In the past decades, respiratory allergic disorders increased dramatically, being the most common chronic diseases of childhood in developed countries. Hereditary predisposition and other factors related to the current lifestyle are responsible for different types of allergy.
The incidence of allergy has also been related to nutrition changes, the indiscriminate use of antibiotics, hygienic measures( Reference Becker 1 ), and the number of births by caesarean section; all these points affect children during the first years of life, making them have less contact with a wide variety of micro-organisms (hygiene hypothesis( Reference Brown, Arrieta and Finlay 2 )) and inducing a complex process characterised by an excessive activation of T helper (Th2) cells against environmental antigens.
It has been established that the colonisation process of the intestinal microbiota early in life is essential to the development of the immune system( Reference Romagnani 3 ). Several studies have shown that the composition of the gut microbiota is different between healthy and allergic children( Reference Bjorksten 4 , Reference Penders, Stobberingh and van den Brandt 5 ). This observation opens an important key in the study of intestinal microbiota and the ability to balance the populations of micro-organisms that exert beneficial features to the host; in this sense, probiotic bacteria are suitable candidates for improving the microbiota, especially when administered in foods without altering the normal feeding behaviour.
Probiotics are defined as live micro-organisms that when administered in adequate amounts confer a health benefit on the host( 6 ). The beneficial properties of probiotics have been reported under different conditions, such as protective effect against intestinal infections( Reference de LeBlanc Ade, Castillo and Perdigon 7 ), decrease in serum cholesterol( Reference Guo and Zhang 8 , Reference Pan, Zeng and Yan 9 ), modulating the immune system( Reference Galdeano and Perdigon 10 ) and reduction in the risk for the development of allergy( Reference Ouwehand 11 ). Lactic acid bacteria, especially lactobacilli and bifidobacteria, are frequently used as probiotics, and studies conducted with these micro-organisms have shown that they are able to suppress allergic reactions in different animal models( Reference Ishida, Bandou and Kanzato 12 , Reference Ohno, Tsunemine and Isa 13 ) as well as in human subjects( Reference Morita, He and Kawase 14 ). There have been many reports indicating that oral administration of probiotics has an effect on the reduction in IgE level in the serum of experimental allergic animals. In this sense, administration of Lactobacillus GG during the prenatal period reduced allergy symptoms in mouse models of allergic airway inflammation( Reference Blumer, Sel and Virna 15 ).
The aim of the present study was to determine whether the oral administration of a probiotic fermented milk (PFM) regulates the immune response to IgE against ovoalbumin (OVA) in a mouse model of airway allergy (airway hypersensitivity), and to study the possible mechanisms involved in this effect by analysing regulatory T (Treg) cells and the modulation of specific IgG and total secretory IgA (S-IgA) antibody responses.
Experimental methods
Experimental animals and probiotic fermented milk administration
Male BALB/c mice (25 ± 2 g), aged 5 weeks old, were obtained from a closed-bred randomised colony, maintained at CERELA (Centro de Referencia para Lactobacilos, San Miguel de Tucumán, Argentina). Mice were kept in separate cages with a controlled atmosphere (22 ± 2°C and 55 ± 2 % relative humidity) and a 12 h light–12 h dark cycle. They were fed with a conventional balanced diet (23 % proteins, 6 % raw fibre, 10 % total minerals, 1·3 % Ca, 0·8 % P, 12 % moisture and vitamins) and water ad libitum throughout the experiment.
PFM used in the present study contained the yogurt starters Lactobacillus bulgaricus (108colony-forming units (CFU)/ml), Streptococcus thermophilus (108CFU/ml) and the probiotic Lactobacillus paracasei ssp. paracasei CNCMI-1518 (108CFU/ml) (former L. casei DN114001). Mice were fed ad libitum with PFM. Consumption of PFM was controlled (3 ml/mouse). Mice received PFM 5 d before the sensitisation procedure, after which they were divided into two groups: mice that continued to receive the treatment until the end of the study (continuous treatment) and mice that received PFM only 5 d before OVA challenge (previous treatment). Control mice (without PFM treatment) were sensitised with OVA.
All animal protocols and trials were carried out in accordance with the current laws in Argentina, and were approved by the Ethical Committee of CERELA (protocol no. CRL-BIOT-LI-2011/1a).
Sensitisation model
In the present study, we selected an OVA sensitisation model because it is an appropriate antigen for studying antibody responses and allows comparison between specific IgE and IgG responses. Therefore, it is one of the most utilised antigens suitable for designing allergy models( Reference Kim, Lee and Kim 16 , Reference Wu, Fu and Yang 17 ).
The antigen was administered by three subcutaneous injections (0·05 ml, 0·1 %) diluted in PBS solution, separated by 1 d. Thereafter, mice were exposed to OVA aerosols (1 % in PBS) for 20 min for 7 consecutive days.
Mice were divided into five experimental groups: (1) normal control (NC, standard diet+water); (2) basal control (BC, standard diet+5 dPFM); (3) sensitisation control (SC, standard diet+OVA); (4) previous treatment (PTREAT, 5 dPFM+OVA+water); (5) continuous treatment (CTREAT, 5 dPFM+OVA+PFM). Samples of serum, broncho-alveolar lavage fluid (BALF), lung tissue and macerated lungs were collected at 7 and 15 d after the last exposure to OVA (days post-sensitisation; dPS). After the last sample collection, mice from each experimental group were restimulated with the antigen as follows: at 15 dPS, mice received one injection of OVA (0·05 ml, 0·1 %), and then they were again exposed to OVA aerosols for 3 consecutive days and received one injection of OVA. The samples were collected 2 d post-restimulus (dPR). The body weight of mice was controlled weekly during the experiment. No differences were found in body weight between the treatment groups (data not shown).
BALB/c mice are a suitable model for sensitisation with OVA, with responses similar to those found for human subjects. This animal model was chosen, as it does not cause severe respiratory outcomes.
Determination of anti-ovoalbumin IgE, IgG, IgG1 and IgG2a, IL-4, IL-10 and interferon-γ levels in serum by ELISA
Before the collection of blood samples, mice were anaesthetised using a ketamine–xylazine mixture. Mice were bled at 7 and 15 dPS, and the blood sample was centrifuged at 800 rpm for 10 min. Serum was collected and stored at − 18°C until analysis. The levels of anti-OVA (a-OVA)-specific IgE and the cytokines IL-10, IL-4 and IFN-γ were measured in serum using commercially available ELISA kits (BD OptEIA™), according to the manufacturer's instructions. Specific IgG, IgG1 and IgG2a antibody responses were determined by ELISA, according to the method described by Maldonado Galdeano et al. ( Reference Maldonado Galdeano, Novotny Nunez and de Moreno de LeBlanc 18 ) and Perdigón et al. ( Reference Perdigón, Maldonado Galdeano and Valdez 19 ), respectively. For the detection of specific IgG, biotin SP-conjugated goat anti-mouse IgG (Jackson Immunoresearch Labs, Inc.) was used. For the detection of specific IgG1 or IgG2a, a rabbit anti-mouse antibody was used, respectively. The reaction was revealed with streptavidin–HRP (BD OptEIA™) and 3,3′,5,5′-tetramethylbenzidine reagent (BD Biosciences). For all cases, absorbance was read at 450 nm. Cytokine levels are expressed as concentration (pg/ml), and the levels of specific antibodies are expressed as optical density.
Determination of anti-ovoalbumin IgE and IgG, IL-4, IL-10, interferon-γ and total secretory IgA levels in the broncho-alveolar lavage fluid and in the supernatants of macerated lungs by ELISA
Mice were killed by cervical dislocation at 7 and 15 dPS. Within the same experimental period, the lungs were washed with 1 ml PBS (0·01 m) by an intra-tracheal cannula, and then the BALF was collected and stored at − 18°C. Subsequently, one lobe of each lung used to collect the BALF was kept for immunofluorescence assays. The second lobe was used to prepare macerated lungs. Briefly, tissue was collected into 1·5 ml of sterile PBS (0·01 m) and disrupted using a homogeniser (MSE). The disrupted organs were centrifuged for 10 min at 1000 rpm (4°C). Lung supernatants (LS) were collected and maintained at − 18°C until analysis. To measure the levels of specific IgE, IL-4, IL-10 and IFN-γ from the BALF or LS, commercial ELISA kits (BD OptEIA™) were used according to the manufacturer's instructions. The level of specific IgG was also measured as described previously( Reference Maldonado Galdeano, Novotny Nunez and de Moreno de LeBlanc 18 ). Total S-IgA level was measured according to the method described by de Moreno de LeBlanc et al. ( Reference de Moreno de LeBlanc, Dogi and Galdeano 20 ), using goat anti-mouse IgA affinity-purified antibody (Bethyl Laboratories, Inc.). Kappa IgA-purified Ig (Sigma) was used as the standard. Detection was performed with anti-mouse IgA (α-chain-specific) peroxidase conjugated developed in goat (Sigma). All reactions were revealed and stopped as described previously. Absorbance was read at 450 nm. Results for total S-IgA and cytokines are expressed as concentration (μg/ml and pg/ml, respectively). Specific IgE and IgG levels are expressed as optical density at 450 nm.
Determination of IgA+, CD4+, CD8+ and F4/80+ cell populations in the lungs by direct immunofluorescence assay
The number of IgA+ cells was determined by direct immunofluorescence assay. After deparaffinisation of histological sections using xylene and rehydration in a decreasing gradient of ethanol, paraffin sections (4 μm) were incubated with a dilution of α-chain monospecific antibody conjugated with fluorescein isothiocyanate (FITC) (Sigma) for 30 min at 37°C. To study the population of CD4+/CD8+ cells, slides were incubated with FITC rat anti-mouse CD4 or CD8 (BD Pharmingen). Macrophages were determined using the FITC anti-mouse F4/80 antigen (eBioscience, Inc.). The cells were observed under a fluorescent light microscope at a magnification of 1000 × . The number of fluorescent cells was counted in thirty fields of vision. Results are expressed as the number of fluorescent cells per ten fields of vision.
Determination of cytokine-positive cells in the lungs by indirect immunofluorescence assay
Cytokine-positive cells were determined using indirect immunofluorescence assay. Paraffin sections were incubated with a blocking solution (1 % bovine serum albumin; Sigma) for 30 min at room temperature. Slices were washed with PBS and incubated with normal goat serum (1:100) for 30 min at room temperature. Anti-murine IL-4, IL-10 and IFN-γ (PeproTech, Inc.) were applied to tissue samples and incubated for 60 min at room temperature. Finally, the slices were washed two times with PBS and incubated with a goat anti-rabbit antibody conjugated with FITC (1:100; Jackson Immunoresearch Labs, Inc.) for 45 min at room temperature, and washed twice in PBS.
The number of fluorescent cells was counted in thirty fields of vision at a magnification of 1000 × . Results are expressed as the number of fluorescent cells per ten fields of vision.
Determination of anti-ovoalbumin-specific IgE and IgG levels after 2 d post-restimulus
Samples of blood and BALF were collected at 2 dPR to measure the levels of a-OVA-specific IgE and IgG according to the techniques described previously.
Histological staining with haematoxylin–eosin
After deparaffinisation, histological sections from lung tissue were stained with haematoxylin–eosin for the analysis of cell infiltration in the lungs, focusing on granulocytes and inflammatory responses. A score was assigned according to Enander et al. ( Reference Enander, Ahlstedt and Nygren 21 ): 0 = no cells around the blood vessels; 1 = three cell layers around the blood vessels; 2 = between four and ten cell layers around the blood vessels; 3 = more than ten cell layers around the blood vessels. The study was performed qualitatively using light microscopy at a magnification of 1000 × . Images are representative of each group.
Determination of inducible CD4+CD25+Foxp3+ regulatory T cells in the lungs by flow cytometry
After sample collection, a portion of each lung was separated to determine the presence of Treg cells. A free cell suspension (1 × 106 cells) was prepared mechanically and filtered using a 35 μm nylon cell strainer (BD Falcon™) after lysing erythrocytes. Cells (1 × 106 cells/ml) were incubated using a FITC rat anti-mouse CD4 and allophycocyanin (APC) rat anti-mouse CD25 (BD Pharmingen™). Then, the cells were prepared with stain buffer (2 % PBS–fetal bovine serum) and mouse Foxp3 buffer set (BD Pharmingen™), according to the manufacturer's instructions, and incubated with PE rat anti-mouse Foxp3 antibody (BD Pharmingen™). The cells were detected using a BD FACSCalibur® (BD Biosciences). Data were analysed using FCS Express 4 Flow Research Edition software (De Novo Software), and are expressed as a percentage of CD4+/CD25+/Foxp3+ cells.
Statistical analysis
Each group and time assayed contained three mice. The sensitisation procedure with or without PFM treatment was carried out three times. Results are presented as means of three independent trials from nine mice with their standard errors of the mean. Statistical analysis was performed using MINITAB 15 software (Minitab, Inc.) by the ANOVA general linear model followed by Tukey's post hoc test. P≤ 0·05 was considered as significant.
Results
Influence of probiotic fermented milk on anti-ovoalbumin-specific IgE, IgG and cytokine levels in serum
The results for specific IgE and IgG antibody responses at 7 and 15 dPS showed that PFM was able to reduce the levels of a-OVA-specific IgE. At 7 dPS, mice that received PFM (PTREAT and CTREAT) had a significant decrease in the level of specific IgE compared with the SC group (P≤ 0·05). At 15 dPS, PFM also reduced the level of a-OVA-specific IgE only in mice that received the treatment until the end of the experiment (CTREAT) compared with the SC and PTREAT groups (Fig. 1(A)).

Fig. 1 Levels of (A) IgE, (B) IgG, (C) IgG1 (T helper (Th)2) and (D) IgG2a (Th1) in serum. Antibody responses were determined by ELISA in the serum of treated and non-treated mice (normal control (![]() ), basal control (
), basal control (![]() ), sensitisation control (
), sensitisation control (![]() ), previous treatment (PTREAT;
), previous treatment (PTREAT; ![]() ) and continuous treatment (CTREAT;
) and continuous treatment (CTREAT; ![]() )) at 5 d with probiotic fermented milk (5 dPFM) and at 7 and 15 d post-sensitisation (dPS). Results are expressed as the absorbance level (optical density (OD) at 450 nm). Values are means (n 9 mice), with their standard errors represented by vertical bars. a,b,c,dMean values with unlike letters were significantly different (P≤ 0·05).
)) at 5 d with probiotic fermented milk (5 dPFM) and at 7 and 15 d post-sensitisation (dPS). Results are expressed as the absorbance level (optical density (OD) at 450 nm). Values are means (n 9 mice), with their standard errors represented by vertical bars. a,b,c,dMean values with unlike letters were significantly different (P≤ 0·05).
For a-OVA IgG, we found that all the sensitised groups exhibited an increase in the level of this antibody, being more significant in mice that received the CTREAT compared with the other groups (NC, BC, PTREAT and SC) at 7 and 15 dps (P≤ 0·05; Fig. 1(B)).
The analysis of the subclasses of IgG antibody showed that the level of IgG2a was predominant in the PTREAT and CTREAT groups compared with the IgG1 level. In particular, the IgG1 level was significantly reduced in the CTREAT group compared with the SC group at 7 and 15 dPS. In the treated groups, the IgG2a level significantly increased at 7 dPS (P≤ 0·05; Fig. 1(C) and (D)).
Concerning IL-10, high levels were observed only at 7 dPS in the serum of treated mice, being remarkable for those in the PTREAT group in comparison with the SC group (P≤ 0·05). At 15 dPS, the level of this cytokine decreased in the sensitised and treated groups (Fig. 2(A)).

Fig. 2 Cytokine levels in the systemic immune response. (A) IL-10 and (B) IL-4 levels were analysed by ELSA in the serum of treated and non-treated mice (normal control (![]() ), basal control (
), basal control (![]() ), sensitisation control (
), sensitisation control (![]() ), previous treatment (PTREAT;
), previous treatment (PTREAT; ![]() ) and continuous treatment (CTREAT;
) and continuous treatment (CTREAT; ![]() )) at 5 d with probiotic fermented milk (5 dPFM) and at 7 and 15 d post-sensitisation (dPS). Results are expressed as concentration (pg/ml). Values are means of three different trials (n 9 mice), with their standard errors represented by vertical bars. a,b,c,dMean values with unlike letters were significantly different (P≤ 0·05).
)) at 5 d with probiotic fermented milk (5 dPFM) and at 7 and 15 d post-sensitisation (dPS). Results are expressed as concentration (pg/ml). Values are means of three different trials (n 9 mice), with their standard errors represented by vertical bars. a,b,c,dMean values with unlike letters were significantly different (P≤ 0·05).
Concerning IL-4, a slight increase in the level was observed with no significant difference between the serum of the SC group and the treated groups (Fig. 2(B)). IFN-γ had no detectable levels (according to the ELISA kit sensibility) in the serum of all the treatment groups.
Study of the mucosal immune response in the lungs: release of antibodies and cytokines in the broncho-alveolar lavage fluid induced by probiotic fermented milk administration after antigen stimulation
In all the treatment groups, we analysed the production of specific IgE, IgG, total S-IgA, IL-4, IL-10 and IFN-γ in the BALF and in the supernatants of the macerated lungs (LS). In the BALF, a significant peak was observed for a-OVA-specific IgE in the SC group at 7 dPS compared with the PTREAT and CTREAT groups, which exhibited similar lower levels to those of the NC and BC groups. At 15 dPS, mice that received the PTREAT showed a higher level of this antibody (Fig. 3(A)). The level of a-OVA-specific IgG significantly increased in the BALF of mice receiving the PTREAT at 7 dPS (P≤ 0·05); however, the levels of this antibody reduced significantly in both PTREAT and CTREAT groups at 15 dPS compared with the SC group (Fig. 3(B)). Specific IgE and IgG levels in the LS were below the detection level. For total S-IgA, PFM induced a higher production of this antibody in the CTREAT group at 7 dPS (P≤ 0·05). At 15 dPS, all the sensitised groups presented similar levels of total S-IgA (Fig. 3(C)).

Fig. 3 Mucosal antibody immune responses in the broncho-alveolar lavage fluid (BALF). (A) Anti-ovoalbumin (a-OVA)-specific IgE, (B) a-OVA-specific IgG and (C) total secretory IgA (S-IgA) were determined by ELISA in the BALF of treated and non-treated mice (normal control (![]() ), basal control (
), basal control (![]() ), sensitisation control (
), sensitisation control (![]() ), previous treatment (PTREAT;
), previous treatment (PTREAT; ![]() ) and continuous treatment (CTREAT;
) and continuous treatment (CTREAT; ![]() )) at 5 d with probiotic fermented milk (5 dPFM) and at 7 and 15 d post-sensitisation (dPS). Results for specific IgE and IgG are expressed as the absorbance level (optical density (OD) at 450 nm), and those for total S-IgA are expressed as concentration (μg/ml). Values are means of three different trials (n 9 mice), with their standard errors represented by vertical bars. a,b,c,dMean values with unlike letters were significantly different (P≤ 0·05).
)) at 5 d with probiotic fermented milk (5 dPFM) and at 7 and 15 d post-sensitisation (dPS). Results for specific IgE and IgG are expressed as the absorbance level (optical density (OD) at 450 nm), and those for total S-IgA are expressed as concentration (μg/ml). Values are means of three different trials (n 9 mice), with their standard errors represented by vertical bars. a,b,c,dMean values with unlike letters were significantly different (P≤ 0·05).
Cytokine analysis for IL-4 showed no statistical differences between the groups (NC, BC, SC, PTREAT and CTREAT) across the study periods (5 dPFM, 7 dPS or 15 dPS) (Fig. 4(A)). For IFN-γ, the CTREAT group showed a higher level of this cytokine in the LS at both 7 and 15 dPS, compared with the control and sensitised groups (P≤ 0·05; Fig. 4(B)). For IL-10, a significant peak was observed in the CTREAT group at 7 dPS compared with the SC and PTREAT groups. However, in the CTREAT group, the level of IL-10 decreased at 15 dPS. In contrast, the SC group exhibited an increased level of IL-10 at 15 dPS (P≤ 0·05; Fig. 4(C)).

Fig. 4 Mucosal cytokine immune responses in the lungs. (A) IL-4, (B) interferon-γ and (C) IL-10 levels were measured by ELISA in the supernatants of the macerated lungs of treated and non-treated mice (normal control (![]() ), basal control (
), basal control (![]() ), sensitisation control (
), sensitisation control (![]() ), previous treatment (PTREAT;
), previous treatment (PTREAT; ![]() ) and continuous treatment (CTREAT;
) and continuous treatment (CTREAT; ![]() )) at 5 d with probiotic fermented milk (5 dPFM) and at 7 and 15 d post-sensitisation (dPS). Results are expressed as concentration (pg/ml). Values are means of three different trials (n 9 mice), with their standard errors represented by vertical bars. a,b,c,dMean values with unlike letters were significantly different (P≤ 0·05).
)) at 5 d with probiotic fermented milk (5 dPFM) and at 7 and 15 d post-sensitisation (dPS). Results are expressed as concentration (pg/ml). Values are means of three different trials (n 9 mice), with their standard errors represented by vertical bars. a,b,c,dMean values with unlike letters were significantly different (P≤ 0·05).
Influence of probiotic fermented milk on the expression of IgA+ cells and CD4+ lymphocytes but not on the expression of CD8+ or F4/80+ cells in the lungs
In the lungs, the expression levels of IgA+ B lymphocytes were increased in mice that received the probiotic treatment until the end of the experiment (CTREAT group, 7 and 15 dPS) compared with the SC and NC groups (P≤ 0·05; Fig. 5(A)). The expression levels of CD4+ T lymphocytes were significantly increased in the three sensitised groups in both study periods in comparison with the NC group (P≤ 0·05), with the levels being higher in the SC group (Fig. 5(B)). OVA-sensitised mice with or without the probiotic treatment did not show significant differences in the number of cells expressing the CD8 marker in all the experimental groups (Fig. 5(C)). Similarly, no significant differences were found between the experimental groups and across the study periods in relation to macrophages that were counted by detecting the F4/80 marker (Fig. 5(D)).

Fig. 5 Mucosal respiratory immune cells. Expression levels of (A) IgA+ cells, (B) CD4+ T lymphocytes, (C) CD8+ T lymphocytes and (D) F4/80+ cells were determined by direct immunofluorescence in the lung tissue of treated and non-treated mice (normal control (![]() ), basal control (
), basal control (![]() ), sensitisation control (
), sensitisation control (![]() ), previous treatment (PTREAT;
), previous treatment (PTREAT; ![]() ) and continuous treatment (CTREAT;
) and continuous treatment (CTREAT; ![]() )) at 5 d with probiotic fermented milk (5 dPFM) and at 7 and 15 d post-sensitisation (dPS). Positive cells were counted in tissue sections using a fluorescence microscope at a magnification of 1000 × . The number of fluorescent cells was counted in thirty fields of vision. Results are expressed as the number of fluorescent cells per ten fields of vision. Values are means of three different trials (n 9 mice), with their standard errors represented by vertical bars. a,b,c,dMean values with unlike letters were significantly different (P≤ 0·05).
)) at 5 d with probiotic fermented milk (5 dPFM) and at 7 and 15 d post-sensitisation (dPS). Positive cells were counted in tissue sections using a fluorescence microscope at a magnification of 1000 × . The number of fluorescent cells was counted in thirty fields of vision. Results are expressed as the number of fluorescent cells per ten fields of vision. Values are means of three different trials (n 9 mice), with their standard errors represented by vertical bars. a,b,c,dMean values with unlike letters were significantly different (P≤ 0·05).
Influence of probiotic fermented milk on cytokine-secreting cells in lung tissue
PFM influenced the expression of IL-4 and IL-10 in different ways. For IL-10, the number of cells slightly increased in mice receiving the CTREAT and PTREAT compared with the SC, BC and NC groups at 15 dPS (P≤ 0·05). The number of IL-4+ cells was significantly increased in the SC group, and the PTREAT and CTREAT groups maintained similar levels to those of the NC and BC groups (P≤ 0·05; Fig. 6(A) and (B) respectively).

Fig. 6 Cytokine-producing cells and regulatory T (Treg) expression in the lungs. Expression levels of (A) IL-10- and (B) IL-4-producing cells were determined by indirect immunofluorescence in treated and non-treated mice (normal control (![]() ), basal control (
), basal control (![]() ), sensitisation control (
), sensitisation control (![]() ), previous treatment (PTREAT;
), previous treatment (PTREAT; ![]() ) and continuous treatment (CTREAT;
) and continuous treatment (CTREAT; ![]() )) at 5 d with probiotic fermented milk (5 dPFM) and at 7 and 15 d post-sensitisation (dPS). Positive cells were counted in tissue sections using a fluorescence microscope at a magnification of 1000 × . The number of fluorescent cells was counted in thirty fields of vision at a magnification of 1000 × . Results are expressed as the number of fluorescent cells per ten fields of vision. (C) Expression of Treg cells was evaluated by the detection of CD4+/CD25+/Foxp3+ cells by flow cytometry. Results are expressed as a percentage of triple positive cells relative to the total number of CD4+ cells (10 % of the total suspension; data not shown). Values are means of three different trials (n 9 mice), with their standard errors represented by vertical bars. a,b,c,dMean values with unlike letters were significantly different (P≤ 0·05).
)) at 5 d with probiotic fermented milk (5 dPFM) and at 7 and 15 d post-sensitisation (dPS). Positive cells were counted in tissue sections using a fluorescence microscope at a magnification of 1000 × . The number of fluorescent cells was counted in thirty fields of vision at a magnification of 1000 × . Results are expressed as the number of fluorescent cells per ten fields of vision. (C) Expression of Treg cells was evaluated by the detection of CD4+/CD25+/Foxp3+ cells by flow cytometry. Results are expressed as a percentage of triple positive cells relative to the total number of CD4+ cells (10 % of the total suspension; data not shown). Values are means of three different trials (n 9 mice), with their standard errors represented by vertical bars. a,b,c,dMean values with unlike letters were significantly different (P≤ 0·05).
Effect of probiotic fermented milk in sensitised and restimulated mice
To analyse the effect of PFM after re-exposure to the antigen at 2 dPR, a-OVA-specific IgE and IgG levels were measured in serum and BALF by ELISA (P≤ 0·05; Table 1). At 2 dPR, we found that the level of a-OVA-specific IgE was decreased in the serum and BALF of mice receiving only the PTREAT. For a-OVA-specific IgG, PFM induced a significant increase in the level of this antibody in the serum and BALF of mice that received a CTREAT with PFM (P≤ 0·05; Table 1).
Table 1 Influence of probiotic fermented milk on the levels of specific IgE and IgG 2 d after a restimulus with ovoalbumin (2 d post-restimulus) measured in serum and broncho-alveolar lavage fluid (BALF)* (Mean values with their standard errors from three different trials; n 9 mice)
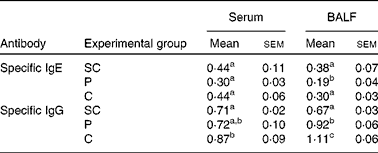
SC, sensitisation control; PTREAT, previous treatment; CTREAT, continuous treatment.
a,b,c,dMean values with unlike superscript letters were significantly different (P≤ 0·05).
* Specific IgE and IgG antibody responses were measured in the serum and BALF of the SC, PTREAT and CTREAT groups. Samples were collected 2 d after a short restimulation with ovoalbumin performed after the last sample collection (15 d post-sensitisation). Results are expressed as the absorbance level (optical density at 450 nm).
CD4+CD25+Foxp3+ regulatory T cells
The analysis of the presence of inducible Treg cells in the lungs showed that mice sensitised with OVA had a reduced percentage of CD4+/CD25+/Foxp3+ cells in comparison with the NC group. However, the percentage of Treg cells increased in the PTREAT and CTREAT groups at 15 dPS compared with the SC group with no significant difference between the groups (Fig. 6(C)).
Histological analysis of lung tissue
Samples from the lungs stained with H–E are shown in Fig. 7. Both the NC group (Fig. 7(A)) and the BC group (Fig. 7(B)) had a score of 0. At 7 dPS, the SC group had score 1 (Fig. 7(C)), the PTREAT group had score 0 (Fig. 7(E)) and the CTREAT group had score 1 (Fig. 7(G)). At 15 dPS, the SC group had score 2 (Fig. 7(D)), the PTREAT group had score 1 (Fig. 7(F)) and the CTREAT group had score 0 (Fig. 7(H)). We observed that mice receiving a CTREAT with PFM at 7 and 15 dPS (Fig. 7(G) and (H), respectively) showed a high frequency of mast cells (indicated by black arrows) and other granulocytic cells (indicated by white arrows); however, the structure of the tissue was conserved similar to that of the NC and BC groups. In contrast, for the SC group at 7 and 15 dPS (Fig. 7(C) and (D), respectively) and for the PTREAT group at 15 dPS (Fig. 7(F)), there was a higher cell infiltration with an increase in cell layers around the blood vessels, inducing a reduction in the alveolar space.

Fig. 7 Histological analysis of lung tissue. Histological analysis was performed by using the Enander score( Reference Perdigón, Maldonado Galdeano and Valdez 19 ), where lung tissue was classified from score 0 to 3 as follows: 0 = no cells around the blood vessels; 1 = one to three cell layers around the blood vessels; 2 = four to ten cell layers around the blood vessels; 3 = more than ten cell layers around the blood vessels. The analysis was performed qualitatively using light microscopy at a magnification of 1000 × . Images are representative of each treated and non-treated group of mice: (A) normal control; (B) basal control; (C, D) sensitisation control at 7 and 15 d post-sensitisation (dPS), respectively; (E, F) previous treatment at 7 and 15 dPS, respectively; (G, H) continuous treatment at 7 and 15 dPS, respectively. Black arrows, mast cells; white arrows, other granulocytic cells.
Discussion
Probiotic studies have demonstrated that these micro-organisms may favour immune responses in different pathologies such as allergic processes( Reference Yu, Jang and Kim 22 – Reference Meijerink, Wells and Taverne 25 ). These studies have reported that oral administration of probiotics reduced the production of specific IgE in the serum of mice( Reference Ohno, Tsunemine and Isa 13 ), suggesting that the consumption of food products containing probiotic micro-organisms may have a positive effect on the regulation of the immune system in the lungs.
Previous studies from our laboratory have demonstrated that oral administration of probiotics or fermented milk containing such micro-organisms induces the activation of the innate immune response to the gut level, with this beneficial effect also being observed in other distant mucosal sites such as lungs( Reference Galdeano and Perdigon 10 ). These previous studies have reported on the effect of PFM consumption for a prolonged period of time (14 weeks) on the cytokine profile of the adult host. In relation to IFN-γ, IL-4 and IL-10 (investigated in the present study), we demonstrated that the levels of IFN-γ and IL-4 increased at the beginning of PFM administration (until 1 week), and then the levels of these cytokine-producing cells were maintained, with a slight increase being modulated by IL-10 throughout the experimental period (14 weeks)( Reference de Moreno de LeBlanc, Chaves and Carmuega 26 ).
In the present study, OVA-sensitised mice were used to evaluate the effect of PFM administration to attenuate the exacerbated Th2 immune response to IgE induced in the airways. We demonstrated that PFM decreases the levels of a-Ova-specific IgE. We analysed whether the reduction in the level of IgE influenced the level of specific IgG for the same T-dependent antigen. Total S-IgA production and the most relevant cytokines involved in the allergic process were studied in order to elucidate the possible mechanisms involved in the decrease of specific IgE levels.
Interestingly, clinical studies have shown that the beneficial effect of probiotic treatment occurs in the absence of IgE reduction( Reference Kalliomaki, Salminen and Arvilommi 27 , Reference Kalliomaki, Salminen and Poussa 28 ). In contrast, Ohno et al. ( Reference Ohno, Tsunemine and Isa 13 ) showed that oral administration of Bifidobacterium bifidum G9-1 to mice reduced the levels of total and allergen-specific IgE in serum, but had no effect on allergen-specific IgG1 or IgG2a levels.
In contrast to these findings, we observed that in our sensitisation model, the main effect exerted by the administration of PFM was the significant reduction in the specific IgE level in the serum and BALF of the PTREAT and CTREAT groups at 7 and 15 dPS, but not for the specific IgG level (Fig. 1(B)), shifting the Th1/Th2 balance towards a Th1 response, as demonstrated by the increase in the specific IgG2a level (Fig. 1(D)). However, after the restimulation of the antigen (2 dPR), PFM administration maintained the IgE and IgG values near to those of the SC group (Table 1).
The analysis of the balance between the IL-10 and IL-4 levels showed high levels of IL-10 and normal levels of IL-4 in the serum of treated mice at 7 dPS (Fig. 2(A) and (B)). However, the effect of PFM on the decreased synthesis of IgE had no effect on the production of specific IgG, which could be mediated by the regulation of B-cell clones to produce IgG instead of IgE through the activation of Th1. This speculation is supported by the fact that PFM mainly activates the innate immune response in the gut( Reference Galdeano, de Moreno de LeBlanc and Vinderola 29 ), with an increased number of cytokine-producing cells (Th1/Th2 populations), as observed in previous work( Reference de Moreno de LeBlanc, Chaves and Carmuega 26 ), and influences the systemic immune response against OVA antigen( Reference Maldonado Galdeano, Novotny Nunez and de Moreno de LeBlanc 18 ).
Previous results led us to analyse the cytokine profile in the lung. In post-sensitised mice, we determined that PFM did not have an effect on IL-4 production, but induced high levels of both IL-10 (7 dPS) and IFN-γ (7 and 15 dPS) (Fig. 4(B) and (C)). The latter cytokine is important in the regulation of IL-4( Reference Hattori, Nishikawa and Watcharanurak 30 ).
It is well known that IL-10 exerts a regulatory effect during inflammation in the mucosa. This could be attributed to the reduction in the level of specific IgE in serum and BALF for 7 d after the last antigen exposure. The high levels of IFN-γ could regulate the immune response at 7 dPS and control the production of IL-10 at 15 dPS, as observed in the present study (Fig. 4).
Recently, new anti-inflammatory/regulatory functions of IFN-γ have been studied. One of its functions is the ability to inhibit the production of IL-17 and promote the maturation of B-lymphocytes and the production of IgG2a( Reference Kelchtermans, Billiau and Matthys 31 ). These observations are consistent with the present results, and could be correlated with the high levels of IFN-γ induced by PFM and specific IgG2a found in the serum of the CTREAT group, favouring the Th1 balance.
This finding led us to explore another hypothesis to explain the origin of IL-10 that is different from Treg (absent in the three sensitised groups) or Th2 cells. The latter cell population (Th2) was excluded, considering that the IL-4 level was not increased in the treated groups, but only in the SC group. Therefore, we reinforce the balance towards a Th1 response correlated with the high levels of IFN-γ released in the lungs and the balance towards IgG2a associated with antibody production. Although Th2 is the key cell to induce the production of antibody against T-dependent antigens such as OVA, Th1 cells are able to initiate immune responses against vaccine antigens such as toxoids (diftericum or tetanicum) with the release of IL-2. We demonstrated that L. casei CRL 431 and the PFM used in the present study increase the release of IL-2( Reference de Moreno de LeBlanc, Chaves and Carmuega 26 , Reference Perdigón, Maldonado Galdeano and Valdez 32 ). There are new findings demonstrating that Th1, macrophages and even mast cells can release IL-10. We believe that IL-10 could be released from the Th1 cell population, more than from Th2 cells, which is in agreement with the results reported by O'Garra & Vieira( Reference O'Garra and Vieira 33 ) and Maldonado Galdeano et al. ( Reference Maldonado Galdeano, Lemme-Dumit and Thieblemont 34 ).
The results found for TCD4+, TCD8+ and F4/80+ cells demonstrated that PFM consumption has an impact only on the profile of cytokine production; however, it does not modify the number of activated immune cells. In lung tissue, IL-4+ cells were increased in the SC group. In the supernatants of the macerated lungs, the level of this cytokine in all the sensitised groups (SC, PTREAT and CTREAT) remained similar to that of the NC group. Probably the regulation observed in the CTREAT group is due to the secretion of IL-10 at 7 dPS.
At the mucosal site, S-IgA is the main antibody produced and plays an important role in protecting the host against antigens that constantly prime the epithelial mucosa surface( Reference Hupin, Rombaux and Bowen 35 ). It is known that defects in the synthesis of the polymeric Ig receptor from mucosal epithelial cells are associated with Th2 inflammation in the airways( Reference Hupin, Rombaux and Bowen 35 , Reference Asano and Komiyama 36 ).
Previous studies have shown that PFM, similar to that used in the present study, increased the total S-IgA level in the gut of normal animals( Reference Galdeano, de Leblanc Ade and Carmuega 37 ). Taking this into account, we evaluated whether PFM administration had the same effect in the lung mucosa of sensitised animals. PFM increased not only the IgA-producing cells, but also the secretion of IgA in the BALF of the CTREAT group at 7 and 15 dPS. In this sense, the increase observed in the level of IFN-γ could favour the release of S-IgA, which is in agreement with a report indicating that IFN-γ is involved in the synthesis of polymeric Ig receptor( Reference Amin, Diebel and Liberati 38 ).
The present results suggest that the regulatory effect of PFM on the immune response in an OVA-sensitised mice model is mainly due to the high levels of IL-10 and IFN-γ, showing a Th1-driven immune response. In addition to the reduction in the specific IgE level, IFN-γ could promote an increase in the IgG2a level in serum and in total S-IgA level in the lungs. Moreover, another function of IFN-γ is the induction of Treg cells that could be involved in the regulatory effect observed for PFM( Reference Kelchtermans, Billiau and Matthys 31 ). Other authors have demonstrated that the administration of Lactobacillus reuteri increased the number of Treg cells in the spleen of asthma-induced mice( Reference Karimi, Inman and Bienenstock 39 ). Feleszko et al. ( Reference Feleszko, Jaworska and Rha 40 ) established in a murine model of asthma that probiotic bacteria-induced Treg cells inhibit subsequent allergic sensitisation and airway disease. According to these findings, we evaluated the expression of Treg cells in the lungs. Interestingly, in our experimental model of allergy, we found that PFM does not affect the expression of CD4+CD25+Foxp3+ Treg cells in the lungs, reinforcing our hypothesis that PFM favours a Th1 balance associated with IFN-γ production. We also demonstrated that PFM favours the improvement of lung tissues with an increase in the alveolar space (Fig. 7).
The most important fact demonstrated in the present study is that PFM intake strongly favours the IgG response to the antigen, probably by the regulation of B-cell clones to produce specific IgG instead of IgE through the activation of IFN-γ.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114515001981
Acknowledgements
The authors thank Azul Zorzoli, Biochemist, for her assistance with the data analysis of Treg cells.
The present study was financially supported by Consejo Nacional de Investigaciones Científicas y Técnicas grant, CONICET PIP 0652, CIUNT 26/D442 (Tucumán University) and by DANONE, Argentina.
The authors' contributions are as follows: E. M. M. V. and C. M. G. carried out the microbiological work, animal studies and immunological determination; G. P. and M. E. B. B. conceived the study; C. M. G. and G. P. designed the experiments; E. C. and R. W. provided PFM and participated in the discussion; E. M. M. V. and C. M. performed the statistical analysis and prepared the figures; E. M. M. V., C. M. G. and G. P. wrote the draft of the manuscript. All authors read and approved the final version of the manuscript.
The authors declare that they have no conflicts of interest.











