Onset of peanut allergy typically occurs in childhood, with 70–100 %(Reference Al-Muhsen, Clarke and Kagan1) of peanut-allergic children being reported to react upon their first known dietary exposure to peanuts. Because IgE-mediated allergic reactions require prior exposure and immunological sensitisation to the allergen, this suggests that sensitisation has already been acquired, either in utero, or by unrecognised oral exposure or non-oral (cutaneous or respiratory) routes. As many as one in fifty-five children in the UK may currently show evidence of an allergic reaction to peanuts(Reference Hourihane, Aiken and Briggs2); indeed, peanut allergy is the most common cause of severe allergic reaction to foods, causing 30 % of all cases of anaphylaxis outside hospital(Reference Hourihane, Kilburn and Dean3). Data from the Isle of Wight UK Birth Cohort Study suggest a threefold rise in the prevalence of peanut sensitisation and allergy in children born between 1989–90 and 1994–6(Reference Grundy, Matthews and Bateman4, Reference Tariq, Stevens and Matthews5). Recent data from children born in 2001–2 show no further increases in the prevalence of peanut sensitisation and allergy(Reference Venter, Pereira and Voigt6).
In 1998 the UK Government issued precautionary advice to mothers whose children have a family history of allergic diseases, that they may wish to avoid peanut consumption during pregnancy and breast-feeding and avoid giving the child peanuts and peanut products until the child is 3 years of age(7). The precautionary advice was based on recommendations from the UK Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT), which advises the UK Government. The recommendations of the COT have since come under scrutiny as further scientific evidence on the development of peanut allergy and other food allergies in children has emerged.
The UK Food Standards Agency therefore commissioned a systematic review of the literature on food allergy published since 1999, in order to assess the relevant evidence base since the COT issued its recommendations in 1998. The systematic review evaluated studies that aimed to investigate dietary food allergen consumption or avoidance behaviour in early life and subsequent development of food allergy, with a particular focus on peanut allergy. The systematic review covered a wide range of food allergies including cows' milk, eggs, fish and nuts, as well as peanut allergy. In this paper, we present the evidence from this systematic review of literature on early-life patterns of exposure to, and avoidance of, peanut allergens and later development of sensitisation and clinical peanut allergy. The role of maternal and childhood diets and the subsequent development of sensitisation and allergy to peanuts was investigated using evidence published since 1998. Non-dietary exposure to peanuts, for example, cutaneous or environmental exposure, was also reviewed. The review included both human and animal studies, and also a review of evidence investigating the response of cord blood mononuclear cells to allergens in order to reflect on the concept that intra-uterine immunological sensitisation can occur and increases the likelihood of subsequent atopic disease.
Methods
Systematic reviews
The research questions related to peanut sensitisation and allergy from human studies are shown below.
Question 1
Does maternal dietary consumption of peanuts or peanut products – or avoidance of dietary consumption of peanuts or peanut products – during pregnancy/lactation have any impact on the subsequent development of sensitisation, or allergy to peanuts by the child?
Question 2
Does dietary consumption of peanuts or peanut products – or avoidance of dietary consumption of peanuts or peanut products in childhood – have any impact on the subsequent development of sensitisation or allergy to peanuts?
Question 3
Does non-dietary exposure to peanuts in childhood, for instance via skin or the respiratory tract, have any impact on the subsequent development of sensitisation or allergy to peanuts?
All types of study design were included except case reports and therapeutic or treatment studies where the aim was to control allergic symptoms. The comparison of interest in each study was avoidance or reduced quantities with non-avoidance of peanuts or peanut products. Both dietary and non-dietary sources (for example, peanut oil in skin creams) were included in the systematic review. Excluded were studies reporting dietary intake as nutrients rather than foods and studies on vitamin and mineral supplements, food preservatives or additives. Outcomes of interest concerned both sensitisation and allergy. Measures of sensitisation included peanut-specific skin prick tests and peanut-specific IgE. Measures of clinical allergy included a positive food challenge to peanuts (open or blind, including double-blind placebo-controlled food challenge), parental reports of suggestive symptoms occurring shortly after eating peanuts and reports of doctor's diagnosis of peanut allergy. Studies with asthma, eczema, atopic dermatitis, rhinitis, atopic wheeze, and other respiratory outcomes, or that did not report specifically on peanut sensitisation or allergy were excluded.
Question 4
Has the current UK Government guidance on dietary consumption of peanuts and peanut products had any impact on sensitisation and allergy rates to peanuts in the UK?
Included were studies that provide information on knowledge of, or adherence to, the 1998 COT recommendations, as well as information on sensitisation or allergy to peanuts in the child. Cohort, case–control and cross-sectional studies assessing the development of sensitisation or clinical peanut allergy in relation to adherence to COT recommendations were included. Subjects were women who became pregnant after June 1998 and infants and children conceived after June 1998. The exposures included maternal knowledge of the COT recommendations, and data on dietary and non-dietary sources of peanuts in the maternal diet and in the child's diet up to the age of 3 years. The outcomes of interest were dietary (maternal and child) intake of peanuts, and sensitisation to peanuts and peanut allergy in the child (using the same measures as previously described).
Study searches
The Cochrane Library (Systematic Reviews and Central Databases), MEDLINE, EMBASE and CAB Abstracts were searched from 1 January 1999 to 7 March 2008. The search strategy included both medical subject heading (MeSH) terms and text terms where possible. A search strategy that included terms to cover all the research questions for human studies was developed. Terms for peanuts (Arachis hypogaea, arachis oil, peanut*) were combined with those for sensitisation and allergy (food hypersensitivity, allerg*, atopy*, atopic* intolerance, hypersensitivity*). The search was restricted to papers published in English and to subjects aged from newborn to 12 years. The reference lists of reviews and included studies were checked for any further papers. Abstracts presented at meetings and/or conferences that were unsupported by a full published paper were not included. A separate search, on authors of included studies, was conducted to find further articles written by these authors.
No study was excluded on the basis of quality. All titles and abstracts of papers identified by the searches were assessed for inclusion by one reviewer. Full copies of papers considered potentially relevant (not clearly excluded) were obtained. The full copies of papers were assessed for eligibility independently by two reviewers; any disagreement between the reviewers was resolved by discussion between the reviewers or by assessment by a third reviewer. Data extraction forms were developed based upon the checklists developed by the Scottish Intercollegiate Guidelines Network(8), and included questions to critically appraise each study. All data extraction forms were completed independently by two reviewers; any disagreement between the reviewers regarding relevant data or quality assessment was resolved by discussion.
Narrative expert-led reviews
Expert-led reviews were conducted of animal studies on peanut sensitisation or allergy, based on questions 1 to 3. Studies on animal models were initially identified using the same search terms, time period and databases as for the human studies, and then the search was expanded to cover a broader time period (studies published from 1980 onwards).
The recommendations of the COT issued in 1998 were in part based on the concept that intra-uterine immunological sensitisation can occur and increases the likelihood of subsequent atopic disease. This concept was primarily based on reports of in vitro proliferative and cytokine responses by allergen-stimulated cord blood mononuclear cells(Reference Miles, Warner and Jones9) and the demonstration that such responses were associated with subsequent atopic disease(Reference Warner, Miles and Jones10). Therefore, a narrative expert-led review on the response of cord blood mononuclear cells to allergens was conducted, in order to assess developments in this area of research since the COT recommendations were issued.
Results
Identification of studies
A total of 5799 references were identified in the full systematic review. After removal of duplicate references (those retrieved by more than one database search), 3518 references remained. From these, 357 papers were deemed potentially relevant and full papers were obtained. Following duplicate assessment, a total of thirty-two papers were judged to be relevant. The κ score for agreement was 0·63 (95 % CI 0·50, 0·76); this result lies in the range (κ 0·6–0·8) for substantial agreement. Of these thirty-two papers, six reported on maternal or childhood exposure to peanuts and either peanut sensitisation or allergy: there were two randomised controlled trials (RCT), two case–control studies and two cross-sectional studies.
Question 1: mother's diet during pregnancy and lactation
Two case–control studies assessed peanut consumption during pregnancy (Table 1). One(Reference Frank, Marian and Visser11) studied a high-risk group (children with suspected food allergy) in South Africa. Those sensitised to peanuts (established by detection of peanut-specific IgE, but no food challenge tests) were compared with controls with milk or egg sensitivity (but no sensitivity to peanuts). The study was small (only twenty-five cases) and the results were not adjusted for potentially confounding factors. The authors reported an OR of 3·97 (95 % CI 0·73, 24·0) for cases compared with controls; this did not reach statistical significance. The second case–control study(Reference Lack, Fox and Northstone12), from the UK, was also small. Twenty-three confirmed cases (diagnosed using reports of reactions plus skin prick test or double-blind placebo-controlled food challenge) of peanut allergy were compared with atopic (non-peanut allergic) and non-allergic controls. Even though the results were not adjusted for potentially confounding factors, there was no association between consumption of peanuts during pregnancy and peanut allergy in the child.
Table 1 Maternal dietary consumption or avoidance of peanuts and subsequent sensitisation or allergy to peanuts in the child
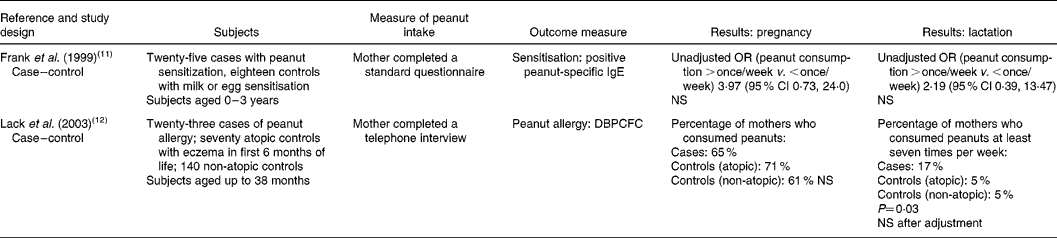
NS, not statistically significant (P>0·05); DBPCFC, double-blind placebo-controlled food challenge.
The same two case–control studies also specifically assessed peanut consumption during lactation. One(Reference Frank, Marian and Visser11) found no association between peanut consumption during lactation and peanut sensitisation in the child (OR 2·19; 95 % CI 0·39, 13·47). The second(Reference Lack, Fox and Northstone12) found that mothers of children with peanut allergy were more likely to consume peanuts at least seven times per week compared with mothers of atopic or normal controls; however, when these results were adjusted for potentially confounding factors, the outcome was no longer statistically significant (the factors adjusted for were not reported).
One other cross-sectional study made reference to maternal peanut consumption and peanut sensitisation in infants(Reference Hourihane, Aiken and Briggs2). The authors reported that there was no statistically significant difference in whether mothers changed their peanut consumption (reduced or avoided, i.e. as a response to the COT recommendations) and whether their children were sensitised to peanuts. There were no detailed data reported in the paper, so it remains unclear whether this statement refers to maternal intake during pregnancy, lactation or both.
No peer-reviewed studies in animals that assessed the impact of maternal dietary exposure to peanuts and the subsequent development of sensitisation or allergy to peanuts in the offspring were found.
Question 2: childhood diet
Two RCT and one case–control study were relevant to research question 2 (Table 2). The two RCT investigated multifaceted interventions comprising avoidance of multiple potentially allergenic foods plus household avoidance measures (such as house dust mite avoidance, no pets, no smoking) in high-risk populations(Reference Arshad, Bateman and Sadeghnejad13, Reference Chan-Yeung, Ferguson and Watson14). Both interventions included advice on the mothers' diets. For one intervention(Reference Chan-Yeung, Ferguson and Watson14) mothers were asked to avoid peanuts, other nuts and fish for the last trimester of pregnancy and during lactation. In the second intervention(Reference Arshad, Bateman and Sadeghnejad13) dairy products, eggs, wheat, nuts, fish and soya were excluded from the mothers' diets during lactation. Both studies included dietary intervention in infants, which was focused on duration of breast-feeding. Recommendations differed for each study: breast-feeding for at least 9 months(Reference Arshad, Bateman and Sadeghnejad13) or for 1 year(Reference Chan-Yeung, Ferguson and Watson14). Both studies included advice to delay introduction of cows' milk, fish and peanuts until 6(Reference Chan-Yeung, Ferguson and Watson14) or 9(Reference Arshad, Bateman and Sadeghnejad13) months.
Table 2 Childhood dietary consumption or avoidance of peanuts and subsequent sensitisation or allergy to peanuts in the child
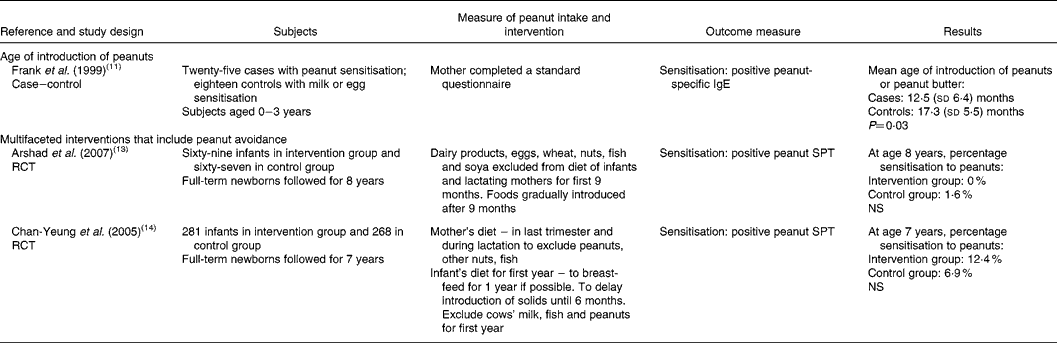
RCT, randomised controlled trial; SPT, skin prick test; NS, not statistically significant (P>0·05).
Both studies reported no statistically significant differences between the intervention and control groups with regard to peanut sensitisation. Rates of sensitisation differed between the two studies and rates were higher in the study from Canada(Reference Chan-Yeung, Ferguson and Watson14) compared with the UK study(Reference Arshad, Bateman and Sadeghnejad13).
One case–control study(Reference Frank, Marian and Visser11) reported on the mean age of introduction of peanuts or peanut butter. On average, peanuts were introduced earlier in cases compared with controls (12·5 (sd 6·4) v. 17·3 (sd 5·5) months). The result was statistically significant (P = 0·03) but it was not adjusted for other factors.
Ten studies investigating oral exposure to peanuts and subsequent development of sensitisation in experimental animals (rodents) were found. The evidence suggests that prior oral exposure to high doses of peanuts can inhibit subsequent development of sensitisation (IgE antibody production) to immunisation with the same allergen(Reference Strid, Hourihane and Kimber15). Under certain conditions (particularly low doses)(Reference Dearman, Caddick and Stone16, Reference De Jong, Knippels and Ezendam17), however, sensitisation and IgE antibody production may be provoked by oral exposure, although oral administration (bolus administration of liquid by syringe into the stomach) may be more effective in this respect than normal dietary exposure. The impact of oral exposure on the development of sensitisation is dependent upon the dose of peanut allergen administered(Reference Strid, Hourihane and Kimber15) and also upon the strain of animal.
Question 3: non-dietary exposure to peanuts in childhood, for instance via skin or the respiratory tract
One study reported on the maternal use of breast creams containing peanut oil and found no association between their use and risk of peanut allergy(Reference Lack, Fox and Northstone12). The study also reported on the use of creams containing peanut oil on infants' skin and it found that children with peanut allergy were statistically significantly more likely to have had these creams containing peanut oil applied, compared with controls. This study had only twenty-three cases but the result is statistically significant even after adjusting for confounders (intake of soya milk or formula and rashes). More than 90 % children with peanut allergy had been exposed to skin creams containing peanut oil, compared with less than 60 % in the control groups. Cases of peanut allergy were also exposed to a greater number of different peanut oil-containing preparations than controls; this finding was highly statistically significant (P < 0·001).
There is also evidence from studies in mice that relatively small amounts of peanut allergen when applied to the skin can induce a (peanut-specific) IgE antibody response(Reference Strid, Hourihane and Kimber15). In these studies, peanut protein was applied to damaged skin, rather than to intact skin, which may be important. However, apparently healthy human skin often contains minor abrasions, and barrier function in skin from atopic individuals in particular is often compromised(Reference Elias, Hatano and Williams18). Moreover, it is possible that even with intact skin, sufficient protein may gain access to cause immunological priming.
Question 4: has the current UK Government guidance on dietary consumption of peanuts and peanut products had any impact on sensitisation and allergy rates to peanuts in the UK?
Two cross-sectional studies were relevant to research question 4 (Table 3) (Reference Hourihane, Aiken and Briggs2, Reference Dean, Venter and Pereira19). One study utilised a birth cohort (658 children) from the Isle of Wight(Reference Dean, Venter and Pereira19); subjects were initially recruited whilst mothers were pregnant. The infants were born between 1 September 2001 and 31 August 2002, 3 years after the COT recommendations were first issued. A questionnaire to assess compliance with the COT recommendations was administered to mothers when children were aged 2 years. Of the mothers who responded, 42 % recalled awareness of the COT recommendations, but this was not associated with maternal atopy or family history of atopy. About two-thirds (65 %) of mothers stated that they avoided peanuts during pregnancy; this was not affected by the presence or absence of maternal atopy or family history of atopy. Mothers having their first child were most likely to change their diet (either avoiding or reducing peanut consumption) during pregnancy, regardless of their atopic status. When the birth cohort reached the age of 2 years, 2 % of the sample were sensitised to peanuts. Of the mothers with sensitised children, 77 % reported that they had avoided peanuts when pregnant. The findings demonstrate that the target population (pregnant or lactating women who were atopic or where another first-degree relative of the child is atopic) did not necessarily take up the advice. The COT recommendations appear to have been misunderstood, as women not in the target group also avoided peanuts.
Table 3 Studies evaluating the impact of the 1998 UK Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) recommendations to avoid peanuts
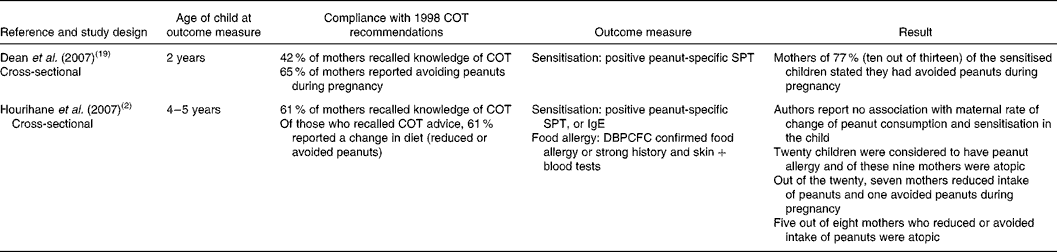
SPT, skin prick test; DBPCFC, double-blind placebo-controlled food challenge.
The other cross-sectional study(Reference Hourihane, Aiken and Briggs2) involved 1072 mother and child pairs. The children were aged 4–5 years, born after the COT recommendations were first issued, and living in Southampton or Manchester. Overall, 61 % of mothers recalled the COT recommendations, and recall was not associated with the mothers' atopy status. Of those who recalled the recommendations, 61 % reported a dietary change, though few women stopped eating peanuts altogether. Most (89 %) women reported reducing peanut consumption and only 10 % reported eliminating peanuts during pregnancy. Of the women who regularly ate peanuts before becoming pregnant and who breast-fed their child, 46 % changed their diet (eliminated or reduced intake of peanuts) while breast-feeding. Of those who breast-fed, 41 % thought that they had eaten peanuts. Two-thirds (65 %) of children were reported to have consumed peanuts; the mean age of introduction was 36 months. There was no statistically significant difference in the age at which peanuts were introduced for those who were peanut sensitised compared with those who were not sensitised (32 v. 29 months). By comparison, a small case–control study(Reference Frank, Marian and Visser11) reported an earlier age of introduction of peanuts in those who were sensitised (12·5 v. 17·3 months; P = 0·03).
Another study also presented information on peanut allergy in relation to compliance with the COT recommendations(Reference Hourihane, Aiken and Briggs2). A total of twenty infants were identified as having peanut allergy, and almost half of these had atopic mothers (9/20). Of these twenty mother–child pairs, eight mothers of children with peanut allergy had reduced their intake or avoided peanuts during pregnancy and lactation. There was no association between take up of the COT recommendations and atopic status of the mother.
Narrative review on cord blood mononuclear cell response to allergens
Research published since the 1998 COT recommendations has demonstrated that it is highly likely that the fetus is exposed to small (but variable) amounts of food proteins derived from the maternal diet and transported across the placenta. However, it remains unclear whether this fetal exposure results in in utero sensitisation of the fetal immune system. Moreover, it is not possible to conclude that in vitro cord blood mononuclear cell responses observed following stimulation with food proteins necessarily reflect in utero exposure(Reference Szepfalusi, Pichler and Elsasser20), in utero sensitisation(Reference Smillie, Elderfield and Patel21), or an increased risk of clinical allergy during later life(Reference Thornton, Upham and Wikstrom22, Reference Rowe, Kusel and Holt23).
Discussion
The systematic review of human evidence found only six relevant studies. These do not suggest that maternal exposure to or avoidance of peanuts modifies the likelihood of subsequent peanut sensitisation or allergy in the child. No relevant studies in animal models were identified. The 1998 COT recommendations embraced evidence from studies of cord blood mononuclear cell responses after stimulation with food allergens and aeroallergens. Studies since 1998 have not related maternal peanut exposure to cord blood mononuclear cell responses after stimulation with peanut allergen but suggest that the cord blood mononuclear cell responses observed after in vitro stimulation with non-peanut food allergens are not necessarily the consequence of fetal exposure to(Reference Szepfalusi, Pichler and Elsasser20), or sensitisation by(Reference Smillie, Elderfield and Patel21), maternally consumed food allergens. More direct evidence of in utero sensitisation of the fetal immune system to peanut allergen, in the form of the detection of peanut-specific IgE in cord blood, remains absent. Furthermore, studies of non-peanut allergens suggest that the sensitivity and positive predictive value of such an observation for subsequent allergic disease would be low(Reference Edenharter, Bergmann and Bergmann24). The one study that attempted to detect peanut-specific IgE in cord blood was unable to do so in twenty-three neonates who subsequently developed peanut allergy(Reference Lack, Fox and Northstone12).
With regard to early childhood, evidence from human studies does not suggest that dietary exposure to, or avoidance or delaying introduction of, food proteins changes the likelihood of subsequently developing atopic sensitisation or clinical allergy. There are few studies that have investigated the timing of introduction of potential food allergens and more research is required in this area. One case–control study reported that peanuts were introduced at an earlier age in cases of peanut sensitisation compared with controls(Reference Frank, Marian and Visser11). However, two RCT(Reference Arshad, Bateman and Sadeghnejad13, Reference Chan-Yeung, Ferguson and Watson14) found no effect on peanut sensitisation when introduction of peanuts was delayed, although these interventions were multifaceted and not specifically designed to investigate the effects of dietary consumption or avoidance of peanuts on outcomes. Evidence from animal studies(Reference Strid, Hourihane and Kimber15) suggests that oral exposure to low doses of peanut protein may induce sensitisation, whereas high doses may result in tolerance. If applicable to humans, these results suggest that incomplete attempts at avoidance of exposure to peanuts (i.e. exposure to low doses) could potentially be harmful, rather than protective. This is a particular concern in light of evidence(Reference Hourihane, Aiken and Briggs2) that incomplete avoidance of peanuts is common. The results of investigations in animals need to be confirmed by further studies and their relevance to human subjects explored, in particular the concept of ‘small’ and ‘larger’ amounts in human terms.
There is little information available from human studies on the effects of non-dietary exposure to peanuts on the development of sensitisation and allergy. However, one study(Reference Lack, Fox and Northstone12) did show an increased risk of peanut allergy in children who were exposed to skin creams containing peanut oil. However, it is possible that differential recall bias by mothers of peanut-allergic children could have contributed to this result. There is evidence from experimental animal studies examining responses to topically applied peanut proteins that is consistent with the human evidence, but further studies are required to elucidate whether topical exposure to peanut protein presents a real risk with respect to sensitisation. There is good evidence from experimental models, using ovalbumin as the allergen, that the skin is an important route for sensitisation(Reference Nelde, Teufel and Hahn25). Currently in the UK, the Committee on Safety of Medicines advises patients known to be allergic to peanuts not to take or use medicines using peanut oil; regulations also state that there should be clear labelling of peanut oil whenever used in skin creams.
Evidence from two studies(Reference Hourihane, Aiken and Briggs2, Reference Dean, Venter and Pereira19) designed to evaluate the impact of the 1998 COT recommendations found that there appears to be confusion among the general public regarding the COT recommendations and that the advice has not been interpreted as intended. More than 60 % of women reported having reduced (rather than avoided) consumption of peanuts during pregnancy and lactation, including those not targeted by the COT recommendations. Results from animal studies indicate that dose, at least in the diets of young animals, is an important determinant of whether tolerance or sensitisation is the result of peanut exposure(Reference Strid, Hourihane and Kimber15). Therefore the degree to which consumption of peanuts is reduced by mothers could be potentially important. There was also no indication that the target group (women with a family history of allergy) were more likely to take up the advice. Of children who developed peanut sensitisation or allergy, a significant number (77 % sensitisation, 40 % allergy) of mothers reported that they reduced their intake or avoided peanuts(Reference Dean, Venter and Pereira19).
The systematic nature of the review means that concerns over bias in the identification, evaluation and reporting of the evidence are minimised. Decisions on inclusion and exclusion of studies, and data extraction, were independently carried out by two reviewers. The review has not included unpublished literature, as this would have been difficult to identify in a systematic way. However, there may be other studies that have been carried out and never published, possibly because they did not find any statistically significant results. The search was restricted to the English language and hence may have missed relevant studies published in languages other than English. Owing to the limited number of studies identified and their heterogeneous nature, meta-analysis was impossible. Studies assessed a heterogeneous range of exposures and interventions that were measured in a number of ways; there was also heterogeneity in the ascertainment of outcome (sensitisation and allergy).
The lack of high-quality human studies and the heterogeneous nature of the evidence have hindered the development of definitive conclusions from the present review. The studies tended to be carried out in high-risk populations with either an atopic family history in parents and/or siblings, attendance of the infant at an allergy clinic, or raised cord blood allergen-specific IgE in the infant. Furthermore, few studies included peanuts as an exposure. Of the six studies, only two were RCT and these both investigated multi-faceted interventions. In general, compliance with the intervention was good in both RCT. Although these RCT did measure sensitisation to peanuts, the intervention included avoidance of other food allergens as well as peanuts; the trials were also too small to be sufficiently powered to detect differences in peanut sensitisation between groups. The remainder of the studies were either small case–control studies that often did not adjust for other factors or cross-sectional studies. Obtaining evidence of sensitisation for each child is problematic (often due to issues of consent) and response rates for skin prick tests were 21 %(Reference Hourihane, Aiken and Briggs2) and 62 %(Reference Dean, Venter and Pereira19) for the cross-sectional studies. Most studies reported information on sensitisation to peanuts; there was little evidence on clinical peanut allergy. Not all children with sensitisation to peanuts experience symptoms, so it is important to recognise sensitisation and clinical allergy as distinct outcomes.
Revised position statements on dietary restrictions in pregnancy and early life and their effects on the risk of atopy have recently been published in Australia(Reference Prescott and Tang26) and the USA(Reference Greer, Sicherer and Burks27, Reference Sicherer and Burks28). Based on a summary of existing evidence, the Australian Society of Clinical Immunology and Allergy concluded that dietary restrictions in pregnancy and lactation are not recommended. They also suggested that the avoidance of peanuts for the first 2–4 years of life might be of value in high-risk children and is unlikely to cause harm, but they emphasise that there is no evidence to support this.
A recent report from the American Academy of Pediatrics(Reference Greer, Sicherer and Burks27, Reference Sicherer and Burks28) on the effects of early nutritional interventions on the development of atopic disease in infants and children, which considered the available evidence on the role of maternal dietary restriction, breast-feeding, timing of introduction of complementary foods, and hydrolysed formulas, also stated that current evidence does not support a major role for maternal dietary restrictions during pregnancy or lactation. This supersedes a consensus document from the USA(Reference Fiocchi, Assa'ad and Bahna29) (developed from a critical review on food allergy and the introduction of solid foods to infants) which suggested that paediatricians and allergists should assess infants with an increased risk of allergy on a case-by-case basis. They suggest that the optimal age for introduction of peanuts is at least 36 months. Furthermore, a systematic review on the relationship between early introduction of solid foods and the development of allergic disease concluded that there are few data supporting an association between early solid feeding and allergy with the exception of eczema(Reference Tarini, Carroll and Sox30). Subsequent to the completion of the present systematic analysis two relevant studies have been published. The first, a comparative study of the UK and Israel(Reference Du Toit, Katz and Sasieni31), showed that despite peanuts being introduced into the diet early in Israel, there is a low prevalence of peanut allergy; this has led to speculation that early introduction of peanuts, rather than avoidance, reduces the risk of peanut allergy. The second has highlighted the potential importance of environmental exposure (cutaneous contact and inhalation in the home) to ‘household peanuts’ in infancy in increasing the risk of peanut allergy(Reference Fox, Sasieni and du Toit32). This study concluded that peanut sensitisation occurs as a result of environmental exposure in infancy; high levels of environmental exposure appeared to promote sensitisation and low levels may be protective in atopic children.
These recently published reports are consistent with the results of our systematic review. They conclude that there is little evidence to support maternal dietary restriction during pregnancy or lactation, and that there is little evidence to support avoidance of peanuts in early infancy.
Conclusions
The systematic review of human studies and the narrative expert-led reviews have not provided clear evidence to suggest that either maternal exposure to or avoidance of peanuts, or early or delayed introduction of peanuts in the diet of children, has an impact upon subsequent development of sensitisation or allergy to peanuts. On completion of the review, the findings were submitted to the COT, which advises the UK Government. The COT has reviewed the evidence from the systematic review, alongside other scientific evidence, to assess the evidence base relevant to the 1998 COT dietary recommendations on peanut allergy; the COT concluded that its previous recommendations (made in 1998) are no longer supported by the current evidence. Following this process, the UK Food Standards Agency Board has considered the conclusions of the COT and advised UK Health Ministers to revise the Government's precautionary advice to mothers regarding peanut consumption during pregnancy and breast-feeding, and early-life peanut consumption by the child, to become in line with the conclusions of the COT.
The impact of maternal exposure and timing of introduction of peanuts into the infant diet on allergic outcomes remains an active area of research. Studies are underway that have the potential to provide more definitive data by 2013. An example is the Learning Early About Peanut Allergy (LEAP) study(33), which is a clinical intervention study testing the hypothesis that high-dose, early introduction of peanuts into infant diets will induce oral tolerance and prevent the development of peanut allergy. Mechanistic analyses of samples from the LEAP cohort could help develop an understanding of the processes in the developing immune system that lead to the acquisition of oral tolerance to peanuts, peanut sensitisation and peanut allergy.
The results from studies such as this could potentially strengthen the nature of the evidence on early exposure to, and avoidance of, peanut allergens and later development of sensitisation or allergy to peanuts. In the meantime, the available evidence does not support the avoidance of peanuts in maternal diets during pregnancy or lactation, or delayed introduction of peanuts in the diets of children.
Acknowledgements
The present review has been funded by the UK Food Standards Agency (grant no. T07052). The authors would like to thank Joelle Buck, Elizabeth Kendall, Susan Hattersley and Ian Kimber for their input on the study protocol.
R. L. T. developed the protocol for the present review. R. L. T., L. M. M. and J. L. selected papers for inclusion in the review and completed data extraction. R. L. T., L. M. M. and J. L. B. interpreted the results of the review. R. L. T. and L. M. M. wrote the manuscript. J. L. B. critically reviewed the manuscript. G. D. and J. S. provided advice on the study protocol and were members of the study steering group. G. D. reviewed and interpreted evidence on cord blood mononuclear cells and critically reviewed the manuscript. R. J. D. reviewed and interpreted the evidence from animal studies and critically reviewed the manuscript.
The authors declare no conflicts of interest.





