to April 2011) and the Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library (issue 4, 2011). We imposed no limit with respect to the language of publication, but certain publication types (i.e., letters to the editor, abstracts, and proceedings from scientific meetings) were excluded.
Quality assessment of trials
The assessment of the quality of the studies that met the inclusion criteria was made separately by CC and MVE, with the knowledge of the authors or journals being assessed. The subsequent strategies associated with good-quality studies were evaluated: generation of allocation sequences and allocation concealment; blinding of investigators, participants, outcome assessors, and data analysts (yes, no, or not reported); intention-to-treat analysis (yes or no); and comprehensive follow-up. The generation of allocation sequences was considered adequate if the resulting sequences were unpredictable, such as computer-generated random numbers, and inadequate if the resulting sequences were predictable. The quality of allocation concealment was considered appropriate only if the randomization method applied unable the investigator or the participant to recognize or influence the intervention group before enrollment in the study. Allocation concealment was considered unclear when researchers claimed to have applied randomization methods but gave no description about it; it was considered inadequate when the randomization methods were unsuitable.
In relation to blinding methods, we have analyzed: double-blind (neither patients, care providers or assessors knew which treatment was being performed), single-blind (either patients or care providers or assessors were aware of treatment), and open (all parties were aware of treatment). For studies with an intention-to-treat analysis, a positive finding on the reviewers' part meant that the authors had specifically reported undertaking this type of analysis or that our own study confirmed this finding or both. On the contrary, a negative finding either meant that authors did not report the use of intention-to-treat analysis, that we could not confirm its use on study assessment, or both. We have established the percentage of participants excluded or lost to follow-up in order to evaluate how complete was patient follow-up. Such was considered adequate if ≥ 80 % of participants were included in the final analysis; if no losses to follow-up were reported we assumed that the study completeness was adequate. Furthermore, we categorized the risk of bias by the number of criteria judged inadequate in each study: low risk of bias ( ≤ 1 inadequate criterion), medium risk of bias ( ≤ 3 inadequate criteria), and high risk of bias (>3 inadequate criteria).
Data collection
Two review authors (CC and MVE) assessed eligibility of studies for inclusion independently. CC and MVE initially screened the title, abstract, and key words of every report identified by the search strategy; the reviewers then retrieved the full text for potentially relevant trials and for reports whose relevance was not clear. Three of us (CC, MVE and TA) independently applied the inclusion criteria to each potentially relevant trial to ascertain its eligibility. If differences in opinion existed, they were resolved by discussion. Differences in the inclusion of trials according to their quality were resolved after consultation with the other review authors (HS and RU). Data from each study were extracted by all of the reviewers using standardized data extraction forms prepared by CC and MVE. After extraction, all data were compared to minimize the possibility of errors.
Data synthesis (Statistical methods)
The mean difference (MD) between the treatment and control groups was selected to represent the difference in continuous outcomes with a 95 % confidence interval (CI). This was feasible when the mean values of the outcomes, the standard deviations of the outcomes, and the number of participants in whom the outcome was assessed in each of the 2 groups were available. If not, the analyses reported by the authors of the original articles are presented.
Results
Recently published systematic reviews and meta-analyses on this topic were identified(Reference Delgado-Noguera, Calvache and Bonfill25–Reference Dziechciarz, Horvath and Szajewska26, Reference Horvath, Koletzko and Szajewska42–Reference Makrides, Duley and Olsen47) and subsequently relevant references of published RCTs were found.
Included studies
All RCTs included in this systematic review were fully peer-reviewed publications.
Included RCTs analyzing the effects of LCPUFA intervention on mothers during pregnancy and/or lactation (Figure 1)
From a total of 105 existing RCTs designed to analyze the effect of LCPUFA intervention on mothers during pregnancy and/or lactation, 16 studies met the selection criteria. The included trials described 11 study populations for neurodevelopment outcome and 13 study populations assessed the growth outcome (including a total of 4524 participants), in infants and children born from supplemented pregnant and/or fed from their supplemented mothers during lactation. The general characteristics of these RCTs Table 2.

Fig. 1 Flow chart of the included RCTs analyzing the effects of LCPUFA intervention on mothers during pregnancy and/or lactation.
Table 2 Characteristics of the included Randomized Clinical Trials (RCTs) related to analyze the effect of supplementation during pregnancy and lactation on growth and neurodevelopment

FO: Fish Oil; OO:Olive oil; HC: Head circumference; LCPUFAs: Long chain polyunsaturated fatty acids; LA: Linoleic acid; ALA: α-linolenic acid; DHA: Docosahexaenoic acid; EPA: Eicosapentaenoic acid; GMDS: Griffiths Mental Development Scales; PPVT: Peabody Picture Vocabulary Test; K-ABC: Kaufman ABC; MPC: Mental Processing Composite; CBCL: Child Behavior Checklist; BSID: Bayley Scales of Infant Development; PDI: Psychomotor Development Index; MDI: Mental Development Index; MacArthur: CDI: Communicative Development Inventory; VEP: Visual evoked potentials; EEG: Electroencephalography. *Included term and preterm infants.
The time of neurodevelopment and physical growth assessment varied from birth to 7 years. The visual evaluations were performed during the age range of 0 to 5 years, and they differed from one another with regard to the vision-testing protocols and the number of vision testers.
Eighteen publications (including 11 sets of RCTs) assessed the effects of the n-3 LCPUFA supplementation during pregnancy on neurodevelopment and physical growth, on the same groups of patients and at different time points(Reference Dunstan, Simmer and Dixon48–Reference Smithers, Gibson and Makrides65), 4 publications (2 sets of RCTs) explored the effect of n-3 LCPUFA supplementation during pregnancy & lactation (Reference Helland, Saugstad and Smith66–Reference Bergmann, Bergmann and Haschke-Becher69) and 5 publications (3 sets of RCTs) exclusively during lactation (Reference Jensen, Voigt and Prager70–Reference Jensen, Voigt and Llorente74).
Six publications were focused on analyzing the supplementation to mothers during pregnancy with n-3 LCPUFA on child neurodevelopment (Reference Dunstan, Simmer and Dixon48, Reference Judge, Harel and Lammi-Keefe53–Reference Campoy, Escolano-Margarit and Ramos56, Reference Makrides, Gibson and McPhee64), 5 papers reported results on visual acuity (Reference Dunstan, Simmer and Dixon48–Reference Innis and Friesen51, Reference Makrides, Gibson and McPhee64), and 14 articles examined the effect on growth as an outcome(Reference Dunstan, Simmer and Dixon48–Reference Judge, Harel and Lammi-Keefe52, Reference Tofail, Kabir and Hamadani54, Reference Olsen, Sorensen and Secher57–Reference Makrides, Gibson and McPhee64).
3 articles, from the same RCT study population, explored the effect of n-3 LC-PUFA supplementation during pregnancy & lactation on the offspring neurodevelopment(Reference Helland, Saugstad and Smith66–Reference Helland, Smith and Blomen68) and 4 published RCTs explored the effect on infant and children growth(Reference Helland, Saugstad and Smith66–Reference Bergmann, Bergmann and Haschke-Becher69).
5 publications (3 sets of RCTs) analyzed the effect of supplementing lactating mothers on their offspring neurodevelopment(Reference Jensen, Voigt and Prager70–Reference Jensen, Voigt and Llorente74), which was assessed using different tests and neurophysiologic procedures, as summarized in Table 2.
Included RCTs analyzing the effects of LCPUFA supplemented infant formulas (Figure 2)
From 21 identified RCTs to analyze the effects of LCPUFA supplemented infant formulas on neurodevelopment and physical growth, 15 met criteria and thus were selected for inclusion in this review. The included RCTs described 15 study populations for neurodevelopment outcome and 13 study populations assessed the physical growth outcome (including a total of 2061 infants and children), from birth to 2 years of age. The general characteristics of these RCTs are shown in Table 3.

Fig. 2 Flow chart of the included RCTs analyzing the effects of LCPUFA supplemented infant formulas on visual acuity, neurodevelopment and growth.
Table 3 Characteristics of the included Randomized Clinical Trials (RCTs) related to analyze the effect of infant formula supplementation on growth and neurodevelopment in term infants

HC: Head Circumference; LCPUFAs: Long chain polyunsaturated fatty acids; LA: Linoleic acid; GLA: γ-Linoleic acid; ALA: α-linolenic acid; DHA: Docosahexaenoic acid; EPA: Eicosapentaenoic acid; IQ: Intelligence Coefficient; GMDS: Griffiths Mental Development Scales; PPVT: Peabody Picture Vocabulary Test; K-ABC: Kaufman ABC; CBCL: Child Behaviour Checklist; BSID: Bayley Scales of Infant Development; PDI: Psychomotor Development Index; MDI: Mental Development Index: BRS: Behavior rating scales; CDI: Communicative Development Inventory; FO: Fish Oil; OO:Olive oil; TG: triglycerides; VEP: Visual evoked potentials; EEG: Electroencephalography.
Seventeen publications (7 sets of RCTs) assessed the effects of the n-3 LCPUFA supplementation of infant formula on neurodevelopment and physical growth, in the same groups of patients and at different time points(Reference Agostoni, Trojan and Bellu32–Reference Hoffman, Birch, Birch and Wheaton34, Reference Drover, Hoffman and Castaneda75–Reference Agostoni, Trojan and Bellu89). Another 9 RCTs which reported the complete study in a single publication were included in this review(Reference Birch, Castañeda and Wheaton90–Reference Makrides, Neumann and Simmer97).
Thirteen papers reported the effect of n-3 LCPUFA supplementation of infant formula on visual acuity(Reference Hoffman, Birch, Birch and Wheaton34, Reference Drover, Hoffman and Castaneda75, Reference Birch, Garfield and Castaneda78, Reference Auestad, Scott and Janowsky80, Reference Birch, Garfield and Hoffman83–Reference Birch, Hoffman and Uauy86, Reference Auestad, Montalto and Hall88, Reference Birch, Castañeda and Wheaton90, Reference Auestad, Halter and Hall92, Reference Carlson, Ford and Werkman96–Reference Makrides, Neumann and Simmer97), 17 publications analyzed the effect on neurodevelopment(Reference Agostoni, Trojan and Bellu32–Reference Lucas, Stafford and Morley33, Reference Drover, Hoffman and Castaneda75, Reference De Jong, Kikkert and Fidler77–Reference Agostoni, Trojan and Bellu89, Reference Ben, Zhou and Zhao91–Reference Auestad, Halter and Hall92, Reference Willatts, Forsyth and DiModugno95, Reference Makrides, Neumann and Simmer97) and 13 articles reported the effect on physical growth from different RCTs(Reference Lucas, Stafford and Morley33, Reference Birch, Carlson and Hoffman76, Reference Auestad, Scott and Janowsky80–Reference Makrides, Neumann and Simmer84, Reference Birch, Hoffman and Uauy86, Reference Auestad, Montalto and Hall88, Reference Birch, Castañeda and Wheaton90–Reference Morris, Moorcraft and Mountjoy94).
LCPUFA supplementation during pregnancy and lactation
Visual acuity (results are summarized in Table 4)
Malcolm et al. found no significant differences in implicit times, amplitudes or parameters of the stimulus-response function in the electroretinogram in the first week of postnatal life comparing infants born to mothers receiving, from 15 weeks of pregnancy til delivery, 200 mg of DHA/day supplements during pregnancy and those receiving placebo(Reference Malcolm, Hamilton and McCulloch49–Reference Malcolm, McCulloch and Montgomery50). There were also no differences in the measures of transient flash VEP waveform at birth and at the ages of 50 and 66 weeks postconceptional age (PCA); However, positive associations were found between infants' DHA status at birth and the sensitivity and maturity of the rod photoreceptors responses at birth(Reference Malcolm, Hamilton and McCulloch49), as well as, with the maturity of the pattern-reversal VEP at the ages of 50 and 66 weeks(Reference Malcolm, McCulloch and Montgomery50).
Table 4 Significant effects found of n-3 LCPUFA supplementation during Pregnancy or Lactation Child Visual Development

CI = confidence interval, PCA = post-conceptional age. VEP = visual evoked potential, cpd = cycles per degree, ms: miliseconds, μV = microvoltium, * = significant difference (P < 0·05).
Innis et al. found no significant differences in the Teller acuity cards at the age of 60 days PCA between infants born from mothers receiving DHA supplements during pregnancy and those who didn't. However, infants in the placebo group were more likely to have a lower visual acuity than those born to DHA-supplemented mothers(Reference Innis and Friesen51). Judge et al. (Reference Judge, Harel and Lammi-Keefe52) showed significant main effects for visual acuity at 4 months of age in those infants whose mothers were supplemented during pregnancy (DHA group), and no differences at 6 months of age; however, in the regression analysis the authors show better visual acuity in the offspring of the DHA supplemented group mothers at 4 months. Smithers et al. (Reference Smithers, Gibson and Makrides65) reported results from the DOMInO trial showing that in those infants whose mothers were supplemented during pregnancy with 800 mg/d of DHA +100 mg of EPA, and were fed only breast milk, the VEP acuity at 4 mo of age was not different compared to the control group. Lauritzen et al. reported no significant differences in Sweep VEP at 2 and 4 mo in babies whose mothers received fish oil supplementation during lactation; however, higher red blood cell n-3 LCPUFA content were associated with better visual acuity at 4 mo of age(Reference Lauritzen, Jorgensen and Mikkelsen71).
Studies based on lactating mothers' supplementation with n-3 LCPUFA have shown also some controversial results. Jensen et al. demonstrated that supplementation to mothers during lactation with 200 mg/d of DHA determines lower amplitude in the Sweep VEP in their infants at 4 & 8 months(Reference Jensen, Voigt and Prager70), but these differences in visual function (Sweep VEP, transient VEP and Bayley-Lovie cards) were not confirmed at 5 years(Reference Jensen, Voigt and Llorente74).
Neurodevelopment
The main results are shown in Table 5.
Table 5 Significant Effects found of n-3 LCPUFA Supplementation during Pregnancy and Lactation on Child Neurodevelopment

CI = confidence interval. K-ABC = Kaufman Assessment Battery for Children, *: significant difference (P < 0·05).
Four studies assessed neurological outcome in the first 2 years of life after n-3 LCPUFA supplementation in pregnancy, none reported significant effects on performance in neurologic tests in the supplemented group; two reported no differences between the supplemented and control group performance in the Fagan Test of Infant Intelligence (FTII) at 6 and 9 months of age(Reference Judge, Harel and Lammi-Keefe53, Reference Helland, Saugstad and Smith66) and three reported no differences in the BSID at 10th and 18th months(Reference Tofail, Kabir and Hamadani54, Reference Jensen, Voigt and Prager70, Reference Makrides, Gibson and McPhee64). Helland et al. (Reference Helland, Saugstad and Smith66) reported no differences in electroencephalogram (EEG) maturity at 2 days and 3 months after birth between groups, but observed that higher EPA and DHA levels were associated with more mature EEG patterns. Judge et al. (Reference Judge, Harel and Lammi-Keefe53) reported a positive effect of supplementation on the 2 step problem solving test at 9th months post birth. Dunstan et al. reported better eye and hand coordination in the Griffiths Mental Development Scales (GMDS) in the supplemented group at 2.5 years, a significant positive association with n-3 LCPUFA and an inverse correlation with AA in cord blood(Reference Dunstan, Simmer and Dixon48). Based on our evaluation of the data to date, 3 trials have reported long term effects of supplementation to date. Helland et al. reported better performance in the K-ABC mental development test in the supplemented group compared to control at 4 years of age, but this effect was not observed when IQ was measured at 7 years. The authors also report a significant positive correlation between IQ at 4 years and DHA levels in infant plasma at 4th weeks of life as well as an association between maternal DHA levels at 35 week gestation and IQ in the children at 7 years(Reference Helland, Smith and Saarem67–Reference Helland, Smith and Blomen68). The NUHEAL trial reported no differences in neurological outcome of children assessed with Hempel (4 years) and Touwen examinations (5.5 years) between children born to mothers receiving fish oil supplements and those who didn't; but, the authors also demonstrated better neurological scores in children at 5.5 yrs with increasing higher DHA levels in cord blood(Reference Escolano-Margarit, Ramos and Beyer55). Moreover, children whose mothers had higher DHA content in erythrocyte phosphatidylethanolamine (PE) at delivery were more likely to have a Mental Processing Composite (MPC) score of the Kaufman ABC over the median at 6·5 yrs. Likewise higher AA/DHA ratio in maternal erythrocyte PE at delivery was associated with greater proportion of low MPC scores (below the median)(Reference Campoy, Escolano-Margarit and Ramos56).
Lauritzen et al. demonstrated that passive vocabulary at 1 yr of age was lower in children whose mothers were supplemented with fish oil during lactation, compared to those babies whose mothers received olive oil; in the same study the authors reported that word comprehension at 1 yr of age was inversely associated with erythrocyte-DHA content at 4 mo additionally a small effect of breast-milk DHA was noted on early language development(Reference Lauritzen, Jorgensen and Mikkelsen71). Jensen et al. in 2005 report a benefit of DHA supplementation during lactation at 2·5 years of age, despite no differences in infancy(Reference Jensen, Voigt and Prager70). The supplemented group from this cohort of children was reported to perform better on a test of sustained attention at the age of 5 years(Reference Jensen, Voigt and Llorente74). Recently, Makrides et al. (Reference Makrides, Gibson and McPhee64) have shown that mean cognitive composite scores and mean language composite scores of children in the DHA group of mothers supplemented during pregnancy did not differ from children in the control group.
Cheatham et al. (Reference Jensen, Voigt and Llorente74) reported that early fish oil supplementation during lactation could have a negative effect on later cognitive abilities, suggesting a need to target an optimun DHA level, below and above which, there could be detrimental consequences to brain development.
Growth
Most studies included in this review showed no statistical differences after supplementation with DHA during pregnancy and/or during lactation in birth weight, length, weight for length or ponderal index at different ages(Reference Tofail, Kabir and Hamadani54, Reference Ramakrishnan, Stein and Parra-Cabrera58–Reference Courville, Harel and Lammi-Keefe59, Reference Smuts, Huang and Mundy61–Reference Stein, Wang and Martorell62, Reference Makrides, Gibson and McPhee64).
Smuts et al. (Reference Smuts, Huang and Mundy61) showed no significant differences between groups, but demonstrated that DHA intake correlates with birth weight. Tofail et al. (Reference Tofail, Kabir and Hamadani54) investigated the effect of fish oil (1·2 g DHA and 1·8 g eicosapentaenoic acid, EPA 20:5 n-3, per day) from 25 weeks gestation until birth on growth and development in 249 mother-infant pairs from a very poor area of Bangladesh. Birth weight, length, head circumference and ponderal index did not differ between groups at birth or at 10 months. Ramakrishnan et al. (Reference Ramakrishnan, Stein and Parra-Cabrera58) and Stein et al. (Reference Stein, Wang and Martorell62) studied the effect of DHA supplementation (400 mg/day) compared with placebo in 1094 pregnant women from Cuernavaca (Mexico), with a low background dietary intake of DHA ( ≈ 55 mg/day). Mean gestational age at birth, birth weight, length and head circumference did not differ between groups. However, the babies from primiparous women who received DHA were heavier (+99 g) and had larger head circumferences (+0·5 cm) at birth compared to controls. These differences were not shown in multigravida women(Reference Ramakrishnan, Stein and Parra-Cabrera58). Makrides et al. (Reference Makrides, Gibson and McPhee64) in a randomised clinical trial on 2399 women, supplemented or not with 800 mg/day of DHA+100 mg/day of EPA, showed no significant differences between groups. Group differences in birth size were largely explained by gestational age at birth. Finally, Lucia et al. (Reference Bergmann, Bergmann and Haschke-Becher69) showed significant lower BMI and birth weight in babies from DHA supplemented mothers, at 1, 3 and 21 mo.
Effects of LCPUFAs supplementation in term infants
Visual acuity
The main results are shown in Table 6.
Table 6 Significant Effects related to n-3 LCPUFA supplementation of infant formula on infant and child visual acuity

PCA = Postconceptional age, VEP = Visual Evoked Potentials, MAR: minimum angle of resolution, HOTV: Amblyopia Treatment Study (ATS) protocol and the Electronic Visual Acuity (EVA) system, CI: Confidence Interval, * = significant difference (P < 0·05).
Some of the included studies exploring the effect of receiving DHA supplementation in early life on visual acuity, using different methodologies (Sweep VEP, Steady state VEP, Teller Acuity Cards, HOTV cards) have reported either no significant effects or minor effects compared to control groups or breast fed infants at different stages of infant development(Reference Auestad, Scott and Janowsky80, Reference Makrides, Neumann and Simmer84–Reference Makrides, Neumann and Simmer85, Reference Auestad, Montalto and Hall88, Reference Auestad, Halter and Hall92). Makrides et al. (Reference Makrides, Neumann and Simmer97) in a randomized control trial to assess the effect of infant formula supplemented with 0·35 % of DHA, given from birth to 30 weeks of postnatal life, reported better visual acuity at 4 mo in the supplemented infants compared to control group.
Birch et al. (Reference Hoffman, Birch, Birch and Wheaton34, Reference Birch, Garfield and Hoffman83, Reference Birch, Hoffman and Uauy86) have consistently demonstrated that DHA (0·36 %) supplementation with or without AA (0·72 %) during the first 17 weeks of life improved visual acuity at 17, 26 and 52 weeks, and steroacuity(Reference Auestad, Scott and Janowsky80) at 52 weeks of postnatal age measured using visual evoked responses and more mature electroretinographic responses at 6 weeks of age(Reference Hoffman, Birch, Birch and Wheaton34). At 4 years, the supplemented group had a small but statistically significant effect on visual acuity using HOTV cards for the right eye (P < 0·03); while the control formula diet group had poorer visual acuity in the right eye compared to the breast-fed group (P < 0·004) as well as lower right eye acuity compared to the DHA containing formula group (P < 0·03); the DHA- and DHA+AA-supplemented groups did not differ significantly from the breast-fed group(Reference Birch, Garfield and Castaneda78). Birch et al. (Reference Birch, Carlson and Hoffman76) recently have published the results of a new large sample size study from 244 healthy, term, formula-fed, singleton-birth infants (37–42 wk gestation; 2490–4200 g birth weight) randomized into four groups (DIAMOND study): 56/85 (66 %) in the control group, 64/83 (77 %) in the 0·32 % DHA group, 59/84 (70 %) in the 0·64 % DHA group, and 65/87 (75 %) in the 0·96 % DHA group. All DHA supplemented formulas contained 0·64 % arachidonic acid (AA). Infants were fed the assigned formulas until 12 months of age, and 141 children completed the 12-month feeding trial(Reference Drover, Hoffman and Castaneda75). Infants fed control formula had significantly poorer VEP visual acuity at 12 mo of age than did infants fed any of the DHA-supplemented formulas (P < 0·001).
Neurodevelopment
The main results are shown in Table 7.
Table 7 Significant Effects related to n-3 LCPUFA supplementation of infant formula on infant and child neurodevelopment. Data from the Bayley Scales for Infant Development (BSID)
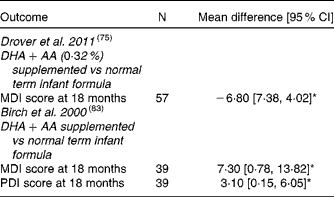
Bayley Scales of Infant Development: MDI = Mental Developmental Index, PDI = Psychomotor Developmental Index, BRS = Behaviour Rating Scales, CI = Confidence Interval, * = significant difference (P < 0·05).
Most studies(Reference Agostoni, Trojan and Bellu32, Reference Birch, Carlson and Hoffman76, Reference Auestad, Scott and Janowsky80, Reference Makrides, Neumann and Simmer84, Reference Auestad, Montalto and Hall88, Reference Auestad, Halter and Hall92, Reference Carlson, Ford and Werkman96–Reference Makrides, Neumann and Simmer97) included in this review were randomised controlled clinical trials reporting on the effect of DHA supplemented infant formula on neurodevelopment using different tests (BSID, Brunetz-Lezine, MacArthur Comunicative Development Inventory, Stanford Binet IQ, Hempel, Touwen, Knobloch, Passamanick and Sherrads' tests) at different ages; no significant differences were found at 6 mo to 9 yrs of age. Willats et al. (Reference Willatts, Forsyth and DiModugno95) demonstrated that babies fed LCPUFA-supplemented infant formula had significantly more intentional solutions than infants who received control formula at 10 mo. Agostoni et al. (Reference Agostoni, Trojan and Bellu32, Reference Agostoni, Riva and Scaglioni82, Reference Agostoni, Trojan and Bellu89) evaluated the effect of infant formula supplemented with 0·3 % DHA plus 0·44 % AA compared to a standard formula, using Brunet-Lezine test, demonstrating higher Developmental Quotient at 4 mo, but not at 1y and 2y in the supplemented infants. Birch et al. (Reference Auestad, Montalto and Hall88) demonstrated 7 points increase of MDI in the DHA supplemented groups and no significant differences in PDI and Behavior Rating Scales scores at 18 mo compared to control group. They also reported that better VEP acuity at 4 mo was associated to a better MDI and PDI scores at 18 mo. Drover et al. (Reference Drover, Hoffman and Castaneda75) confirmed the previous results assessing cognitive function in a new study including 131 children at 18 mo of age using the more up to date BSID II reporting that MDI scores of DHA-supplemented children were higher than those who did not received DHA supplementation at 18 mo of age; moreover, DHA concentration of 0·32 % was adequate to improve cognitive function and higher concentrations did not confer additional benefit.
Growth
Some of the randomized clinical trial included in this review, using DHA plus or not in combination with AA(Reference Lucas, Stafford and Morley33, Reference Birch, Carlson and Hoffman76, Reference Birch, Garfield and Castaneda78, Reference Auestad, Scott and Janowsky80–Reference Lucas, Morley and Stephenson81, Reference Birch, Garfield and Hoffman83–Reference Makrides, Neumann and Simmer84, Reference Birch, Hoffman and Uauy86, Reference Auestad, Montalto and Hall88, Reference Birch, Castañeda and Wheaton90–Reference Morris, Moorcraft and Mountjoy94) reported this outcome. No studies found statistically significant differences between LCPUFA and control groups, even if the studies included a breast fed reference group.
Discussion
The aim of this systematic review of RCTs was to assess whether n-3 LCPUFA supplementation to pregnant and/or lactating mothers, and to infant formula, could benefit term infants in their psychomotor, mental, visual acuity development and their physical growth. Evidence from the data obtained in the present review do not demonstrate that n-3 LCPUFA supplementation during prenatal or early life exert a clear and consistent short or long-term benefit of the offspring. Some recent large sample size studies included in this review reported favorable effects of n-3 LCPUFA supplementation on one specific domain of child development, or on visual acuity development using electrophysiological assessment. The effect on different measures of physical growth is null or minor in virtually all studies. Transient early differences tend to disappear in subsequent assessments. However, there is great heterogeneity among studies regarding the timing, type, concentration and duration of LCPUFA supplementation and of the outcomes assessed and methods used to assess the outcomes. Thus results of most studies are not comparable. For example, visual acuity development was measured at 4, 6, 12 months, 3 and 4 years, using sweep VEP, steady state VEP or teller cards. The neurodevelopmental outcomes were assessed at 3, 4, 6,1 2 months and at 2, 3, 4, 7 and 9 years in different studies, using different age-adapted tests. The only exception was physical growth was assessed at 4, 6, 12 months and 2, 3, 4 years in different studies, using standard physical measurements or z scores of such measurements.
SomeRCTs during pregnancy suggest that prenatal DHA status might have subtle positive effects on neurodevelopmental and behaviour outcome(Reference Jensen, Voigt and Prager70–Reference Jensen, Voigt and Llorente74), but whether these effects are maintained beyond early infancy remains a matter of discussion(Reference Makrides, Neumann and Simmer97–Reference Dangour and Uauy100). Trials specifically addressing n-3 LCPUFA supplementation during pregnancy and/or lactation consistently show a direct dose-response relationship between DHA intake and mothers' DHA concentration in plasma or erythrocyte phospholipids or in human milk, respectively, but have not consistently demonstrated benefits on developmental outcomes in children(Reference Dunstan, Simmer and Dixon48–Reference Campoy, Escolano-Margarit and Ramos56, Reference Makrides, Gibson and McPhee64–Reference Smithers, Gibson and Makrides65, Reference Helland, Smith and Saarem67–Reference Helland, Smith and Blomen68). It is important to note that some of these RCTs have agreed with the observational and epidemiological results(Reference Blau-Hospers and Hadders-Algra46), indicating that a better AA/DHA status during pregnancy and lactation is related to a better neurodevelopment outcome in children born at term.
Postnatal supplementation to improve neurodevelopment, has shown conflicting results in term infants. The follow up results in young children suggest that neurodevelopment and cognitive abilities are also enhanced by early provision of n-3 LCPUFAs through breast milk or DHA-fortified foods. Breast fed infants also require n-3 LCPUFAs after weaning to achieve optimal visual acuity at 12 months of age(Reference Agostoni, Trojan and Bellu32, Reference Uauy, Hoffman and Mena36, Reference Koletzko, Agostoni and Carlson101). Jensen et al. (Reference Jensen, Voigt and Prager70, Reference Jensen, Voigt and Llorente74) reported significant differences in psychomotor development and sustained attention at long term (30 mo and 5 years), favouring the breastfeeding mothers supplemented with DHA.
Makrides et al. (Reference Makrides, Gibson and McPhee64, Reference Makrides, Neumann and Simmer84, Reference Makrides, Neumann and Simmer97) and Birch et al. (Reference Hoffman, Birch, Birch and Wheaton34, Reference Birch, Carlson and Hoffman76, Reference Birch, Garfield and Castaneda78, Reference Birch, Hoffman and Uauy86, Reference Birch, Castañeda and Wheaton90) have conducted the largest studies and have shown, in different studies with multiple repeated measures, that n-3 LCPUFA supplementation to infant formula benefits visual acuity and mental development indices for up to 4 years. In addition, it has been reported benefits of LCPUFA supplementation on MDI scores at 18 months(Reference Jensen, Voigt and Llorente74, Reference Birch, Garfield and Hoffman83) and better problem solving skills at 10 months of age(Reference Willatts, Forsyth and DiModugno95). However, these beneficial effects on vision and neurodevelopment have not been replicated in other good quality studies(Reference Agostoni, Trojan and Bellu32–Reference Lucas, Stafford and Morley33, Reference De Jong, Kikkert and Fidler77, Reference Bouwstra, Dijck-Brouwer and Boehm79, Reference Lucas, Morley and Stephenson81–Reference Agostoni, Riva and Scaglioni82, Reference Makrides, Neumann and Simmer85, Reference Auestad, Montalto and Hall88, Reference Auestad, Halter and Hall92, Reference Morris, Moorcraft and Mountjoy94). In 2003, a meta-regression analysis of seven trials in term infants showed that the DHA dose in milk formula was positively related to visual acuity measurements at age 4 months(Reference Uauy, Hoffman and Mena36). In 2010, Beyerlein et al. (Reference Beyerlein, Hadders-Algra and Kennedy44) analysed the results of 4 large randomised clinical trials in a meta-analysis and they could not find a clinically meaningful effect on the neurodevelopment and visual acuity in children that received LCPUFA supplemented formula. This served to further raise the discussion on whether it was valid to continue supplementing DHA to breastfeeding mothers(Reference Lauritzen, Jørgensen and Olsen72–Reference Cheatham, Nerhammer and Asserhoj73) and to term infants fed infant formula(Reference Scott, Janowsky and Carroll87). Furthermore these investigators reported potential adverse effects, suggesting that there is an optimum DHA level below and above which DHA might be detrimental to the developing brain. We conclude that based on present evidence there is still no clear evidence of long-term beneficial or harmful effect of LCPUFA supplementation on neurodevelopment or visual function in term infants.
In summary the results shown in this systematic review demonstrate evidence that n-3 LCPUFA supplementation to pregnant women determines a modest increases of birth size in their neonates born at term, especially in primigravidas(Reference Ramakrishnan, Stein and Parra-Cabrera58, Reference Stein, Wang and Martorell62), independently of low- or high-income populations; Previous meta-analysis reached similar conclusions, basically a small but significant increase in the length of gestation ( ≈ 2·5 days)(Reference Delgado-Noguera, Calvache and Bonfill25, Reference Horvath, Koletzko and Szajewska42–Reference Szajewska, Horvath A and Koletzko43, Reference Makrides, Duley and Olsen47) and a modest increase in infant birth weight (+50 g), birth length (+0·48 cm) and head circumference (+0·69 cm) in the offspring's of women taking DHA supplements(Reference Delgado-Noguera, Calvache and Bonfill25). The new data emerged from recent randomised clinical trials have not changed these conclusions, suggesting that the observed increases in birth weight and birth length could be the result of the increased duration of gestation(Reference Makrides, Collins and Gibson27, Reference Horvath, Koletzko and Szajewska42–Reference Szajewska, Horvath A and Koletzko43).
Despite numerous randomized controlled trials, meta-analysis and meta-regression analysis, data after the addition of a specific amount of LCPUFAs to term infant remain probable but not convincing for a robust effect(Reference Simmer, Patole and Rao45). Post-natal supplementation with LCPUFA does not appear to influence infant growth(Reference Makrides, Collins and Gibson27, Reference Beyerlein, Hadders-Algra and Kennedy44). The present systematic review concludes there is no effect of prenatal or postnatal n-3 LCPUFA supplementation on physical growth.
Considerations about other new important confounders
Common polymorphisms of the genes FADS2 (FADS2, encoding Δ-6 desaturase) and FADS1 (FADS1, encoding Δ-5 desaturase) found in about one quarter of the European population, encoding for the key enzymes regulating endogenous LCPUFA synthesis, i.e. delta-6-desaturase and delta-5 desaturase, are associated with markedly reduced plasma LCPUFA concentrations(Reference Lattka, Eggers and Moeller102). First results suggest marked effects of genetic variation in the FADS gene cluster on relevant clinical end points, including cognitive development, with potentially major importance for public health(Reference Glaser, Lattka and Rzehak103). Koletzko et al. (Reference Koletzko, Lattka and Zeilinger104) showed a consistent significant association of rare SNP alleles with lower amounts of DHA in red blood cell phospholipids of pregnant women; a modulation of DHA status during pregnancy by frequently occurring FADS genotypes may be of major relevance for child outcomes. These data are results from the NUTRIMENTHE EU Project (Grant Agreement: 212652). Further results will clarify the real power of this genetic effect, present in the 30% of the population, on the cognitive outcome during development. It is tempting to speculate that genetic heterogeneity in fatty acid metabolism may be one of the reasons, besides differing study design and variable quality, for the apparent inconsistent results of different studies that investigated effects of a perinatal supply of DHA sources on developmental outcome.
Considerations about methodological procedures
Concerns pertaining to the impact of nutrition on neurodevelopment and cognitive performance have emerged over the past decade, in most cases the focus has been on methodological considerations and limitations of these studies(Reference Burgard105–Reference Hughes and Bryan107). The sensitivity of the neuropsychological tests to subtle effects of nutrition(Reference Schmitt, Benton and Kallus106–Reference Hughes and Bryan107) have been addressed and the need to consider the timing of effects relative to the critical periods involved in brain developmental process have been raised(Reference Hughes and Bryan107). In addition, it is now well recognized that neuropsychological tests should assess the specific neuropsychological domains (perceptual, motor, attention, learning and memory, and executive functions) instead of global cognitive performance; first, to avoid hiding or masking specific effects of the nutritional intervention and secondly to address the need to consider the specific biological mechanisms involved. Cultural local factors need to be considered when comparing similar neuropsychological tests administered in different countries, as well as the practice/learning effect(Reference Schmitt, Benton and Kallus106), especially if the intention is to test subtle improvements after a nutritional intervention.
A number of novel approaches are now available for the assessment of nutrition-related variations in brain structure and function. RCTs with modest sample sizes (N < 100) should consider a combination of structural MRI and functional MRI with EEG; these would provide the most-comprehensive assessment of brain structure and function and, hence, offer insights into possible neural mechanisms underlying the effect of nutrients on cognition and mental well-being(Reference Tomas108). These new techniques in combination with the neuropsychological assessment offer new opportunities to unravel the interaction between nutrition and brain development in the near future.
Implications for future research
The complexity of brain development process requires special attention. The absence of positive results obtained so far should be analyzed carefully; the existing neuropsychological tests used up to now are likely not sufficiently sensitive to conclude there is “no effect”. Novel more function specific approaches that combine the assessment of different neuropsychological domains should be developed considering the potential biological mechanism involved for the specific nutrient-effect to be explored. Furthermore, the combination of new imaging techniques and electrophysiological responses must be promoted as gold-standard methodologies to detect objective nutrient effects on brain development.
Regarding the recent emerging results from different studies, it seems that there is really an optimum DHA level below and above which there may be detrimental consequences to the developing brain; this should be explored in low income populations where risk of abnormal brain development is greatest considering the need for large sample sizes and the evaluation of a dose response.
Analyses of FADS gene variants should be mandatory in all sizeable cohort and intervention studies in order to address the diet and endogenous individual metabolism interactions in defining the potential biological effects of LCPUFA; this should enhance study sensitivity and precision.
Acknowledgements
The publication of the supplement was supported by PULEVA Food, S.L. No conflicts of interest are declared. All coauthors contributed to the initial protocol of the review. CC, VE and TA were responsible for the literature searched, study selection, methodological quality assessment, and data extraction. HS and RU supervised the methodological quality assessment and all authors contributed to the data interpretation and writing of the final report. CC (coordinator), VE, TA and HS are researchers in the EU Project funded by FP7 European Commission - DG Research. Directorate E - Life Sciences: Theme 2 Food, Agriculture and Fisheries, and Biotechnology. Grant agreement no: FP7-212652-NUTRIMENTHE.











