Hidden hunger is a nutritional deficiency that occurs in the presence of an otherwise nutritionally or energetically appropriate diet but one that is deficient in essential vitamins and minerals( Reference Burchi, Fanzo and Frison 1 , Reference Cole 2 ). It is quite prevalent in underdeveloped countries, and this becomes more relevant during periods of economic depression when emphasis on cost rather than quality usually means that the consumption of inexpensive energy-dense foods increases, whereas vegetables, fruits, fish and meat consumption, needed to provide a balanced diet, is clearly reduced( Reference Cole 2 ).
At present, Guatemala has one of the lowest rates of development in Latin America( Reference Lee, Houser and Must 3 ); it is an ethnically diverse country with large inequalities in the socio-economic and nutritional status of its population. Guatemala has the highest rate of chronic malnutrition in Latin America and the sixth highest worldwide, affecting 49 % of the child population( Reference Davis, Fischer and Rohloff 4 ). The hidden hunger risk due to deficient mineral intake is also high( Reference Montenegro-Bethancourt, Vossenaar and Kuijper 5 , Reference Bui, Marcinkevage and Ramakrishnan 6 ). An adequate mineral intake during infancy, childhood and adolescence is essential for normal growth and immune function, as well as to prevent chronic diseases in adulthood. Trace elements such as Mn, Se and Cr have received little attention among specialists, in spite of their essential role in various metabolic pathways and the severity of their nutritional deficiency among child populations( Reference Cole 2 , Reference Anderson 7 ).
In this sense, Mn is a constituent of several enzymes such as mitochondrial glutamine synthetase, pyruvate carboxylase and superoxide dismutase, a primary enzyme in the antioxidative defence system( Reference Anderson 7 ). Mn deficiency has been related in children and adolescents to skeletal abnormalities, osteoporosis, impaired growth and alterations of lipid and carbohydrate metabolism( Reference Bae and Choi 8 , Reference Ravelo, Rubio and González 9 ). Rodríguez-Rodríguez et al.( Reference Rodríguez-Rodríguez, Bermejo and López-Sobaler 10 ) reported the importance of ensuring an adequate Mn intake in children in order to prevent insulin resistance and diabetes type 2 in the future. Se is involved in the prevention of hepatocyte damage and CVD due to its antioxidant activity as a component of enzymes such as glutathione peroxidase. Some interesting findings reported an association of Se intake with a reduced prevalence and risk for prostate and colon cancers. However, randomised controlled trials for other cancer types are inconclusive( Reference Navarro-Alarcón and Cabrera-Vique 11 ). Cr is involved in lipid and carbohydrate metabolism, and the most frequent manifestation of Cr deficiency is altered glucose tolerance. Deficiency of this nutrient has also been associated with obesity, hypertension, CVD and neurological diseases and gene expression( Reference Anderson 7 , 12 ).
Based on FAO/WHO recommendations( 13 , 14 ), three basic approaches may be used to assess mineral intake: (a) total diet studies, (b) duplicate diet studies and (c) diary studies. The implementation of a duplicate diet study offers the advantage of providing more realistic exposure data for a particular population group, as all foods are directly analysed ‘as-consumed’; in addition, its economic cost is adequate( Reference Domingo, Perelló and Bordonaba 15 ).
The aim of this study was to determine the dietary intake of Mn, Se and Cr by institutionalised children and adolescents from Guatemala, in order to test their adequacy compared with dietary reference intakes and to detect possible mineral deficiency risks. A duplicate diet study, which represents the habitual diet, was carried out for 7 consecutive days. Diets were analysed under validated conditions by electrothermal atomic absorption spectrophotometry following acid digestion. Combined weighed and estimated dietary records were completed in parallel with the duplicate diet collection in order to evaluate the intake of energy and other nutrients.
Methods
Chemicals
Atomic absorption standard solutions for Mn, Se and Cr (Tritisol grade; Merck) were used to obtain calibration curves from a stock solution of 1000 mg/l for each metal by successive dilutions. Aqueous solutions of reagents and standards were prepared using a Milli-RO 12 plus Milli-Q purification system for bi-distilled deionised water (Millipore). All the chemicals used were of analytical reagent grade. High-quality concentrated nitric acid (65 %, v/v) (Merck) and perchloric acid (37 %, v/v) (Merck) were used for sample mineralisation. Ammonium molybdate (Merck) was used to precondition graphite furnace tubes, and magnesium nitrate (Merck) was used as the chemical modifier for Cr and Mn determination.
Instrumentation
A Perkin-Elmer 2100 double-beam atomic absorption spectrophotometer, equipped with a graphite furnace, deuterium-arc-background correction, an AS-800 autosampler and hollow cathode lamps (PerkinElmer), was used. Pyrolytically coated graphite tubes (ref. B013-5653) and pyrolytic graphite platforms (ref. B012-1092) were obtained from PerkinElmer. Ar of 99·999 % purity at 300 ml/min was used as the internal gas during all stages, except during atomisation, when the flow was stopped. Background-corrected integrated absorbance was used as the analytical signal. An Ultra Turrax homogeniser, Ika Labortechnik T25 (IKA), was used for sample homogenisation. Diet samples were subjected to a microwave-assisted mineralisation procedure (Multiwave 3000; Anton Paar GmbH). To avoid contamination, glass material, polypropylene vessels and pipette tips were cleaned by soaking in 30 %, v/v nitric acid for 24 h and were rinsed several times with bi-distilled deionised water.
Diet sampling strategies
For the duplicate diet study, duplicates (average size) of all foods and beverages served for breakfast, mid-morning snack, lunch, afternoon snack and dinner were obtained on 7 consecutive days at an orphanage school in Guatemala, which provides full board to over 250 indigenous children and adolescents aged between 4 and 14 years. These meals were the only food and beverages consumed, including nutritional supplements. Owing to the impossibility of quantifying water consumption, it was not included in the study. The food samples were subjected to a simulated eating procedure using normal knives and forks. The food items were sliced and the inedible parts were discarded. The remaining parts were weighed and then homogenised in a blender.
Combined weighed and estimated dietary records were completed in parallel with the duplicate diet collection to evaluate the energy and other nutrient intakes. All foods and beverages making up the diet were recorded and weighed and were transformed into energy and nutrient values using the Spanish Food Composition Tables and Dietsource 3.0® software. Local products and nutritional supplements were included in the software programme using the Food Composition Tables published by the Institute of Nutrition for Central America and Panama( 16 ).
Permission was obtained beforehand from the orphanage school authorities to conduct this study, and details of the procedures involved were explained. It was emphasised that dietary habits should not be changed over the study period, and furthermore samples had to be carefully collected. The study protocol was reviewed and approved by the Ethics Committee of the University of Granada (Spain).
Sample mineralisation
Before the mineral analysis, an aliquot of 0·4 g of homogenised samples was microwave mineralised in a closed quartz vessel with 6 ml of concentrated nitric acid and 1 ml of concentrated hydrochloric acid. The microwave oven was programmed at 1400 W and 80 bar as power and pressure limits, respectively (ramp time 10 min; hold time 10 min; cooling time 20 min). The mineralised solutions were then adequately diluted with bi-distilled deionised water. In these solutions, Mn, Se and Cr were determined in accordance with the analytical conditions that we had previously optimised for similar matrices( Reference Cabrera, Lloris and Giménez 17 – Reference Cabrera-Vique and Bouzas 19 ). The reliability of the method was further corroborated using standard reference materials: SRM 1548a NIST typical diet, purchased from the National Institute of Standards and Technology, and SRM 63 BCR Powdered skimmed milk, purchased from the Community Bureau of Reference. Both were subjected to the same analytical procedure. Paired t tests showed good agreement between the certified and the obtained values at a significance level of 0·05 %. All the analyses were performed in triplicate at the laboratories of the Mariano Gálvez University.
Statistical analysis
Data were interpreted using the statistical software package SPSS version 20.0 for Windows. Results are expressed as the arithmetic mean and standard deviation. The normal distribution of the variables and the homogeneity of the variances were checked using the Kolmogorov–Smirnov and the Bartlett’s test, respectively. A paired t test was applied for analytical method validation. In addition, correlations by Pearson’s or Spearman’s test (for parametric and non-parametric conditions, respectively) were used.
Results and discussion
The Guatemalan traditional diet has been characterised for centuries by a reliance on whole grains, such as the maize in tortillas, and low micronutrient density( Reference Montenegro-Bethancourt, Vossenaar and Kuijper 5 ). In recent decades, health authorities have promoted the consumption of nutritional supplements in the diet of children at risk for malnutrition( Reference Montenegro-Bethancourt, Vossenaar and Kuijper 5 , Reference Bui, Marcinkevage and Ramakrishnan 6 ). Institutionalised children are a population group with particular nutritional risk. As a result of poverty-related conditions, the socio-political situation and the high crime rate, a large number of children are institutionalised in orphanages, which largely subsist on the basis of donations from corporations and foreign economic aid channelled through non-governmental organisation and other humanitarian organisations. However, the severe economic crisis that is currently affecting Europe and North America has significantly reduced this foreign economic aid, and rates of child malnutrition have risen. These data are in contrast with the large numbers of children with overweight and obesity detected among the urban population( Reference Lee, Houser and Must 3 , Reference Swinburn, Sacks and Hall 20 , Reference Mancipe, García and Correa 21 ).
Table 1 shows the food composition of the analysed diets. It was noticed that diets were quite monotonous and were in accordance with the dietary pattern of this population group. The habitual diet contains locally available foods, mainly rice, black beans, tortillas and some fruits and vegetables. The consumption of eggs, meat or fish (river fish) was limited to 1–2 times/week. The intake of milk, dairy products and nuts was negligible. Five nutritional supplements enriched in minerals (Ca, Fe and Zn, according to the labelling information), and referred to as S1–S5 in Table 1, were consumed as a part of the diet at a rate of 2–4 servings/d. No information concerning Mn, Se and Cr contents was depicted on the label of the nutritional supplements. The S1–S4 supplements are prepared from soya flour and maize flour and consumed as ‘atol’ adding water and sugar. The name ‘atol’ refers to a pre-Hispanic beverage originally brewed with boiled maize and water; it is quite viscous and consumed very hot. The S5 supplement is made out of texturised soya protein and is added to rice during food preparation as a substitute for meat.
Table 1 Composition of the analysed diet samples

S1–S5, mineral-enriched nutritional supplements.
* Tilapia (river fish).
In the analysed diets, the overall daily contribution of energy in the 7-d diet was 8418·2 kJ (2012 kcal) (Table 2). The energy distribution of macronutrients was as follows: carbohydrates 69·4 %, proteins 12·3 % and fats 18·3 %. These values are not in accordance with the recommendations of the Food and Nutrition Board of the American Institute of Medicine( 22 ), according to which 45–65 % of energy should be obtained from carbohydrates, 10–30 % from proteins and 25–35 % from fats.
Table 2 Mean energy intake and mean nutrient content provided by the analysed diets, according to the data from food composition tables
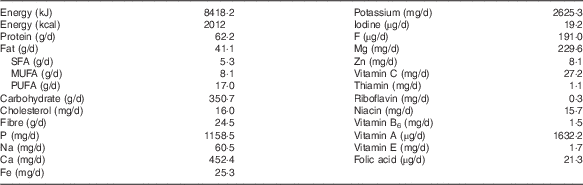
The daily contents of Mn, Se and Cr in the duplicate diets and the dietary reference intakes are summarised in Table 3. The average Mn content of the analysed menus was 1·96 mg/d, which is within the recommended level for children under 14 years of age, but not for boys over 14 years of age. Bibliographic data from childhood and adult populations show variable values of Mn intake, which are generally higher than those found in the present study – for example, Santos et al.( Reference Santos, Lauria and Da Silveira 24 ) estimated the mean dietary intake of Mn in Rio de Janeiro (Brazil) as 2·5 mg/d, Domingo et al.( Reference Domingo, Perelló and Bordonaba 15 ) in Catalonia (Spain) as 2·72 mg/d, Nasreddine et al.( Reference Nasreddine, Nashalian and Naja 25 ) in Beirut (Lebanon) as 2·04 mg/d and Velasco-Reynold et al.( Reference Velasco-Reynold, Navarro-Alarcón and López-G de la Serrana 26 ) in South-Eastern Spain as 3·05 mg/d. Ravelo et al.( Reference Ravelo, Rubio and González 9 ) determined the content of Mn in menus served in a public nursery school in Tenerife (Spain) and found an average content of 37 µg/kg. The authors suggest that this is insufficient, as this level is likely to cover only around 35 % of the daily recommended amount. Additional data on Mn dietary intake in similar recent studies are summarised in Table 4. Human Mn deficiency is rare because a varied and balanced diet normally provides adequate amounts of this element( Reference Ravelo, Rubio and González 9 ). Mn deficiency has been related in children and adolescents to skeletal abnormalities, osteoporosis, impaired growth and alterations of lipid and carbohydrate metabolism( Reference Bae and Choi 8 ). The major sources of Mn are cereals, vegetables and legumes, and these are the most commonly consumed foods by the studied population group, but the influence of vegetable species and the Mn levels in soil is high( Reference Ravelo, Rubio and González 9 ). The Mn content is also high in nuts, fish and green tea( Reference Bae and Choi 8 ), but the consumption of fish was infrequent and of nuts was negligible.
Table 3 Manganese, selenium and chromium contents in the analysed diet, consumed by institutionalised children and adolescents from GuatemalaFootnote * (Mean values and ranges)
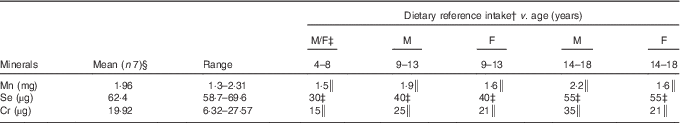
Table 4 Manganese dietary intake in several children and adolescent population groups according to recent bibliographical data

M, male; F, female.
* High intake of fats, proteins, free sugars, Na and cholesterol.
Approximately 80 % of the total Se dietary intake is absorbed( Reference Navarro-Alarcón and Cabrera-Vique 11 ); however, in several regions of the world, the content of Se in the diet has been estimated to be insufficient for the appropriate activity of selenoproteins( Reference Pedrero and Madrid 31 ). The average contribution of the analysed menus was 62·4 µg/d, which meets the recommended intake for this population group( 23 ). Results of the present study were higher than those reported by Gagné et al.( Reference Gagné, Blanchet and Lauzière 28 ) for Canadian Inuit children, but lower than data from studies carried out in Saudi Arabia( Reference Al-Daghri, Al-Othman and Alkharfy 27 ) and in USA( Reference Ervin, Wang and Wright 32 ) with a reported Se dietary mean intake in these studies of 164·8 and 88·5–101·2 µg/d, respectively. Data from other similar studies are summarised in Table 5. Mensink et al.( Reference Mensink, Fletcher and Gurinovic 38 ) reviewed the intake of micronutrients across Europe and reported that Se daily dietary intake in children (1–17 years) ranged from 16 to 29 µg in countries such as Belgium, UK, Poland, Serbia and Denmark. For adults, the mean daily intake of this element has been reported to be 66 µg in French diets( Reference Noël, Leblanc and Guérin 39 ), 54–57 µg in the UK( Reference Ysart, Miller and Crews 40 ) and 38 µg in forty-six different African countries according to a global study using food supply and composition data( Reference Joy, Ander and Young 41 ). The concentration range in which Se is considered toxic or essential is very narrow. It has been estimated that the ingestion of foodstuffs with a Se content above 1 mg/kg can induce toxicity, meanwhile a concentration below 0·1 mg/kg leads to a deficient status( Reference Pedrero and Madrid 31 ). In addition, it is very important to remark that the beneficial effects of Se on human health are strongly dependent on its chemical form and concentration( Reference Navarro-Alarcón and Cabrera-Vique 11 , Reference Pedrero and Madrid 31 ). In general, fish and shellfish, meat and related products, legumes, fruits and dry fruits are considered good Se sources, but the Se levels in local products are related to the Se concentration in the soil of the area. Se supplements can be beneficial for subjects living in regions with very low environmental levels of Se. Several strategies have been followed: (1) employment of Se-enriched fertilisers; (2) supplementation of farm animals with Se; and (3) consumption of multi-micronutrient supplements with Se. Nevertheless, detailed investigations of possible interactions between Se supplements and other food components and their influence on Se bioavailability are needed( Reference Navarro-Alarcón and Cabrera-Vique 11 , Reference Pedrero and Madrid 31 ).
Table 5 Selenium dietary intake in several children and adolescent population groups according to recent bibliographical data

M, male; F, female.
* Data from the USA National Health and Nutrition Examination Survey 2003–2006.
† Data from the National Health and Nutrition Examination Survey 1999–2000 for the US population (Mexican-Americans, black or African-American persons and low-income persons were included).
The average content of Cr in the menus analysed in the present study was 19·92 µg/d. Therefore, the diet only met the needs of children of 4–8 years of age, possibly because the serving size was not enough for older children (Table 3). Higher Cr intake has been reported by Rubio et al.( Reference Rubio, González and Gutiérrez 42 ) in children (6–17 years) from the Canary Islands (Spain); these authors evaluated the dietary intake by a total diet study and encountered values that varied between 85 and 106 µg/d according to the age. However, recent publications focusing on Cr dietary intake in children are very scarce, and future investigations are needed. Data on Cr dietary intakes higher than that of our study have also been reported in the literature for adults or for the general population. For example, Cr intakes between 50 and 120 µg/d have been reported in Italy( Reference Alberti-Fidanza, Burini and Perriello 43 ), France( Reference Roussel, Andriollo-Sanchez and Ferry 44 ), Australia( Reference Ashton, Barclay and Louie 45 ) and Brazil( Reference Santos, Lauria and Da Silveira 24 ). As a result of a duplicate diet study, Domingo et al.( Reference Domingo, Perelló and Bordonaba 15 ) reported a daily intake of 275 µg Cr/d in Catalonia (Spain) and Gimou et al.( Reference Gimou, Charrondière and Leblanc 46 ) of 230 µg Cr/d in Yaoundé (Cameroon). Cabrera-Vique & Mesías( Reference Cabrera-Vique and Mesías 18 ) in a study on females aged between 18 and 24 years in a university residence in Spain analysed duplicate meals (breakfast, lunch and dinner included), which showed optimal adherence to the Mediterranean diet, and reported Cr contents ranging from 98·50 to 120·80 µg/d (mean=110·00 µg/d). These authors attributed the elevated Cr values to the high consumption of legumes, cereals, fish, fresh fruits and vegetables, which is characteristic of the Mediterranean patterns. González-Weller et al.( Reference González-Weller, Rubio and Gutiérrez 47 ) analysed the Cr content in food and beverages consumed by the population of the Canary Islands (Spain) and found a mean dietary intake of 87 µg/d. These authors also found cereals to constitute the majority of dietary Cr. Dietary food sources of Cr include meat, brown sugar, brewer’s yeast, nuts, whole cereals, prunes, asparagus, mushrooms, egg yolk, beer and wine( Reference Cabrera, Lloris and Giménez 17 , Reference Cabrera-Vique and Bouzas 19 ). Except for cereals, these foods were consumed very little by the studied population group. Storelli( Reference Storelli 48 ) indicated that seafood consumption (except some cephalopods) does not represent an important contribution to daily dietary Cr intake. The fish occasionally included in the analysed meals was tilapia, a river fish.
In relation to the data variability in the Cr dietary intake, it is interesting to note that the toxicity of Cr(III), the chemical form present in foods, is low enough to provide a sufficient safety margin between usually consumed and harmful amounts, as humans cannot oxidise Cr(III) to potentially carcinogenic Cr(VI) compounds( Reference Anderson 7 , 12 ).
The intake of a given mineral could be related to that of other nutrients, particularly minerals and vitamins( Reference Anderson 7 ). In the analysed diets, we observed a significantly positive correlation between Cr and energy (P<0·01), carbohydrates (P<0·05), proteins (P<0·05), Zn (P<0·01) and Fe (P<0·01) intakes. Positive correlations were also observed between Mn and Se levels with the energy intake (P<0·05 in both cases).
Conclusions
It is widely accepted that in childhood and adolescence diet influences not only immediate health but also has an important impact on adult health. Minerals are essential to support adequate growth and development, but children in developing countries are at high risk of mineral deficiency, often referred to as hidden hunger, which is usually caused by a low mineral content in the diet and/or by low bioavailability. To determine the dietary intake of minerals and trace elements, study designs that include the collection and preparation of ready-for-consumption foods are believed to produce the most realistic and reliable data.
The results obtained from our duplicate diet study, the most reliable method to evaluate dietary mineral intake, demonstrated low intake of Mn and Cr in the studied population group. Nutritional strategies and interventions required to reduce hidden hunger include nutrition education, food fortification and the use of nutritional supplements. The present findings are of potential use to design new nutritional strategies for this and similar population groups; however, additional studies including mineral bioaccessibility assays are advisable. Our results demonstrate once again the impact of the socio-economic status and cultural conditions on the nutritional quality of the habitual diet.
Acknowledgements
The authors thank University Mariano Gálvez of Guatemala for technical support.
The present study was supported by a CICODE-University of Granada (Spain) International Cooperation Project of and by the Research Groups AGR141 and AGR177, financed by Junta de Andalucía.
C. C.-V. and R. G. designed the present study. M. B. analysed the diet samples and collected the data in Guatemala. All the other co-authors contributed to the data analysis and participated in the writing of the manuscript.
The authors have no financial or personal conflicts of interest to declare.








