Non-alcoholic fatty liver disease (NAFLD) has evolved as the most common form of chronic liver disease, and has become a major public health issue as it increasingly appears as a systemic disease in the context of the metabolic syndrome( Reference Tarantino 1 ). Hepatic fat accumulation induces not only cellular stresses such as oxidative stress and endoplasmic reticulum stress but also insulin resistance (IR) and inflammation, which lead to the development of non-alcoholic steatohepatitis (NASH)( Reference Pinzani 2 , Reference Zhang, Xu and Yu 3 ). Visceral fat accumulation is regarded as a significant risk factor for the development of NAFLD and its progression to NASH( Reference Takaki, Kawai and Yamamoto 4 ). NASH can further deteriorate into cirrhosis and hepatocellular carcinoma( Reference Pinzani 2 ). Energy imbalance and an ‘unhealthy’ dietary pattern have been claimed to be key factors in the development and progression of steatosis to NASH( Reference Volynets, Küper and Strahl 5 ). High-fat, high-fructose diets, also called Western diets, have been shown to induce IR and de novo lipogenesis in the liver with further deterioration in liver function( Reference Sobrecases, Lê and Bortolotti 6 ). Several recent studies have also pointed to alterations in intestinal microbiota and gut barrier function as important contributors to the development of NASH( Reference Ilan 7 , Reference Chassaing, Etienne-Mesmin and Gewirtz 8 ). The composition of intestinal microbiota in NASH patients is characterised by a higher level of Clostridium coccoides and a lower level of Bacteroides/Prevotella ( Reference Takaki, Kawai and Yamamoto 4 ). Dysbiosis in turn alters gut permeability and increases bacterial translocation, which results in endotoxin-induced activation of toll-like receptor 4 (TLR4) in the liver, leading to the production of pro-inflammatory cytokines( Reference Thuy, Ladurner and Volynets 9 ). Thus, the intestinal environment may be at least as important as hepatic metabolic stress in the progression of the disease( Reference Takaki, Kawai and Yamamoto 4 ).
Besides lifestyle modifications (weight loss, reduction in dietary fat and sweetened-drink consumption, etc.), which are difficult to implement, or vitamin E treatment, for which there is a safety concern in terms of cancer risk, effective treatment strategies are limited( Reference Chalasani, Younossi and Lavine 10 ), probably because NASH is a multifactorial disease. Amino acid (AA) or protein supplementations may offer a useful alternative, as they may target several mechanisms involved in disease progression such as steatosis and IR, as well as dysbiosis and altered gut function. They have been shown to improve steatosis via their effects on lipid metabolism, insulin sensitivity and glucose tolerance( Reference Theytaz, Noguchi and Egli 11 , Reference Lee, Han and Yim 12 ). The mechanism proposed may be (i) decreased de novo lipogenesis through down-regulation of genes involved in lipogenesis, (ii) increased lipid oxidation or (iii) modulation of lipid-induced inflammation. However, although protein supplementation has been shown to improve gut function and microbiota equilibrium( Reference Lopez-Legarrea, Fuller and Zulet 13 , Reference Rhoads and Wu 14 ), its effectiveness in terms of gut/liver interactions remains to be demonstrated. Recent studies emphasise the importance of specific AA such as citrulline (Cit) in this setting( Reference Ginguet, Cynober and De Bandt 15 – Reference Lai, Lee and Hung 17 ). From a metabolic point of view, we previously showed that Cit improves hepatic lipid metabolism, insulin sensitivity and steatosis in a model of fructose-induced NAFLD( Reference Jegatheesan, Beutheu and Ventura 18 , Reference Jegatheesan, Beutheu and Ventura 19 ) where gut function is preserved. Moreover, Cit supplementation is associated with an improvement in plasma AA and lean body mass suggesting improved protein metabolism. Interestingly, Cit may also be protective at the gut level and on gut/liver interactions. Indeed, Cit attenuates mucosal damage and improves intestinal permeability in a model of 5-fluorouracil-induced mucositis in mice. Recently, it has also been shown that Cit mitigates intestinal inflammation( Reference Lai, Lee and Hung 17 ) and promotes intestinal adaptation to massive intestinal resection( Reference Osowska, Moinard and Neveux 20 ). Finally, as already shown for arginine (Arg), its precursor/derivative, which regulates intestinal microbiota( Reference Ren, Chen and Yin 21 ), Cit may also possibly affect microbiota composition in the large intestine. This led us to wonder about the potential interest of Cit in the prevention of the disease aggravation into NASH.
This present study’s hypothesis was that Cit might improve some of the factors involved in disease progression not only at the liver but also the gut and peripheral organ level.
The aims of this study were thus to assess the effects of oral Cit administration on liver and gut function in a rat model of NAFLD induced by an 8-week Western diet and to elucidate the mechanisms involved.
Methods
Animals
In total, forty-three 6-week-old male Sprague–Dawley rats (190–220 g) were purchased from Charles River. They were housed individually in a temperature-controlled room with a 12 h light–12 h dark cycle. They were given free access to water and standard rodent chow (UAR A04; SAFE) ad libitum for a 1-week acclimatisation period.
For the feeding experiments, the rats received either standard rodent chow (UAR A04) or a Western diet combining a 45 % fat diet (824053; Special Diets Service) with 30 % fructose in drinking water (F0127; Sigma-Aldrich). Compositions of the diets are given in the online Supplementary Table S1.
Animal care and experimentation complied with both French and European Community regulations for animal care and experimentation. All the procedures were conducted in accordance with the guidelines for animal care of the regional ethics committee (Comité régional d’éthique en matière d’expérimentation animale, Ile-de-France), and the study protocol was approved by the same committee (registration no. 00737.02).
Experimental design
The rats were randomly assigned to four groups (10–13 per group) to receive for 8 weeks either standard rodent chow (C group) or a Western diet either alone (WD group) or supplemented with non-essential amino acids (NEAA, WDA group) or with Cit 1 g/kg per d (WDC group). The NEAA supplement contained isomolar amounts of six NEAA (alanine, glycine, proline, aspartate, histidine and serine) and was isonitrogenous to the Cit supplement. The animals received their respective food and drinks ad libitum. Body weight (BW) was measured at the beginning of the experiment and then once a week throughout the study. In addition, once a week throughout the study, food and drink intakes were monitored, 24 h urine samples were collected and N excretion was measured by pyrochemiluminescence (Antek 9000; Antek). At the end of the feeding period and after an overnight fast, the rats were anaesthetised by isoflurane inhalation. Portal and aortal blood samples were taken, and the rats were euthanatised by exsanguination for liver and gut sample collection as described previously( Reference Jegatheesan, Beutheu and Ventura 18 ). Body composition, particularly localisation and size of fat deposits, was determined by dissection and weighing. To study gut microbiota, samples of caecal content and colon mucosa were collected and frozen at −80°C until analysis.
Metabolic assessment
For AA analysis, plasma samples were de-proteinised with 30 % (w/v) sulphosalicylic acid solution, separated and quantified by ion-exchange chromatography with post-column ninhydrin derivatisation using a JLC-500/V AminoTac™ analyzer (Jeol Ltd). Plasma liver enzyme activities (alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP)) and plasma levels of bilirubin, total cholesterol, TAG, glucose and uric acid were determined using standard techniques on a multi-parameter analyzer (Cobas C 6000; Roche).
Plasma insulin was measured by ELISA, using a commercial kit (Insulin Rat Ultrasensitive ELISA; ALPCO), following the manufacturer’s protocol. Insulin sensitivity was evaluated using the homoeostasis model assessment of insulin resistance (HOMA-IR): (fasted insulin (mU/l)×fasted glucose (mm))/22·5.
Hepatic steatosis and inflammation
Frozen liver tissue samples were homogenised in 5 % NP-40 to extract lipids. Hepatic TAG content was assessed using a commercially available TAG quantification kit (Abcam).
For histological evaluation of hepatic lipid accumulation, frozen liver sections (10 µm) were fixed with 10 % formalin in PBS for 30 min, stained with Oil Red O (Sigma-Aldrich) for 30 min and then washed four times with sterile water. Representative photomicrographs were captured at a 20× magnification using a camera-equipped microscope (DM 4000B, Leica Microsystems CMS GmbH).
Liver samples were homogenised in (ten times their volume) 10 % TCA–0·5 mm-EDTA, using an Ultra Turrax blender (IKA-Labortechnik). GSH and GSSG level was determined in the supernatant by reverse-phase HPLC coupled with MS (Thermo Fisher Scientific).
RNA isolation and real-time PCR
Total RNA from liver and colon mucosa samples was extracted with TRIzol reagent (Invitrogen), and complementary DNA was synthesised using the QuantiTect Reverse Transcription kit (Qiagen). Real-time PCR was performed using the QuantiTect SYBR-Green PCR kit (Qiagen) according to the manufacturer’s instructions. To check for variations in the reactions, all PCR data were normalised against β-actin expression. PCR primers for Tlr4, carbohydrate-responsive element-binding protein (Chrebp), microsomal TAG transfer protein (Mtp), Tnfα, Il6 and C/EBP homologous protein (Chop) are described in the online Supplementary Table S2.
The comparative C
t
method was used to determine the amount of target gene expression, normalised to an endogenous reference gene and relative to a calibrator
![]() $(2^{{{\minus}\Delta \Delta ^{{C_{t} }} }}\!)$
.
$(2^{{{\minus}\Delta \Delta ^{{C_{t} }} }}\!)$
.
Determination of endotoxin levels and intestinal inflammatory status
Portal plasma samples were heated at 75°C for 5 min. Plasma endotoxin levels were determined using a commercially-available end point Limulus Amebocyte Lysate assay (Charles River) with a concentration range of 0·005–0·5 EU/ml.
Myeloperoxidase (MPO) activity, a marker of polymorphonuclear infiltration, was determined in colon mucosa as described by Barone et al. ( Reference Barone, Hillegass and Price 22 ). In brief, colon mucosa was homogenised in 50 mmol/l potassium phosphate buffer at pH 6 containing 0·5 % hexadecyltrimethyl ammonium bromide. The homogenates were centrifuged for 30 min at 12 500 g and 4°C. The supernatants were collected and incubated with 0·167 mg/ml o-dianisidine dihydrochloride (Sigma-Aldrich) and 0·0005 % H2O2 in 50 mmol/l PBS at pH 6. The measurements were made at 460 nm using a microplate reader (MRX; Dynex Technologie) in duplicate; 1 unit of MPO activity was defined as the quantity of enzyme hydrolysing 1 mmol of H2O2/min.
Intestinal tight-junction protein expression
Colon mucosa was homogenised in lysis buffer (50 mm-Hepes, 150 mm-NaCl, 10 mm-EDTA, 10 mm- β-glycerophosphate, 100 mm-NaF, 2 mm-orthovanadate, 1 % Triton X-100, anti-protease and phosphatase inhibitor cocktail). After centrifugation for 30 min at 13 000 g and 4°C, the supernatants were collected. Protein levels were determined using the bicinchoninic acid assay (Protein Assay kit; Interchim). Claudin-1, zonula occludens 1 (ZO-1) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expressions were determined according to the technique previously described( Reference Beutheu Youmba, Belmonte and Galas 23 ) using primary antibodies: rabbit anti-claudin-1 (1:1000; Invitrogen), rat anti-ZO-1 (1:500; Millipore) and rabbit anti-GAPDH (1:2000; Cell Signaling). After three washes with TBS-1 % Tween 20 buffer, the membranes were incubated with peroxidase-conjugated goat anti-rabbit or anti-rat IgG (Dakocytomation) for 1 h at room temperature and revealed using the enhanced chemiluminescence detection system (GE Healthcare). Protein bands were quantified by densitometry using a CCD camera (ImageQuant LAS 4000; GE Healthcare) and ImageQuant TL software (GE Healthcare). Protein expression levels were normalised to GAPDH.
Caecal and mucosa-associated microbiota analysis
For mucosa-associated microbiota analysis, colons were flushed with sterile PBS, and the mucosa was scraped. Caecal microbiota and mucosa-associated total bacteria and main bacterial groups were quantified using real-time PCR. Total DNA was extracted using guanidium isothiocyanate and the mechanical bead beating method as previously described( Reference Kalach, Kapel and Waligora-Dupriet 24 ). Total bacteria populations, Clostridium leptum group, Bacteroides/Prevotella group and Bifidobacterium, were quantified using TaqMan® qPCR. C. coccoides group, enterococci, Lactobacillus/Leuconostoc/Pediococcus group and Akkermansia muciniphila were quantified using SYBR-Green® qPCR. The primers and probes used are described in the online Supplementary Table S3. When a species or bacterial group was not detected, a value of 3 log10 colony-forming units/g of mucosa – that is, corresponding to about half the detection limit – was used for statistical analysis.
Data analysis
Results are expressed as mean values and standard deviations. Statistical analysis was performed using Prism 6.0 (GraphPad® software). Homogeneity of variance of the data was assessed using Bartlett’s test. Differences between groups were analysed using ANOVA followed by Fisher’s protected least significant difference test. In case of heterogeneity of variance, a Kruskall–Wallis test followed by Dunn’s test was performed. For daily food intake and BW, ANOVA for repeated measurement was used, and differences were analysed using Bonferroni’s test. For all the tests, P<0·05 was considered significant.
Results
Effect of diet on food intake, body weight and adiposity (Table 1)
BW did not differ among groups. Although food intake was lower in all the Western diet-fed rats (P<0·05), total energy intake was similar in the four groups. However, there were significant differences in body composition: Western diet resulted in higher visceral fat in WD (P=0·003) and WDA (P=0·002) groups than in controls and in lower lean mass (P<0·05). In rats fed Western diet supplemented with Cit, the gain in visceral fat mass was lower (C v. WDC: NS) and the loss in lean mass tended to be lower compared with the C group. Cutaneous fat mass and N balance were not modified by the Western diet.
Table 1 Body composition and food intake of rats fed a control diet (C; n 10), a Western diet (WD; n 13), a Western diet and non-essential amino acids (WDA; n 10) or a Western diet and citrulline 1 g/kg per d (WDC; n 10) (Mean values and standard deviations)

BW, body weight.
* Mean value was significantly different from that of the control group (P<0·05).
† Body weight, body composition and N balance at 8 weeks.
‡ Mean daily intake
Effect of diet on metabolic parameters
Western diet-fed rats had higher plasma TAG (P<0·05 WD), cholesterol (P<0·01), insulin (P<0·05) and glucose (P<0·001) than the control rats (Table 2). NEAA or Cit supplementations lowered plasma TAG by 39 and 32 %, respectively, and plasma insulin (P<0·05) compared with the WD group. Only the WD group developed IR as shown by the significantly higher HOMA-IR (P<0·05) compared with the control group. Insulin sensitivity was not different from the control group in WDA and WDC groups; it was significantly improved (P<0·05) in the WDA group and tended to improve (P=0·1) in the WDC group compared with the WD group (Table 2). Plasma uric acid did not differ among the groups (Table 2).
Table 2 Effects of citrulline and non-essential amino acid supplementation on metabolic parameters in rats fed a control diet (C; n 10), a Western diet (WD; n 13), a Western diet and non-essential amino acids (WDA; n 10) or a Western diet and citrulline 1 g/kg per d (WDC; n 10) (Mean values and standard deviations)
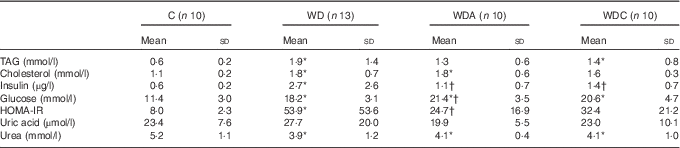
HOMA-IR, homoeostatic model assessment – insulin resistance.
* Mean value was significantly different from that of the control group (P<0·05).
† Mean value was significantly different from that of the WD group (P<0·05).
Western diet intake resulted in decreased plasma urea (P<0·05), and this was not modified by NEAA or Cit (Table 2). In parallel, the plasma AA profile was modified by the Western diet (Table 3) with a significant increase (P<0·05) in plasma glutamine (Gln), threonine, alanine, proline and lysine (Lys), and a significant decrease (P<0·05) in plasma glycine and isoleucine. Supplementation of the Western diet with NEAA or Cit prevented the modifications in threonine and proline levels (P<0·05), whereas only Cit prevented the modifications in Gln levels. Arg plasma concentration was 14 % higher in the WDC group than in the WD group. In parallel, the ratio of Arg:(ornithine (Orn)+Cit), which can be considered as a marker of systemic Arg bioavailability( Reference Sailer, Dahlhoff and Giesbertz 25 ), was higher with Cit than in the WD group, but this did not reach significance (Fig. 1(a)). Moreover, we observed a significant increase (P<0·05) in plasma Lys in all the Western diet-fed groups, and the Arg:Lys ratio, which is an important factor for Arg entry into cells( Reference Wu, Bazer and Davis 26 ), was decreased in WD (P=0·0005) and WDA (P=0·002) groups (Fig. 1(b)). Only in the Cit-supplemented group this ratio was not different compared with controls (P=0·2) and was significantly increased compared with WDA (P=0·01). Finally, we observed an increase in glutathione turnover, characterised by the ratio of glutamate:(serine+glycine)( Reference Vanni, Rosso and Mezzabotta 27 ) (Table 3) in all the Western diet-fed groups (P<0·05).

Fig. 1 Effect of amino acids on arginine homoeostasis in rats fed a control diet (C; n 10), a Western diet (WD; n 13), a Western diet and non-essential amino acids (WDA; n 10) or a Western diet and citrulline 1 g/kg per d (WDC; n 10). (a) Plasma Arg:(Orn+Cit) ratio, a marker of Arg metabolic utilisation. (b) Plasma Arg:Lys ratio, a marker of Arg bioavailability. Values are means, with standard deviations represented by vertical bars. Mean values were significantly different (P<0·05): * v. C, † v. WD, or ‡ v. WDA.
Table 3 Effects of citrulline and non-essential amino acid supplementation on plasma amino acids in rats fed a control diet (C; n 10), a Western diet (WD; n 13), a Western diet and non-essential amino acids (WDA; n 10) or a Western diet and citrulline 1 g/kg per d (WDC; n 10) (Mean values and standard deviations)

* Mean value was significantly different from that of the control group (P<0·05).
† Mean value was significantly different from that of the WD group (P<0·05).
Effects of non-essential amino acids and citrulline on Western diet-induced hepatic steatosis
Chronic consumption of Western diet resulted in a significant increase (P<0·05) in liver weight (Fig. 2(a)), and this was associated with a significant increase in hepatic TAG content (Fig. 2(b)) in the WD (P=0·004) and WDA (P=0·02) groups. Hepatic TAG content did not differ in the WDC group from the C group, and liver weight was significantly lower (P<0·05) in the WDC group compared with the WD group (Fig. 2(a)). In parallel, plasma bilirubin, ALP, ALT and AST levels were not modified by the different diets (Table 4).

Fig. 2 Effect of amino acids on hepatic lipid accumulation in rats fed a control diet (C; n 10), a Western diet (WD; n 13), a Western diet and non-essential amino acids (WDA; n 10) or a Western diet and citrulline 1 g/kg per d (WDC; n 10). (a) Liver weight. (b) Hepatic TAG content. (c) Representative photomicrographs of liver sections stained with Oil Red O showing microvesicular and macrovesicular lipid droplets. ![]() , Micro- and macro-vesicular lipid droplets. (d) Expression of genes involved in hepatic lipid accumulation. Values are means, with standard deviations represented by vertical bars. Mean values were significantly different (P<0·05): * v. C, † v. WD or ‡ v. WDA.
, Micro- and macro-vesicular lipid droplets. (d) Expression of genes involved in hepatic lipid accumulation. Values are means, with standard deviations represented by vertical bars. Mean values were significantly different (P<0·05): * v. C, † v. WD or ‡ v. WDA.
Table 4 Effects of citrulline and non-essential amino acid supplementation on the gut–liver axis in rats fed a control diet (C; n 10), a Western diet (WD; n 13), a Western diet and non-essential amino acids (WDA; n 10) or a Western diet and citrulline 1 g/kg per d (WDC; n 10) (Mean values and standard deviations)

MPO, myeloperoxydase; ZO1, zonula occludens 1; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase.
* Mean value was significantly different from that of the control group (P<0·05).
† Mean value was significantly different from that of the WD group (P<0·05).
‡ Mean value was significantly different from that of the WDA group (P<0·05).
§ Normalised to glyceraldehyde 3-phosphate dehydrogenase.
On liver histological examination, WD and WDA rats presented macrovesicular and microvesicular lipid droplets, whereas in Cit-supplemented animals hepatic lipids were limited to microvesicular lipid droplets (Fig. 2(c)).
Hepatic steatosis in Western diet-fed rats was associated with an increased expression of Chrebp (P<0·05) and a trend towards increased expression of Mtp (Fig. 2(d)). Only Cit administration abolished WD-induced increase in Chrebp expression.
Western diet feeding was associated with liver inflammation, as suggested by the induction of Tlr4 expression in the WD group (P<0·05) and Il6 expression in the WD and WDA groups (P<0·05), as well as the slight increase in Tnfα (Fig. 2(d)). Only Cit administration significantly decreased (P<0·05) Tlr4 and Il6 mRNA expressions.
The Western diet significantly decreased (P<0·05) the GSSG:GSH ratio, a marker of oxidative stress, without any effect of NEAA, whereas the ratio tended to increase (P=0·09 v. WD) with Cit supplementation (Table 4).
The Western diet also led to ER stress, as shown by the significant increase (P<0·05) in Chop expression, and it was prevented by NEAA and Cit supplementation (Fig. 3(c)).

Fig. 3 Effect of amino acids on microbiota in rats fed a control diet (C; n 10), a Western diet (WD; n 13), a Western diet and non-essential amino acids (WDA; n 10) or a Western diet and citrulline 1 g/kg per d (WDC; n 10). Quantification of all bacteria populations (a), Bacteroides/Prevotella group (b), Clostridium leptum group (c), Cluster XI (d), Coccoides group (e), enterococcus (f), Lactobacillus/Leuconostoc group (g) and Akkermansia muciniphila (h) in the colon mucosa in all groups. CFU, colony-forming units. ![]() , Rat;
, Rat; ![]() , median. * Mean values were significantly different compared to that in C group (P<0·05).
, median. * Mean values were significantly different compared to that in C group (P<0·05).
Effect of diet on gut microbiota
We did not observe any modification in total caecal microbiota or Bifidobacteria count. Compared with the control diet, the main effect of the Western diet on caecal microbiota was a marked increase in A. muciniphila (online Supplementary Fig. S1). NEAA and Cit did not modify Western diet-induced changes in caecal microbiota.
In contrast, the Western diet induced dramatic changes in the colon mucosa-associated microbiota, with a significant decrease (P<0·05) in total bacteria, C. leptum, Bacteroides/Prevotella and Lactobacillus/Leuconostoc (Fig. 3(a)–(h)). Colonisation with A. muciniphila was lower in the WD group than in the controls (Fig. 3(h)), but this did not reach significance. Although NEAA or Cit failed to significantly counterbalance the effect of the Western diet on total bacteria and C. leptum, only Cit restored Bacteroides/Prevotella colonisation to control levels, but only at the mucosal level.
Effect of diet on gut function (Table 4)
At gut level, the Western diet did not increase plasma endotoxin levels.
MPO activity slightly increased in colon mucosa in Western diet-fed rats compared with the controls. It was significantly lower in the WDC group compared with the WDA group (P=0·03).
Expression of the tight-junction protein claudin-1 in the colon tended to be decreased by the Western diet (P=0·06), and this was associated with increased Tnfα and Tlr4 expressions (P<0·05) (Fig. 4(a)). NEAA and Cit administration significantly decreased Tlr4 expression (P<0·05), whereas Cit decreased Tnfα expression (P<0·05), and tended to increase claudin-1 protein expression (ANOVA P=0·1, WD v. WDC P=0·02). Cytoplasmic protein expression of ZO-1 was preserved among groups.

Fig. 4 Effect of amino acids on gut function in rats fed a control diet (C; n 10), a Western diet (WD; n 13), a Western diet and non-essential amino acids (WDA; n 10) or a Western diet and citrulline 1 g/kg per d (WDC; n 10). Analysis of the mRNA expressions of genes involved in colon mucosa inflammation. (a) Tlr4; (b) Tnfα. Values are means, with standard deviations represented by vertical bars. Mean values were significantly different (P<0·05): * v. C or † v. WD. Tlr4, Toll-like receptor 4.
Discussion
The progression of non-alcoholic fatty liver (NAFL) to its severe necro-inflammatory stage – that is, NASH – may be prevented through the management of lipid metabolism and inflammatory processes. The benefits of AA supply on the development of NAFL have been repeatedly demonstrated, and we have previously shown a preventive effect of Cit on fructose-induced NAFL. In the present study, we evaluated whether Cit may also be effective during Western diet feeding, which is known to affect gut function and NAFLD progression. Our present results showed that in a situation of chronic Western diet feeding, Cit supplementation presents some specific beneficial effects on several factors of disease progression and this could probably decrease the severity of steatosis.
First, as expected, the Western diet induced hepatic steatosis, as shown by increased liver weight and TAG content, via increased Chrebp expression and the induction of de novo lipogenesis. Liver histological examination showed macrovesicular and microvesicular lipid droplet accumulation in the WD group, suggesting progression of the disease( Reference Ibrahim, Singh and Ganie 28 ). Only in the Cit-supplemented group, a lower TAG content was observed with lipid accumulation limited to microvesicular lipid droplets, and this was probably related to the lower expression of Chrebp, a key regulator of de novo lipogenesis. As a consequence, Cit significantly decreased liver weight compared with the WD group. The effect of Cit on lipid metabolism may be the result of decreased de novo lipogenesis, but also to a better VLDL-TAG release given the improved peripheral TAG oxidation previously demonstrated( Reference Moussaoui, Binimbi and Cottart 29 ).
Chronic Western diet consumption in our experiments did not induce weight gain. Owing to the higher energy density of their diet and to fructose in their drinking water, the Western diet-fed rats had a energy intake similar to control rats, despite significantly decreased food intake, probably related to the satiety effect of fructose( Reference Moran 30 ). In the literature, WD-induced increase in BW is only observed with a higher energy intake( Reference Tsuchiya, Ebata and Sakabe 31 , Reference Zelber-Sagi, Nitzan-Kaluski and Goldsmith 32 ).
NAFLD is commonly associated with higher adiposity. In this study, the Western diet increased visceral fat mass, and this was dampened by Cit supplementation in keeping with several studies from our group( Reference Jegatheesan, Beutheu and Ventura 18 , Reference Moinard, Le Plenier and Noirez 33 ). The effect of Cit may occur through enhanced visceral adipose tissue lipolysis( Reference Joffin, Jaubert and Durant 34 , Reference Joffin, Jaubert and Durant 35 ), fat oxidation( Reference Joffin, Jaubert and Durant 34 ) and uncoupling of the respiratory chain, resulting in energy expenditure that favours fat mass reduction( Reference Joffin, Jaubert and Bamba 36 ). It will be of interest to evaluate whether Cit may modify the fatty acid profile in adipose tissue, and thereby regulate fat cell size. Indeed, Garaulet et al. ( Reference Garaulet, Hernandez-Morante and Lujan 37 ) showed that fatty acid composition of adipose tissue is correlated to adipocyte size and number in overweight/obese humans. An additional mechanism may also be involved. Indeed Vasanthakumar et al. ( Reference Vasanthakumar, Moro and Xin 38 ) showed that an increase in visceral fat mass may also be associated with a reduction in regulatory T cells, which prevents obesity-associated inflammation and preserves insulin sensitivity and glucose tolerance. As Cit is able to limit the polarisation of macrophages to the M1 phenotype in the peritoneal tissues( Reference Breuillard, Bonhomme and Couderc 39 ), the influence of Cit on regulatory T cells deserves to be studied.
Western diet-induced visceral adiposity was associated with hepatic and peripheral IR as shown by the significant increase in HOMA-IR, in agreement with literature data( Reference Gaggini, Morelli and Buzzigoli 40 ). Indeed, dysfunctional adipose tissue, especially the more metabolically active visceral fat tissue, plays a central role in the pathogenesis of NASH and IR through the release of cytokines and fatty acids( Reference Gaggini, Morelli and Buzzigoli 40 , Reference De Moura, Ribeiro and de Oliveira 41 ). In our experiments, NEAA and Cit corrected the higher plasma insulin level and improved insulin sensitivity. In addition to its action on body fat distribution, Cit may act on IR via its effects on Arg availability and nitric oxide synthesis, which plays an important role in the regulation of energy metabolism( Reference Cynober, Moinard and De Bandt 42 ). Although we have not investigated nitric oxide production, Cit did prevent Western diet-induced alterations in Arg metabolism, as shown by the increase in plasma Arg:Lys and Arg:(Orn+Cit) ratios( Reference Sailer, Dahlhoff and Giesbertz 25 , Reference Wu, Bazer and Davis 26 ). Interestingly, Cit-supplemented rats did not present a higher plasma concentration of this AA compared with non-supplemented rats, suggesting its rapid utilisation in a situation of metabolic disorder.
Western diet feeding was also associated with a decrease in lean body mass. Although the mechanisms remain to be investigated, hepatic fat accumulation may alter the liver–muscle axis – for example, through the production of lipids( Reference Stephens, Chee and Wall 43 ), methylglyoxal( Reference Dhar, Dhar and Wu 44 ), hepatokines such as Fetuin A( Reference Srinivas, Wagner and Reddy 45 ) and uric acid( Reference Fabbrini, Serafini and Colic Baric 46 ) by the liver. All these metabolites and lipid infiltration in myocytes decrease insulin sensitivity in muscle, and may contribute to muscle anabolic resistance and loss in lean body mass( Reference Stephens, Chee and Wall 43 ). The preventive effect of Cit on hepatic lipid accumulation probably occurs through its beneficial effect on visceral fat mass, but also at least in part through an activation of muscle protein synthesis( Reference Osowska, Duchemann and Walrand 47 ), taking into account the role of muscle mass on metabolic homoeostasis. Of note, the Western diet-induced loss in lean body mass in our experiments was associated with modifications in the plasma levels of several AA, suggesting disturbances in AA inter-organ exchanges. The decrease in plasma urea in the absence of modifications in N balance suggests preserved urinary N elimination and increased renal ammonia excretion. This might be related to a lower gluconeogenic flux from AA, elevated plasma glucose resulting from gluconeogenic fructose utilisation and probably explains the increase in the plasma levels of some gluconeogenic AA. These data are in contrast with our previous observation in fructose-induced NAFLD, where Cit corrected fructose-induced perturbations in plasma AA. This is probably related to the high fat content of the diet and requires to be further investigated.
Interestingly, plasma Gln was significantly increased in the WD group and normalised by Cit supply. The increase in Gln may be related to (i) its decreased utilisation in liver ureagenesis and/or (ii) its lower utilisation by the gut. Although IR is associated with increased plasma Cit, plasma Cit was not altered by the Western diet in our experiments. As plasma Cit depends on its intestinal production in part from Gln, this may be related to the deterioration in intestinal function discussed below. Only Cit normalised plasma Gln levels; this may due to its protective effects on the gut leading to improved Gln intestinal utilisation.
Liver inflammation is a hallmark of NASH, and is associated with increased production of pro-inflammatory cytokines such as TNFα or IL6. Increased plasma cytokine levels may originate from the liver and the gut but also from dysfunctional adipose tissue as mentioned above or other immune organs such as the spleen( Reference Tarantino, Savastano and Capone 48 ). The exact role of IL6 remains to be investigated, as some studies have clearly demonstrated that IL6 plays a key role in determining IR and other NAFLD-related diseases( Reference Tarantino, Savastano and Capone 48 ), whereas other studies have shown an hepatoprotective effect of IL6( Reference Gao 49 ). Focusing on the liver, hepatic lipid overload increases macrophage recruitment leading to a higher production of cytokines such as TNFα and IL6. Our present study shows that the Western diet increased hepatic Tlr4 and Il6 mRNA expressions, suggesting liver inflammation, and this is prevented by Cit supplementation. Increase in Tlr4 expression may also reflect an increase in macrophage infiltration in the liver( Reference Bian, Peng and You 50 ). The Western diet could induce a hepatic macrophage switch to an M1 phenotype, and Cit could promote an M2 phenotype as shown by the lower IL6 mRNA expression. Further studies are needed to investigate this point. Interestingly, the Western diet was associated with higher hepatic expression of the pro-apoptotic Chop gene, suggesting endoplasmic reticulum stress, which was significantly reduced by NEAA and Cit. Whatever the mechanism involved, our data showed that Cit prevented Western diet-induced hepatic inflammation. In addition, oxidative stress could be both a cause and a consequence of the progression of NAFLD to NASH. It can mediate liver injury through at least two major pathways: direct cell injury and cell signalling. Hepatic lipid accumulation and IR can produce endoplasmic reticulum stress, leading to activation of NF-κB, which plays a critical role in the production of pro-inflammatory cytokines. This study showed that the Western diet increased glutathione turnover, suggesting an induction of antioxidant defences, and decreased hepatic GSSG:GSH ratio, a marker of oxidative stress. Cit tended to normalise GSSG:GSH ratio, whereas NEAA were ineffective.
Alterations in gut permeability and microbiota play a critical role in disease progression( Reference Ritze, Böhle and Haub 51 , Reference Spruss and Bergheim 52 ). The Western diet has been shown to be associated with reduced bacterial colonisation, but increased numbers of mucin-degrading bacteria of the Bacteroidetes and Lachnospiraceae family/Firmicutes phyla( Reference Hold 53 ). In our study, an increase in mucin-degrading bacteria was observed in the caecal contents of the Western diet-fed rats without modification in total bacteria, whereas we observed reduced mucosal colonisation. An increase in the Firmicutes:Bacteroidetes ratio has been repeatedly demonstrated to develop with excess weight( Reference De Bandt, Waligora-Dupriet and Butel 54 ) and in NAFLD as a result of decreased Bacteroidetes( Reference Mouzaki, Comelli and Arendt 55 ) or an increase in some members of Firmicutes( Reference Raman, Ahmed and Gillevet 56 ). In the mucosa, in our study, despite decreased Lactobacillus/Leuconostoc and C. leptum groups, some other members of Firmicutes (i.e. other Clostridium clusters such as C. coccoides and enterococci) remained at high levels. On the other hand, the Bacteroides/Prevotella group was significantly decreased in the WD group compared with controls. This bacterial group, a major component of the Bacteroidetes phylum, is recognised for its immunomodulatory properties and its role in gut homoeostasis( Reference Mazmanian, Liu and Tzianabos 57 ). Finally, A. muciniphila level was not affected by the Western diet. As A. muciniphila has been shown to be able to restore the gut mucus layer, and its abundance is inversely correlated with weight gain( Reference Everard, Belzer and Geurts 58 , Reference Zhang, Shen and Fang 59 ), the fact that all our rats exhibited normal weight probably explains this latter observation. In our study, this dysbiosis was associated with inflammation as shown by the higher MPO activity and expression of TNFα and TLR4 in colon mucosa. We also noted a lower expression of the tight-junction protein claudin-1 in the colon of Western-diet fed rats. The increased caecal content and the mucosal preservation of A. muciniphila probably help in explaining why we did not observe any Western diet-induced endotoxaemia.
Although Cit had little impact on total bacteria and C. leptum, it had a positive effect on the Bacteroides/Prevotella count, which was restored to a level similar to the control. Colonisation of the gut by damaging bacteria could thus be limited by Cit in favour of protective bacteria, leading to a lesser colon mucosa inflammation as observed in our Cit-supplemented rats. The resulting prevention of intestinal inflammation could in turn protect gut barrier function. Several mechanisms, either direct or indirect, may contribute to these effects of Cit on microbiota. First, as already demonstrated for Arg, a closely related AA that affects gut microbiota, we cannot rule out a direct effect of oral Cit on the large intestine. Indeed, as the absorption rate of these AA is relatively slow a small part may arrive in the large intestine and be metabolised by the microbiota. In this respect, Bacteroides/Prevotella are known to synthesise Arg from Cit( Reference Morizono, Cabrera-Luque and Shi 60 , Reference Xu, Labedan and Glansdorff 61 ) and Arg, at least in part as a precursor for polyamine synthesis, may protect intestinal mucosa and also play a role in cell growth( Reference Noack, Kleessen and Proll 62 ). Second, systemic effects of Cit may be involved. As already mentioned, Cit displays trophic and anti-inflammatory properties on the gut and this is further supported by our study. Through these effects, Cit may thus affect the interaction between gut barrier (immune cell modulation, secretory antimicrobial peptide production) and the microbiota. In addition, the improvement of peripheral insulin sensitivity by Cit may also affect gut barrier/microbiota interactions; indeed insulin has been shown to play an important role, for example, in the production of mucin and the integrity of gut mucous layer. As a result, whatever the underlying mechanisms, Cit supplementation was associated with decreased gut inflammation as indicated by lower MPO activity and pro-inflammatory gene expression.
Finally, in terms of specificity of the effects of Cit, some of our data show that our NEAA supplement, isonitrogenous to Cit, may in part prevent some of the metabolic effects of the Western diet. Although this suggests a possible effect of extra N supply, the effect of the NEAA mixture may be debated. Indeed, although in our study, only low dose of each AA was used, a specific effect of one of the six AA cannot be ruled out. For example, one study showed that histidine significantly decreases gene expression and activity of transcription factors SREBP and CHREBP and lipogenic enzymes Fas and HMG-CoA reductase, leading to decreased hepatic TAG content and cholesterol and improved insulin sensitivity( Reference Mong, Chao and Yin 63 ). In the same way, Glycine may prevent lipid accumulation in the liver and bloodstream( Reference Senthilkumar, Viswanathan and Nalini 64 ), preserve muscle mass, stimulate loss of adipose tissue and also exert anti-inflammatory effects( Reference Caldow, Ham and Godeassi 65 ). Further studies are needed to clarify this point.
In conclusion, beneficial effects of both NEAA and Cit on some aspects of lipid metabolism confirm that AA supply by itself may be protective on NAFLD. In addition, besides a possible N supply-related effect, Cit seems to play an important specific role. Cit showed a direct effect on several factors of disease progression at the liver level such as lipid accumulation, inflammation, oxidative stress and ER stress. Cit supply is also associated with specific effects at the peripheral level on visceral adipose tissue and gut function. Cit supplementation may thus be an interesting nutritional strategy in the prevention of NAFLD progression to NASH.
Acknowledgements
The authors thank Servane Le Plenier, Radji Ramassamy and Christelle Vicente for expert technical assistance, and acknowledge the imaging platform and animal facility of Paris Descartes University.
This project was supported by an Institut Appert grant and the French National Research Agency (ANR) grant (ANR-11-BSV1-0015, NAFLD-citrulline).
Study concept and design: J.-P. D. B., S. B. and I. B. Acquisition of data, analysis and interpretation of the data: P. J., K. F., E. N., A.-J. W.-D., M.-J. B. Drafting of the manuscript: P. J. and J.-P. D. B. Critical revision of the manuscript for important intellectual content: all authors.
Professor J.-P. D. B. is a shareholder of Citrage Company.
Supplementary Material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114516001793











