Partially hydrolysed guar gum (PHGG) is a unique, soluble, functional dietary fibre derived from guar gum. PHGG (with nearly 85 % dietary fibre content) is derived by controlled hydrolysis of guar gum while targeting a blend of 1:7 ratio of short chain (3–8 monomers) and long chain (>9 monomers) galactomannans, respectively. The high viscosity of parent guar gum is nearly decimated after hydrolysis, making PHGG an ideal addition to liquid foods and nutritional formulas. Recently, attention has been given to the characteristics of foods and beverages containing dietary fibre that results in a high level of satiety. A feeling of long-lasting post-meal satiety or a feeling of fullness is an important factor to suppress inter-meal appetite and additional energy intake for the prevention of weight gain and obesity.
Generally, satiety is associated with postprandial sensations related to the activation of intestinal chemoreceptors, such as insulin, glucocorticoid hormones, hypothalamic neuropeptide Y, leptin, and cholecystokinin( Reference Batterham, Cowley and Small 1 – Reference Verdich, Flint and Gutzwiller 4 ). Such postprandial sensitisation, which is largely responsible for the phenomenon of satiation after a meal is consumed, has a longer-lasting effect on satiety or hunger than gastric distention.
Nevertheless, subjective measures such as visual analogue scales (VAS) wherein hunger rate, desire to eat and other appetite parameters are assessed to evaluate the perception of satiety have been proved to be ideal tools for understanding the satiety-inducing compositions( Reference Flint, Raben and Blundell 5 , Reference Parker, Sturm and MacIntosh 6 ). The VAS results might be variable due to variable response patterns among subjects, but were found to be reproducible within a subject for comparison( Reference Flint, Raben and Blundell 5 ).
Most of the beneficial effects of PHGG are probably due to the fact that the fibre is nearly completely fermented in the colon( Reference Greenberg and Sellman 7 ), and it produces significantly high amounts of SCFA, in particular butyrate, compared to those produced by other types of soluble dietary fibre( Reference Pylkas, Juneja and Slavin 8 ). Butyrate is known to be the preferred fuel for colonocytes, and it plays an important role in gut health, including immunity and production of satiety hormones( Reference Hamer, Jonkers and Venema 9 ). Unlike other types of soluble dietary fibre, which are rapidly fermented in the proximal colon, PHGG does not significantly increase stool weight.
Thus, it is beneficial in normalising bowel function, and it radially prevents or alleviates both constipation( Reference Patrick, Gohman and Marx 10 ) and diarrhoea( Reference Alam, Ashraf and Sarker 11 ), especially in populations receiving enteral nutrition, and/or sensitive to intestinal intolerance. Due to its slow fermentation, PHGG is also considered as a strong prebiotic fibre, as it increases the concentration of the beneficial bacterial strains bifidobacteria and lactobacilli( Reference Okubo, Ishihara and Takahashi 12 ), and reduces flatulence/bloating( Reference Furnari, Parodi and Gemignani 13 ).
PHGG may also produce various beneficial effects during digestion, like binding sterol compounds to help lower elevated serum cholesterol and slowing the rate of glucose absorption( Reference Gu, Yamashita and Suzuki 14 , Reference Trinidad, Perez and Loyola 15 ). It can thus prevent diabetes through lowering glucose response( Reference Peters and Davidson 16 ) and related metabolic factors( Reference Dall'Alba, Silva and Antonio 17 ). In addition, PHGG has negligible influence on mineral and protein absorption( Reference de Cassia Freitas, Amancio and Ferreira Novo 18 , Reference Takahashi, Sung and Kim 19 ). Earlier, Pasman et al ( Reference Pasman, Saris and Wauters 20 ) reported that the consumption of 40 g of PHGG/d for 1 week reduced the subsequent energy intake by 19 % compared to the effect of its non-consumption.
Later on further studies showed that the consumption of PHGG resulted in the increasing of blood cholecystokinin and delaying of gastric emptying, thus suggesting its physiological satiety effects( Reference Meier, Beglinger and Schneider 21 , Reference Heini, Lara-Castro and Schneider 22 ). In a more recent study, 2 g of PHGG in combination with protein intake was found to reduce inter-meal appetite through VAS assessment and observation of energy intake of a subsequent meal( Reference Lluch, Hanet-Geisen and Salah 23 ).
However, there is lack of evidence on the effect of PHGG, when administered in isolation at very low doses, on the subjective ratings of perception of the post-meal satiety and subsequent energy intake. Therefore, the present study explored further the PHGG-induced satiety phenomenal behaviour. Satiety effects of PHGG were found to be closely associated with its mobility, fermentation ability and production of SCFA. Acetate, propionate and butyrate could be readily absorbed by intestinal epithelial cells, providing energy and signals, and aiding the modulation of several transport systems.
The galactomannans in PHGG may thus be expected to support the aforementioned physiological and mechanistic approaches to the perception of sustained post-meal satiety effects and reduced subsequent energy intake. The VAS methodology employs a self-report scale, which consists of questions that assess subjective appetite-related sensations in a controlled setting, in response to an eating occasion right before and after consuming a preload or a test meal, and then at regular time intervals. Therefore, it was hypothesised that the variations which exist in VAS corresponding to subjective sensation of satiety could be quantified in context to acceptable degrees of validity and reliability, in response to the administration of satiation-inducing products. Thus, the present study was an attempt to evaluate the effects of acute and long-term consumption of small amounts of PHGG in a daily meal on subjective post-meal perception of satiety through VAS assessment. The satiety effects of PHGG were evaluated in comparison with those of its non-consumption or of consumption of equal amounts of carbohydrates and/or with other types of soluble fibre in a sequence of studies 1 to 3 (Fig. 1). The effect of intake of PHGG on subsequent food and energy intake was assessed.

Fig. 1 Schematic flow chart of studies (1, 2 and 3). PHGG, partially hydrolysed guar gum; VAS, visual analogue scale; ID, indigestible dextrin.
Materials and methods
Three different studies were conducted with volunteers at the Department of Health and Nutrition Sciences, Faculty of Human Life Sciences, Nagoya Keizai University, Nagoya, Japan with written informed consent of the subjects and approval of human ethical committee of the university as per the Declaration of Helsinki. In these studies, PHGG (Sunfiber®; Taiyo Kagaku Company, Limited), dextrin (SD#70; Sanwa Starch Company), indigestible dextrin (Fibersol-2®; Matsutani Chemical Industry Company) and inulin (Orafti® HSI; BENEO GmbH) were used. The perception of before- and after-meal satiety was evaluated through hourly measurements of various satiety parameters on the VAS (1–10 cm, representing low to high activity) as referred to by Lluch et al ( Reference Lluch, Hanet-Geisen and Salah 23 ). Before each study, subjects were screened on the basis of their hunger ratings on a VAS. Subjects who showed a difference of not less than 3 cm between maximum and minimum hunger ratings, or a difference of less than 3 cm between the maximum and the minimum, but with the maximum hunger rating at or above 9 cm on a VAS were considered to have good expression of hunger ratings, and were selected for the study. The others who had less than 3 cm difference between maximum and minimum hunger ratings, or who had a maximum hunger rating of less than 9 cm were considered to have weak expression of their perception of hunger on a VAS. Such subjects were excluded from the study as their responses might impose limitations on significant differences among the treatments. Those who had gastrointestinal disorders and other health issues were also excluded.
Study 1: perception of hunger after consumption of partially hydrolysed guar gum at different times of the day
This was an observational study to assess subjective perception of hunger post-breakfast/-lunch/-dinner with PHGG each occasion separately to find an ideal time for consumption of PHGG for sustained satiety effects (Fig. 1(a)). Initially, twenty subjects were recruited and screened for their hunger levels through VAS assessment as mentioned above. Finally, a total of twelve healthy subjects (ten male and two female) were selected. The subjects were divided into three groups (four subjects per group), and each group was assigned a diet with or without PHGG at breakfast, lunch or evening snack as described below.
Consumption with breakfast
On the 1st day of study, four subjects (three male and one female; age 36·8 (se 3·6) years; BMI 22·1 (se 1·3) kg/m2) were given a control dietary regimen of 170 g of yogurt (Natural Yogurt; Meiji Dairy Japan, 569 kJ) only as breakfast at 09.00 hours. From the 2nd day onwards, every day the subjects took 170 g of yogurt plus 2 g of PHGG (16·7 kJ) as breakfast consecutively for a week. The hourly hunger ratings on the VAS were measured for each subject after breakfast on the 1st day (control), 2nd day (PHGG-D1) and last day (PHGG-D7) for 4 h. The day before the VAS measurements, the subjects were restrained from taking any food (except water) after 22.00 hours.
Consumption with lunch
A lunch box along with the Japanese traditional miso (fermented soya) soup (total 1799 kJ) was the regimen served to the four subjects (three male and one female; age 37·2 (se 2·6) years; BMI 21·8 (se 1·7) kg/m2) at 12.00 hours. On day 1 the subjects were served the soup without PHGG along with the lunch box (control). On day 2, the subjects were served the soup containing 5 g of PHGG (41·8 kJ). Hourly hunger rating of each subject was assessed on the VAS before and after lunch for 5 h. On both days, the subjects took a similar breakfast (1381 kJ) at 09.00 hours in the morning and refrained from taking any additional food (except water) until lunch at 12.00 hours. The lunch box contained 100 g of rice (628 kJ), meat and vegetables (1004 kJ) and soup (167 kJ).
Consumption with evening snack
The remaining four subjects (four men; age 36·0 (se 3·7) years; BMI 21·8 (se 1·7) kg/m2) were served a snack (five pieces of cereal bars; 969 kJ) at 15.00 hours. On day 1, the snack did not contain fibre (control) and on the 2nd day the snack contained 5 g of fibre (PHGG; 42 kJ). Pre- and post-snacking hourly hunger rating of each subject was recorded on the VAS for 5 h. On both days, the subjects took similar lunch (1674 kJ) at 12.00 hours and refrained from taking anything except water until completion of the VAS assessments.
Study 2: perception of satiety with intake of partially hydrolysed guar gum or equal amount of carbohydrates
Based on the results of study 1, a randomised, double-blind, cross-over study was designed to assess the long-term administration of low doses (2 g) of PHGG or equal amounts of carbohydrates (as dextrin) on the subject's perception on post-breakfast satiety when compared with control consumption (Fig. 1(b)). Subjects were chosen according to Flint et al. ( Reference Flint, Raben and Blundell 5 ) to attain the power of 0·8 for most of the satiety parameters. Thirty-four subjects (twenty male and fourteen female) with an average age of 32·6 (se 8·8) years (BMI 21·2 (se 2·6) kg/m2) were recruited and subjected to screening for hunger ratings as described above. Finally, twenty-four healthy subjects (twelve male and twelve female) with an average age of 33·4 (se 8·7) years (BMI 20·7 (se 1·7) kg/m2) were selected for the study.
The study was conducted for a period of 7 weeks, wherein the 1st week was used for preparation of the subjects with daily intake of yogurt (Yogurt Natural; Glico Dairy Japan, 125 g, 561 kJ) only as breakfast at 09.00 hours. On the last day of the week-long preparation period, hourly VAS measurements of various satiety parameters were recorded before and after breakfast with yogurt (125 g). In the present study, each subject was employed to provide his or her own control or baseline value. After this the subjects were randomly assigned to two groups of different dietary regimens – to consume yogurt (125 g) with PHGG (2 g; 16·7 kJ; n 12) or to consume yogurt with dextrin (2 g; 33·5 kJ; n 12) as breakfast. The same dietary regimen was followed every day by each subject for two consecutive weeks (2nd and 3rd week). The VAS measurements for various satiety parameters were recorded on days 1, 7 and 13 during this dietary treatment. Then, the next 2 weeks (4th and 5th week) were considered as the washout followed by control periods, when the subjects were given none of these two dietary regimens except yogurt as breakfast. On the last day of the control period (i.e. the last day of the 5th week), VAS measurements were recorded again before and after breakfast with yogurt alone. Following these periods, the subjects were put on cross-over treatment of dietary regimens for the next two consecutive weeks (6th and 7th weeks), when the subjects who consumed PHGG or dextrin during the 2nd and 3rd weeks were put on reverse dietary regimens with yogurt as breakfast. Again the pre- and post-breakfast hourly VAS measurements under the revised dietary regimen were recorded on days 1, 7 and 13 during this reverse dietary treatment period.
In the present study the VAS measurements for different satiety parameters such as satiety, hunger, appetite, desire to eat and appetite score (average of all the above scores) were recorded as referred to previously( Reference Lluch, Hanet-Geisen and Salah 23 ) on a 1–10 cm scale immediately before breakfast, immediately after breakfast and then hourly for 4 h. The VAS measurements taken with the consumption of yogurt alone on the last day of the 1st week (preparation period) and the 5th week (washout followed by control period) were treated as control measures. Except on the previous day of VAS measurements, the subjects were free to consume their regular diet at dinner, but with restrictions on performing heavy work during day, and consumption of heavy food and alcohol during the entire period of the present study. The day before VAS measurements, all subjects were provided with a uniform dinner, and then were restrained from taking any additional food except water after 21.00 hours. During the entire period of dietary regimens of PHGG (14 d) and dextrin (14 d), energy intake through breakfast, lunch and snacking was recorded separately every day for each subject.
Study 3: comparison of perception of satiety with intake of different types of dietary fibre against the control diet
Subjective perception of hunger post-breakfast/-lunch/-dinner with PHGG was assessed in study 1, each occasion separately. Study 2 focussed on the perception of satiety with intake of PHGG or equal amount of carbohydrates. And study 3 was a randomised single-blind, single dose, comparative study performed to assess the differences in the acute effects of different types of soluble fibre on the subjective perception of post-meal satiety (Fig. 1(c)).
Initially ten subjects were recruited and screened as described before. Finally, six subjects (four male and two female; age 29·2 (se 4·7) years; BMI 20·6 (se 1·2) kg/m2) were selected. The study was conducted in a span of 12 d by providing similar lunch boxes (1979 kJ) every day to all subjects. On days 3, 6, 9 and 12 the subjects were randomly given a lunch box in which rice was mixed with either control diet (no fibre addition), or with the addition of PHGG (6 g), indigestible dextrin (6 g) or inulin (6 g) at 12.00 hours. Each one of these soluble fibre types provided an energy intake of 8·4 kJ/g. VAS readings for satiety, hunger, appetite, desire to eat and appetite score were recorded before lunch, immediately after lunch and hourly for 5 h. Each subject was required to consume all the four diets in the study period; and each subject was employed to provide his or her own control or baseline value to compare the effects of the other three diet regimens. The subjects were allowed to consume their regular diet at dinner but with restrictions on performing heavy work during day and consumption of heavy food or alcohol during the entire period of the present experiment. On the day of VAS measurements, all subjects were given uniform breakfast (1707 kJ) at 09.00 hours, and then restrained from taking any additional food except water until lunch and during the VAS measurements after lunch.
Statistical analysis
The hourly VAS measurements recorded before and after consumption of food with or without the addition of PHGG or other treatments (dextrin or other types of soluble fibre) were analysed with ANOVA. The effect of each treatment was compared with that of the control diet (with no addition) or among themselves (with addition) using paired t tests. In study 1, the hourly hunger ratings of VAS measurements of the three experiments conducted separately at different timings (breakfast, lunch and snack) of the day were used to compare between the effects of the PHGG and control diets. In study 2, VAS measurements for satiety, hunger, appetite, desire to eat and appetite score were recorded. The VAS measurements of the two control periods (end of 1st week and 5th week) were similar, and hence the average values were drawn and used as the control value. The VAS results of PHGG and dextrin interventions were compared with the control, and then among themselves. The average energy intake through breakfast, lunch and snack were compared between PHGG and dextrin interventions.
In study 3, the VAS measurements of satiety, hunger, appetite, desire to eat and appetite score for PHGG and other types of soluble fibre were compared with the control or among themselves. All values represent means with their standard errors. Statistical significance was assessed at the 0·05 and 0·01 level. The Statistical Package for the Social Science (version 15.0; SPSS, Inc.) was used for the statistical calculations. Post hoc power calculations with sample size used in each study were performed using online StatWeb system version 1.1.1 (Department of Statistics, The University of British Columbia, Canada) developed by Brant( Reference Brant 24 ) to assess the power and validation of each study.
Results
Study 1: perception of satiety with consumption of partially hydrolysed guar gum at different times of the day
The hourly hunger ratings after intake of 2 g of PHGG along with yogurt as breakfast was not significantly different (P>0·23) from that of control on the 1st day. However, after regular consumption of PHGG along with yogurt as breakfast consecutively for 1 week, the post-breakfast hourly VAS hunger ratings for PHGG were significantly low (P= 0·016 at 1 h, P= 0·009 at 2 h, P= 0·022 at 3 h and P= 0·044 at 4 h) when compared to the control (Fig. 2(a)). The consumption of 5 g of PHGG during lunch significantly (P< 0·05) reduced the hourly VAS hunger ratings only after 3 h (P= 0·010 at 3 h, P= 0·046 at 4 h and P= 0·024 at 5 h, Fig. 2(b)).

Fig. 2 Visual analogue scale (VAS) hunger ratings after consumption of breakfast (a), lunch (b) and evening snack (c) with control and partially hydrolysed guar gum (PHGG). Values are means, with their standard errors represented by vertical bars. The hourly VAS ratings of hunger with the consumption of PHGG mixed with breakfast were measured on day 1 (PHGG-D1) and day 7 (PHGG-D7) and compared with those of the control. Mean value was significantly different from that of the control: * P< 0·05, ** P< 0·01. ![]() , Control (a, b and c);
, Control (a, b and c); ![]() , PHGG-D1 (2 g) (a);
, PHGG-D1 (2 g) (a); ![]() , PHGG-D7 (2 g) (a);
, PHGG-D7 (2 g) (a); ![]() , PHGG (5 g) (b and c). IBI, immediately before intake; IAI, immediately after intake.
, PHGG (5 g) (b and c). IBI, immediately before intake; IAI, immediately after intake.
Also, the consumption of 5 g of PHGG during snack time (15.00 hours) in the afternoon significantly (P< 0·05) reduced the hunger ratings after 2 h (P= 0·035 at 2 h, P= 0·0004 at 3 h, P= 0·009 at 4 h and P= 0·018 at 5 h, Fig. 2(c)).
Overall the consumption of PHGG along with food may be responsible for the reduction of the post-meal hunger ratings at any time of the day. The intake of low amounts (2 g) of PHGG may require longer periods to exhibit its satiety effects probably through modulation of intestinal physiological activities. The retrospective power analysis suggests an average power of 0·99 (se 0·0) at breakfast (day 7), 0·31 (se 0·11) (with the range of 0·10–0·43) at lunch and 0·49 (se 0·08) (with the range of 0·39–0·65) at snack for the hunger ratings after 3 h of PHGG consumption.
Study 2: perception of satiety with intake of partially hydrolysed guar gum or equal amount of carbohydrate (dextrin)
All twenty-four subjects successfully completed the study. The satiety parameters measured on the VAS at the end of both the control periods (end of 1st week and 5th week) were not significantly different (P>0·31), and so the average values for each subject were drawn to compare with the values of PHGG and dextrin. The ANOVA results suggest that the hourly VAS ratings for most of the satiety parameters at 2 and 4 h on days 1 and 13 were significantly different (P< 0·05) among the groups (Table 1). With reference to the control, the post-breakfast satiety response with consumption of PHGG or dextrin was comparable in all the satiety parameters. PHGG showed better satiety effects than dextrin when compared with the control. Post-breakfast satiety effects of PHGG for all the satiety parameters were significantly different (P< 0·05) after 2 h on days 1, 6 and 13 when compared with the control. Whereas, dextrin showed significant difference (P< 0·05) for satiety, hunger, appetite and appetite score on day 1 only (Table 1). There was no significant difference of comparison between PHGG and dextrin on days 1 and 6 for all the parameters (data not shown). The clearly distinct satiety effects of PHGG and dextrin were observed in comparison to the control on day 13 (Table 1, Fig. 3).
Table 1 Average visual analogue scale ratings for various satiety parameters at 2 and 4 h after consumption of the control diet, dextrin (2 g) or partially hydrolysed guar gum (PHGG) (2 g) with breakfast on days 1 and 13 in study 2 (Mean values with their standard errors)

Mean value was significantly different from that of the control: * P< 0·05, ** P< 0·01 (t test).

Fig. 3 Hourly visual analogue scale (VAS) measurements on the 13th day for satiety (a), hunger (b), appetite (c), desire to eat (d) and appetite score (e) before and after consumption of control (![]() ), dextrin (2 g) (
), dextrin (2 g) (![]() ) or partially hydrolysed guar gum (PHGG) (2 g) (
) or partially hydrolysed guar gum (PHGG) (2 g) (![]() ) with breakfast. Values are means, with their standard errors represented by vertical bars. The hourly satiety VAS ratings of dextrin and PHGG were compared with those of the control. Mean value was significantly different from that of the control: * P< 0·05, ** P< 0·01. IBB, immediately before breakfast; IAB, immediately after breakfast.
) with breakfast. Values are means, with their standard errors represented by vertical bars. The hourly satiety VAS ratings of dextrin and PHGG were compared with those of the control. Mean value was significantly different from that of the control: * P< 0·05, ** P< 0·01. IBB, immediately before breakfast; IAB, immediately after breakfast.
The satiety effects of PHGG on day 13 were significantly different for satiety (P< 0·01 at 2 and 4 h; Fig. 3(a)), hunger (P< 0·05 at 2 h and P< 0·01 at 4 h; Fig. 3(b)), appetite (P< 0·05 from 1 to 4 h; Fig. 3(c)), desire to eat (P< 0·05 at 1, 2, 4 h; Fig. 3(d)) and appetite score (P< 0·05 at 1–3 h and P< 0·01 at 4 h; Fig. 3(e)) when compared with the control. Whereas dextrin did not show any significant difference at any hour after breakfast for any of the satiety parameters when compared with the control (Fig. 3). While comparing the PHGG against the dextrin intervention, a significant difference was observed for satiety (P< 0·05) and hunger (P< 0·05) after 4 h. Overall, the results suggest that PHGG has better satiety effects through VAS measurements for all satiety parameters when compared to the control and the dextrin intervention. There was no specific difference between men and women in their responses (data not shown) with PHGG or dextrin for all the parameters.
The energy intake through breakfast, lunch and whole day snacking was not monitored during the control period, and hence the data was compared between PHGG and dextrin only. The average energy intake through breakfast was not much different between PHGG and dextrin, because the subjects took fixed amount of breakfast (yogurt+PHGG or dextrin). However, the energy intake was significantly lower through lunch (P< 0·05) and whole day snacking (P< 0·01) with PHGG in comparison to dextrin (Fig. 4). The results suggest that the energy intake via whole day snacking was about 20 % lower with the consumption of PHGG when compared to that of dextrin.
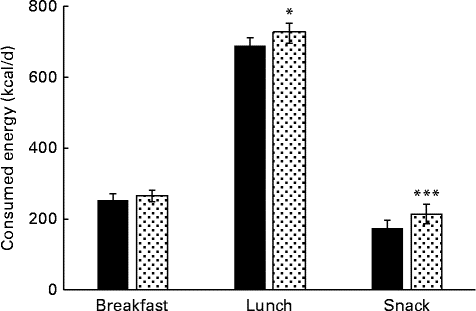
Fig. 4 Average daily energy intake via breakfast, lunch and whole day snacking after consumption of 2 g of either dextrin (![]() ) or partially hydrolysed guar gum (PHGG,
) or partially hydrolysed guar gum (PHGG, ![]() ) for 14 d. Values are means, with their standard errors represented by vertical bars. Mean value was significantly different from that of PHGG: * P< 0·056, *** P< 0·009. To convert values in kcal to kJ, multiply by 4·184.
) for 14 d. Values are means, with their standard errors represented by vertical bars. Mean value was significantly different from that of PHGG: * P< 0·056, *** P< 0·009. To convert values in kcal to kJ, multiply by 4·184.
The retrospective power analysis suggests an average power of 0·76 (se 0·03) (with the range of 0·50–0·94) to PHGG, and 0·33 (se 0·06) (with the range of 0·03–0·87) to dextrin, while compared with the control for all satiety parameters.
Study 3: comparison of perception of satiety with intakes of different types of soluble fibre
The post-lunch satiety effects of different types of soluble dietary fibre were compared hourly for 5 h. All theses types of fibre showed some degree of satiety effects compared to the control, but none of them had a significant effect until 5 h post-meal for any of the satiety parameters on the VAS. ANOVA results (Table 2) suggest significant differences among the groups for appetite (P= 0·02), desire to eat (P= 0·05) and appetite score (P= 0·03) after 5 h. A clear differentiation among the different types of soluble dietary fibre was observed only after 5 h. PHGG showed a difference of more than a centimetre on the VAS for all the parameters when compared with the control (Fig. 5). PHGG showed significant difference for appetite (P= 0·023), desire to eat (P= 0·011) and appetite score (P= 0·026) when compared with the control. On the other hand, other types of soluble fibre did not show any significant (P= 0·14–0·92) difference when compared with the control (Fig. 5). The retrospective power analysis suggests an average power of 0·82 (se 0·09) (with the range of 0·50–0·99) to PHGG, 0·56 (se 0·08) (with the range of 0·42–0·87) to indigestible dextrin and 0·13 (se 0·05) (with the range of 0·03–0·31) to inulin, when compared with the control for all satiety parameters.
Table 2 Average visual analogue scale ratings for various satiety parameters at 5 h after consumption of control, partially hydrolysed guar gum (PHGG) (6 g), indigestible dextrin (6 g) or inulin (6 g) with lunch in study 3 (Mean values with their standard errors)
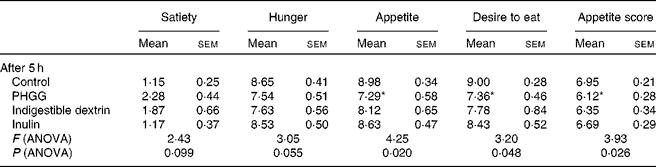
Mean value was significantly different from that of the control: * P< 0·05 (t test).

Fig. 5 Visual analogue scale (VAS) measurements of satiety, hunger, appetite, desire to eat and appetite score after 5 h of intake of lunch with control (![]() ) or different types of soluble fibre (partially hydrolysed guar gum (
) or different types of soluble fibre (partially hydrolysed guar gum (![]() ) or indigestible dextrin (
) or indigestible dextrin (![]() ) or inulin (
) or inulin (![]() )). Values are means, with their standard errors represented by vertical bars. The VAS ratings of different types of soluble fibre were compared with those of the control. Mean value was significantly different from that of the control: * P< 0·05.
)). Values are means, with their standard errors represented by vertical bars. The VAS ratings of different types of soluble fibre were compared with those of the control. Mean value was significantly different from that of the control: * P< 0·05.
Discussion
Post-meal satiation and inter-meal satiety are crucial factors to reduce the additional energy intake through snacking, which is reported to increase the risk of weight gain in many people( Reference Nicklas, Yang and Baranowski 25 , Reference Savige, MacFarlane and Ball 26 ). On average snacking is reported to contribute an addition of 1255–3347 kJ/d, which is a significant portion of the average daily total energy of about 10 042 kJ/d of a person. Additionally, most of these snacks are rich in fat, sugar or salt, which when taken in excess may cause harmful effects on health( Reference Astrup, Bovy and Nackenhorst 27 ).
An individual typically requires at least 30 min of exercise to burn 418 kJ. So any simple effort that could help to reduce the intake of inter-meal snacking may be beneficial to maintain good health and to control weight gain. Reports suggest that foods rich in proteins( Reference Fischer, Colombani and Wenk 28 – Reference Harper, James and Flint 30 ), thickening agents like guar gum( Reference French and Read 31 – Reference Lavin and Read 33 ), gel-forming dietary fibre such as pectins( Reference Wanders, Jonathan and van den Borne 34 ) and nuts rich in fat, protein and fibre( Reference Tan and Mattes 35 ) could provide good perception of satiation in human subjects.
Several biomarkers such as cholecystokinin, glucagon-like peptide-1 and peptide YY are considered physiological indicators of satiety( Reference de Graaf, Blom and Smeets 2 , Reference Hellstrom, Geliebter and Naslund 3 ). The VAS measurements of various satiety parameters such as satiety, hunger, appetite and desire to eat are noted as good indicators of the perception of one's psychological behaviour of hunger and satiety( Reference Flint, Raben and Blundell 5 , Reference Parker, Sturm and MacIntosh 6 ). Findings of previous studies noted that PHGG, a soluble fibre, promoted many physiological health benefits( Reference Yoon, Chu and Juneja 36 – Reference Quartarone 38 ), and showed satiety effects through physiological indicators such as increased cholecystokinin concentration in blood( Reference Meier, Beglinger and Schneider 21 , Reference Heini, Lara-Castro and Schneider 22 ) and delayed gastric emptying( Reference Meier, Beglinger and Schneider 21 ). A few other studies evaluated the perception of satiety effects through the VAS measurements of fructose( Reference Van de Ven, Westerterp-Plantenga and Wouters 39 ) and protein( Reference Lluch, Hanet-Geisen and Salah 23 ) inerventions.
In the present studies (studies 1 and 3), the acute intake of 5–6 g of PHGG along with lunch and evening snacks significantly increased feelings of satiety. In studies 1 and 2, the consumption of a small amount (2 g) of PHGG along with yogurt also induced satiety feelings, but it took prolonged adaptation periods to show sustained effects. The acute satiety effect with high amounts (5–6 g) of PHGG might be related to more of a physical effect than physiological, whereas the effect after prolonged adaptation with small amounts (2 g) of PHGG might be considered as both physiological and physical. Unlike other types of soluble fibre, PHGG has high amounts of long chain polysaccharides with more than nine monomers, which may form a matrix of the food, probably delaying digestion and colonic transit time. An earlier report suggested prolonged colonic transit time with adaptation of PHGG for a week( Reference Meier, Beglinger and Schneider 21 ). Another study indicated the acute effects of guar gum on delayed gastric emptying( Reference French and Read 31 ). These features might be considered as typical nature of the structure and function of guar galactomannans.
Consumption of small amounts of both PHGG and dextrin in study 2 showed acute satiety effects, but that of PHGG in isolation persisted with sustained effects throughout the prolonged adaptation period. Several studies have suggested the role of fermentability of different types of dietary fibre and formation of SCFA in triggering satiety effects. PHGG with many long chain polysaccharides was found to promote the prolonged and high production of SCFA, in particular butyrate( Reference Pylkas, Juneja and Slavin 8 , Reference Valazquez, Davies and Marett 40 ), which in turn was found to play a crucial role in a variety of colonic mucosal functions and promote satiety through energy homeostasis and production of satiety inducing hormones( Reference Hamer, Jonkers and Venema 9 ). Other studies indicated the direct involvement of SCFA in gastrointestinal mobility( Reference Cuche, Cuber and Malbert 41 , Reference Cuche and Malbert 42 ) and colonic brake( Reference Ropert, Cherbut and Rozé 43 ). The prolonged adaptation of small amounts of PHGG may thus increase the physiological effects in the gut leading to sustained satiety effects.
In the acute intake test (studies 1 and 3), the onset of significant satiety effect with PHGG was perceived to vary between lunch and evening snack. It took 2 h with evening snack, but took more than 3 h with lunch. This differential response may be associated with the energy consumed at the background at the respective times. Naturally foods that take up more enteral space and that provide more energy tend to be more satiating. At lunch subjects had high volume consumption of food with high energy (>1799 kJ) compared to evening snack with low volume consumption of food with low energy (971 kJ), so the satiation effect of the meal and the duration of post meal satiety effect might be longer for lunch than for evening snack.
In study 3, acute intake of different types of soluble fibre showed varied levels of satiety effects: PHGG showed significant satiety effects over other types of fibre when compared with the control. According to Wills et al. ( Reference Wills, Eldridge and Beiseigel 44 ), the efficacy of dietary fibre on satiety may vary in relation to specific chemical structure or fermentation capacity. The difference in acute effects might be due to the difference in chemical structure more than that in fermentation capacity, because the former might have had a greater physical effect to delay the gastric emptying/mobility than the latter. Lyly et al. ( Reference Lyly, Licukkonen and Salmenkallio-Marttila 45 ) also found that a beverage containing guar gum showed better acute satiety effects through VAS measurements compared to a beverage containing wheat bran and oat β-glucan.
Most of the available literature suggest that dietary fibre which has high viscosity and high fermentability properties triggered strong perception of satiety effects though physical and physiological effects( Reference Clark and Slavin 46 , Reference Slavin and Green 47 ). Non-viscous types of soluble fibre showed some degree of physiological satiety effects through the production of varied amounts of SCFA and satiety-inducing hormones, but they often failed to exhibit the perceivable satiety effects through VAS measurements. In this context, PHGG, a non-viscous soluble fibre, was found to exhibit both perceivable and physiological satiety effects probably through its unique composition of short and medium-chain polysaccharides of galactomannans. The high fermentability of PHGG may be of interest for further studies examining its effects on weight management and appetite control. Prolonged intake of small amounts of PHGG was found to reduce the subsequent energy intake (study 2). The results suggest that PHGG could be an ideal natural product to incorporate into daily food to cut the intake of additional energy, salt and sugar through snacking.
The addition of PHGG in food led to perceivable satiety effects after-meal in all the three studies. However, the post hoc power analysis suggests an under power especially in studies 1 and 3 for most of the satiety parameters, which constrains valid conclusions on the acute effects of PHGG and its comparison with other types of soluble fibre. Thus, it warrants further investigations with larger number of subjects.
Conclusion
Dietary fibre available in soluble or insoluble forms, differing in physical and chemical properties may exert differential functional and health benefits. High-viscous and high-fermentable types of dietary fibre do cause acute satiety effects, but high viscosity limits their use in many kinds of foods.
The non-viscous types of soluble fibre with high functionality might be ideal for use in a number of applications, but many of these were found to be ineffective in exhibiting acute psychological satiety effects. PHGG, a non-viscous soluble fibre, has been demonstrated in various clinical studies to be one of the largest producers of SCFA including butyrate and propionate which are known to exert a healthy effect on the intestine and overall immune well-being.
PHGG which helps a high production of critical SCFA in the intestine may therefore help produce both short and long-term satiety effects even when administered in small amounts. PHGG could thus be the ideal fibre to add to various food products or to be taken as a single supplement for appetite control, arresting of temptation to snack and reduction of subsequent energy intake.
We thank all the volunteers who have participated in the studies. Also we thank the staff of the Department of Health and Nutrition Sciences at Nagoya Keizai University, Nagoya, Japan for their excellent support to the study.
All the foods (breakfast, lunch and dinner) and treatment materials (different types of dietary fibre and dextrin) for these studies were sponsored with a research grant of Taiyo Kagaku Company, Limited. The sponsor had no role in the design, conduct or results of these studies
Acknowledgements
The authors' responsibilities are as follows: T. P. R. initiated the project and wrote the manuscript; M. H. designed and conducted all the studies; T. M. and N. I. provided technical and physical support to M. H. during the period of the studies; M. P. K. provided assistance in statistical analysis; T. O. was the scientific advisor; L. R. J. and K. W. were collaborators.
The authors have no conflict of interest to declare.










