Gestational weight gain (GWG), which is a modifiable factor influencing maternal and neonatal outcomes, remains a highly controversial topic, mainly reflected in the difficulty in balancing the risks of inadequate against excessive gain. Meta-analyses have shown that inadequate GWG is associated with increased risks of small for gestational age (SGA), low birth weight (LBW) and preterm birth(Reference Goldstein, Abell and Ranasinha1–Reference Xu, Wen and Zhou3), while excessive GWG is associated with increased risks of large for gestational age (LGA), macrosomia and caesarean delivery(Reference Goldstein, Abell and Ranasinha1,Reference Tian, Hu and He4) . However, previous studies had some limitations – most studies used total GWG as the exposure measure, but few used rate of weight gain per week of gestation (GWG rate)(Reference Carnero, Mejia and Garcia5–Reference Sharma, Vesco and Bulkley10). Unlike total GWG, GWG rate does not heavily rely on the duration of pregnancy(Reference Gilmore and Redman11), and thus is less likely to bias the association of GWG with pregnancy outcomes. An improved understanding of the association may provide insight into normative GWG in Chinese population. In addition, most studies focused on morbidity outcomes, and few dealt with mortality outcomes, such as neonatal and infant death(Reference Chen, Feresu and Fernandez6,Reference Bodnar, Siminerio and Himes12–Reference Langford, Joshu and Chang15) , which are of greater concern worldwide. Most previous studies were also conducted in Caucasian populations, especially in American women, and there have been few such studies in non-Caucasian populations. The risk profiles between Caucasian and non-Caucasian populations might be different, as race–ethnicity factor was associated with both pattern of GWG(Reference Abrams, Carmichael and Selvin16) and pregnancy outcomes(Reference Singh and Yu17).
Therefore, we performed a prospective cohort analysis to comprehensively assess the associations of GWG rate in the second/third trimester with a spectrum of adverse pregnancy outcomes, including mortality and morbidity indicators, among Chinese nulliparous women.
Methods
Study population
Data for this prospective cohort analysis were retrieved from a randomised control trial (clinicaltrials.gov identifier: NCT00133744). The trial was conducted in five counties of Hebei province in China during 2006–2009 to investigate the effects of prenatal micronutrient supplementation on pregnancy outcomes. The details of the trial, such as inclusion criteria of participants, have been presented elsewhere(Reference Liu, Mei and Ye18). Briefly, 18 775 nulliparous women were enrolled before gestational week (GW) 20 and individually randomised to receive a daily supplement containing folic acid, Fe–folic acid or multiple micronutrients from early pregnancy to delivery. The women were followed up monthly from enrolment through delivery, and their infants were followed up until 1 year after birth. A total of 4556 women were excluded due to moving out of the hospital catchment area (n 28), spontaneous or induced abortions (n 815), dropout (n 33), maternal death (n 2), multiple birth (n 67), missing end pregnancy weight (n 23), end pregnancy weight measured over 2 weeks prior to delivery (n 3561), extremely low ( ≤ 10 kg) or extremely high ( > 35 kg) GWG (n 23) or death of infant due to an accident (n 4). Thus, 14 219 women were included in the analysis (Fig. 1). There were no significant differences in demographic characteristics between the women who were excluded and those who were included in the study (Supplementary Table S1). Among the 14 219 included women, 7378 were enrolled before GW 12, and 6841 were enrolled during GW 12–20. The demographic characteristics and adverse pregnancy outcomes were not substantially different between women enrolled before GW 12 and those enrolled during GW 12–20 (Supplementary Table S2). Women enrolled before GW 12 had slightly longer gestational age at delivery (39·6 v. 39·4 weeks), higher Hb concentration at enrolment (126·4 v. 122·5 g/l) and lower rates of preterm (4·5 v. 7·6 %) and LBW (1·9 v. 2·4 %) than those enrolled during GW 12–20. Because the GWG was lower before GW 12 but higher thereafter, different calculation methods for GWG rate in second/third trimester were applied in the following analyses.
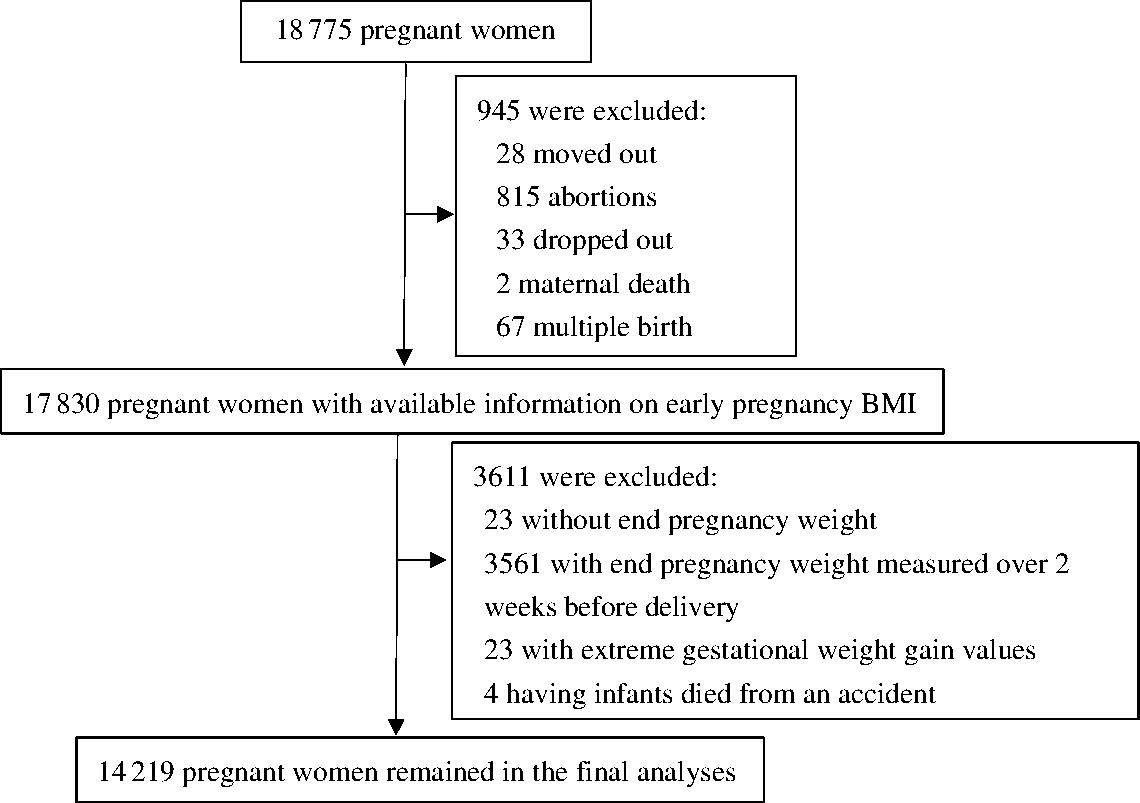
Fig. 1. Flowchart of study participants.
The institutional review boards of the US Centers for Disease Control and Prevention (Atlanta, GA) and Peking University (Beijing, China) approved the trial. All women gave informed consent to participate in the trial. The analyses of already collected data were exempt by the institutional review boards.
Rate of gestational weight gain
The exposure measure used in the present analysis was rate of GWG in second/third trimester which is preferable over the total GWG, as the latter is intrinsically associated with gestational age(Reference Gilmore and Redman11) and, thus, with pregnancy outcomes, particularly preterm and birthweight outcomes. Because the GWG rate was low in first trimester, and high and linear in second/third trimester(Reference Abrams, Carmichael and Selvin16), the GWG rate in second/third trimester was respectively calculated for women enrolled before GW 12 and those enrolled during GW 12–20. For women enrolled before GW 12, the GWG from enrolment to delivery was firstly calculated by subtracting the weight at enrolment from the end pregnancy weight, which was defined as the last recorded weight measured within 2 weeks before delivery. Then their exclusive GWG in second/third trimester was determined as the difference between GWG from enrolment to delivery and the estimated first-trimester GWG(Reference Durie, Thornburg and Glantz7); the latter was calculated as: (12 weeks – GW at enrolment) × 0·11 kg/week, the GWG rate in first trimester for Asians(Reference Abrams, Carmichael and Selvin16). Finally, their GWG rate in second/third trimester was calculated by dividing the GWG in second/third trimester by the number of weeks between GW 12 and end-pregnancy weight. For women enrolled during GW 12–20, the GWG rate was calculated by subtracting the weight at enrolment from the end-pregnancy weight and then dividing by the number of weeks between the two measurements. As GWG rate differs with maternal BMI(19), the GWG rate in second/third trimester was divided into quintiles within each BMI category.
Maternal weight and height were measured at enrolment before GW 20, using standardised equipment by trained staff. If weight at enrolment was used to calculate early-pregnancy BMI, some women enrolled during GW 12–20 would be classified into wrong BMI categories, due to the weight gain during GW 12–20. Therefore, for women enrolled during GW 12–20, we estimated the weight in first trimester as weight at enrolment minus estimated weight gain during GW 12–20; the latter was calculated as: (GW at enrolment – 12 weeks) × 0·56 kg/week, the GWG rate in second trimester for Asians(Reference Abrams, Carmichael and Selvin16). Early-pregnancy BMI (kg/m2) was estimated as first trimester weight in kg divided by the square of height in m. Women were then classified into four BMI categories, according to the WHO guidelines for Asians(20): underweight, < 18·5 kg/m2; normal weight, 18·5–23 kg/m2; overweight, 23·1–27·5 kg/m2; and obese, > 27·5 kg/m2.
Furthermore, an additional exposure measure was the rate of total GWG. The total GWG was firstly calculated by subtracting the first-trimester weight from the end-pregnancy weight, and the total GWG rate was calculated by dividing the total GWG by the number of weeks between the last menstrual period and the end-pregnancy weight(Reference Oken, Kleinman and Belfort9) and then divided into quintiles in the same manner as previously described. The mean total GWG of the included women was 12·9 (4·7) and 11·5 (4·6) kg for their GWG in second/third trimester.
Adverse pregnancy outcomes
The outcomes of interest included mortality indicators and other adverse pregnancy outcomes. The mortality indicators included stillbirth, neonatal death, early neonatal death, perinatal death, infant death and total mortality from GW 28 to 1 year after birth. Stillbirth was defined as fetal death occurring on or after GW 28. Neonatal death was defined as death occurring within 28 d after birth, early neonatal death as death occurring within 7 d after birth, and infant death as death occurring within 365 d after birth. Perinatal death was defined as the sum of stillbirth plus early neonatal death, and total mortality as the sum of stillbirth plus infant death. All mortality events were determined through active community surveillance and validated by registration certificates or hospital records(Reference Liu, Mei and Ye18).
The other adverse pregnancy outcomes included preterm birth, LBW, macrosomia, SGA and LGA. Preterm birth was defined as live birth within 37 completed GW from the first day of the last menstrual period; early preterm birth as birth occurring within 32 completed GW; and late preterm birth as birth occurring within 32–37 completed GW. LBW was defined as birth weight < 2500 g, and macrosomia as birth weight ≥ 4000 g. SGA and LGA were defined as birth weight below the 10th percentile for gestational age and above the 90th percentile for gestational age, respectively.
Statistical analysis
Continuous variables are presented as means and standard deviations, while categorical variables are shown as numbers and percentages. Differences across the GWG rate quintiles were assessed by ANOVA for continuous variables and the χ 2 test for categorical variables. Multivariable logistic regression models were used to estimate the adjusted OR and 95 % CI for pregnancy outcomes across different GWG rate quintiles, with the middle quintile serving as the reference group. In multivariable models, we adjusted for maternal age (< 25, 25–29 or ≥ 30 years), educational level (high school or above, middle school, or elementary school or less), occupation (farmer or other), ethnicity (Han or other), micronutrient supplementation (folic acid, Fe–folic acid or multiple micronutrients), BMI in early pregnancy (< 18·5, 18·5–23, 23·1–27·5 or > 27·5 kg/m2), Hb level at enrolment (100–109, 110–119, 120–129 or ≥ 130 g/l) and gestational age at enrolment (as a continuous variable). We further adjusted for gestational age at delivery (< 37 or ≥ 37 weeks) in models for mortality indicators, LBW and macrosomia. When we estimated the adjusted OR and 95 % CI for a given pregnancy outcome, only women at risk of that outcome were taken into consideration. That is, all 14 219 women were included in analyses of stillbirth, then women whose pregnancy ended in stillbirth (n 49) were excluded from analyses of other pregnancy outcomes.
To assess the robustness of our results, we repeated the main analyses using the rate of total GWG. To compare estimates of association with those reported in previous studies, we further repeated our main analyses using the recommended GWG rates in the second/third trimester provided in the 2009 Institute of Medicine (IOM) guidelines(19). Women were then divided into three groups: less than, within or greater than the guideline in each BMI category, stratified as follows: underweight, < 18·5 kg/m2; normal weight, 18.5 to < 25 kg/m2; overweight, 25 to < 30 kg/m2; and obese, ≥ 30 kg/m2. All analyses were performed with SPSS software (version 20.0; SPSS Inc.). Statistical tests were two-sided, and P < 0·05 was taken to indicate statistical significance.
Results
The mean maternal BMI in early pregnancy was 21·9 (sd 2·8) kg/m2. Among the 14 219 nulliparous women, 1187 (8·3 %) were categorised as underweight, 8897 (62·6 %) as normal weight, 3522 (24·8 %) as overweight and 613 (4·3 %) as obese. Overall, the mean rate of GWG in the second/third trimester was 0·47 (sd 0·19) kg/week, with values of 0·58 (sd 0·23) kg/week for underweight, 0·48 (sd 0·18) kg/week for normal weight, 0·42 (sd 0·18) kg/ week for overweight and 0·38 (sd 0·19) kg/week for obese (Table 1). Maternal characteristics, including maternal age, height, education level, occupation, gestational age at enrolment and Hb level at enrolment across quintile groups of GWG rate in second/third trimester, are shown in Table 2.
Table 1. Characteristics of gestational weight gain (GWG) rate and Institute of Medicine (IOM) guidelines in second/third trimester
(Mean values and standard deviations)
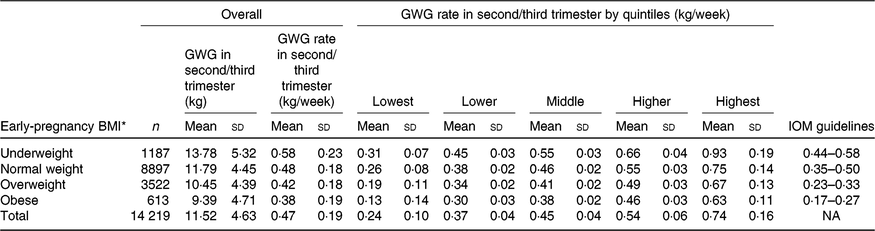
NA, not available.
* For presenting ‘Overall’ and ‘GWG rate in second/third trimester by quintiles’ portions, the early-pregnancy BMI was categorised as underweight (< 18·5 kg/m2), normal weight (18·5–23·0 kg/m2), overweight (23·1–27·5 kg/m2) and obese (> 27·5 kg/m2); for presenting ‘IOM guideline’ portion, the early-pregnancy BMI was then categorised as underweight (< 18·5 kg/m2), normal weight (18·5 to< 25 kg/m2), overweight (25 to < 30 kg/m2) and obese (≥ 30 kg/m2).
Table 2. Maternal characteristics according to quintiles of gestational weight gain (GWG) rate in second/third trimester
(Numbers and percentages; mean values and standard deviations)
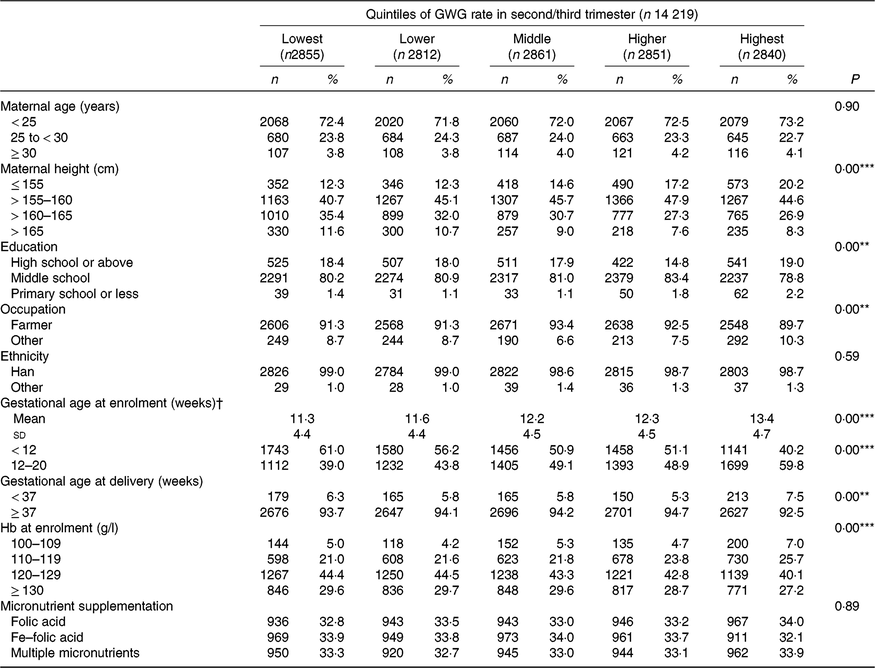
** P<0·01, *** P<0·001.
† Because gestational age at enrolment was adjusted as a continuous variable, both its means and standard deviations, and n and % are presented.
Among all women included in this study, forty-nine (3·4 ‰) pregnancies ended in stillbirth, while 14 170 ended in live births. Among the live births, there were sixty-two (4·4 ‰) early neonatal deaths, seventy-six (5·4 ‰) neonatal deaths and 109 (7·7 ‰) infant deaths. Of the seventy-six neonatal deaths, twenty-two died of asphyxia, twenty-two of premature birth, fifteen had birth defects, ten had infection, and seven died of other or unknown causes; the corresponding numbers for the 109 infant deaths were twenty-seven, twenty-two, twenty-seven, seventeen and sixteen, respectively. The associations between GWG rate in the second/third trimester and mortality indicators are presented in Table 3. Compared with women in the middle quintile, those in the lowest quintile had higher risks of infant death (adjusted OR 1·85; 95 % CI 1·02, 3·37) and neonatal death (adjusted OR 2·27; 95 % CI 1·03, 5·02), and those in the lower quintile also had a higher risk of neonatal death (adjusted OR 2·34; 95 % CI 1·06, 5·17).
Table 3. Risk for offspring mortality outcomes by quintiles of gestational weight gain (GWG) rate in second/third trimester*
(Adjusted odds ratios and 95 % confidence intervals)
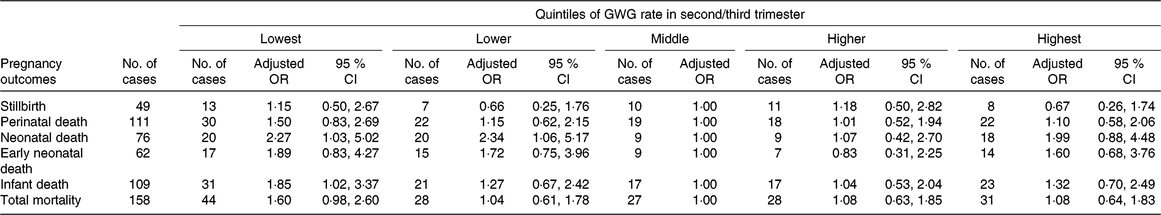
* Adjusted for the following variables: maternal age, educational level, occupation, ethnicity, micronutrient supplementation, BMI in early pregnancy, Hb level at enrolment, gestational age at enrolment and gestational age at delivery.
Of the live births, 845 (6·0 %) were preterm, 307 (2·2 %) were LBW, 570 (4·0 %) had macrosomia, 1366 (9·6 %) were SGA and 1383 (9·8 %) were LGA. The associations between GWG rate in second/third trimester and these adverse outcomes are shown in Table 4. Compared with women in the middle quintile, those in the lowest quintile had increased risks of early preterm birth (adjusted OR 2·33; 95 % CI 1·13, 4·77), while those in the highest quintile had increased risks of overall preterm birth (adjusted OR 1·28; 95 % CI 1·04, 1·59), late preterm birth (adjusted OR 1·25; 95 % CI 1·00, 1·56), macrosomia (adjusted OR 1·89; 95 % CI 1·46, 2·45), LGA (adjusted OR 1·56; 95 % CI 1·31, 1·85) and LBW (adjusted OR 1·48; 95 % CI 1·02, 2·15).
Table 4. Risk for preterm- and birthweight-related outcomes by quintiles of gestational weight gain (GWG) rate in second/third trimester*
(Adjusted odds ratios and 95 % confidence intervals)
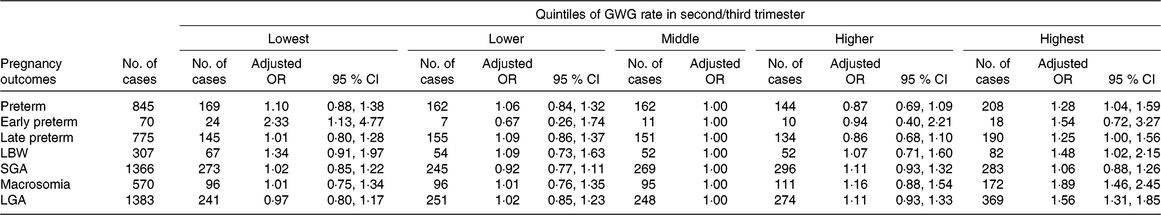
LBW, low birth weight; SGA, small for gestational age; LGA, large for gestational age.
* Adjusted for the following variables: maternal age, educational level, occupation, ethnicity, micronutrient supplementation, BMI in early pregnancy, Hb level at enrolment, gestational age at enrolment. We further adjusted for gestational age at delivery in models for LBW and macrosomia.
When we repeated the main analysis using the total GWG rate, the results regarding preterm and birthweight outcomes were not substantially changed, while no positive association was found for mortality indicators (Supplementary Table S3).
In an analysis of GWG rate in the second/third trimester classified according to the 2009 IOM guidelines, the proportions of women with weight gain less than, within and greater than the recommendations were 23·0, 35·7 and 41·3 %, respectively. In comparison with women who showed weight gain within the recommendation, those with less weight gain had increased risks of offspring total mortality (adjusted OR 1·55; 95 % CI 1·04, 2·32), infant death (adjusted OR 1·63; 95 % CI 1·01, 2·63), LBW (adjusted OR 1·41; 95 % CI 1·02, 1·94) and SGA (adjusted OR 1·15; 95 % CI 1·01, 1·31), while those with greater weight gain showed increased risks of macrosomia (adjusted OR 1·38; 95 % CI 1·13, 1·67) and LGA (adjusted OR 1·28; 95 % CI 1·12, 1·45) (Supplementary Table S4).
Discussion
In this prospective cohort analysis of nulliparous Chinese women, the GWG rate in the second/third trimester was associated with increased risks of various adverse pregnancy outcomes, independent of maternal BMI in early pregnancy. In comparison with women in the middle quintile of GWG rate in second/third trimester, those in the lowest quintile had increased risks of infant death, neonatal death and early preterm birth, while those in the highest quintile had increased risks of preterm birth, macrosomia, LGA and LBW.
Previous studies regarding infant mortality were limited by the use of either total GWG(Reference Bodnar, Siminerio and Himes12–Reference Langford, Joshu and Chang15) or GWG rate(Reference Chen, Feresu and Fernandez6) as the exposure measure. All of these studies were performed in American women, but the results were inconsistent. One case–control study(Reference Chen, Feresu and Fernandez6) and one retrospective cohort study(Reference Bodnar, Siminerio and Himes12) using self-reported GWG indicated that, in comparison with average GWG rate or GWG z-scores, low and high GWG were associated with an increased risk of infant death, mainly driven by early neonatal(Reference Bodnar, Siminerio and Himes12) or neonatal death(Reference Chen, Feresu and Fernandez6). The third study reported an increased risk of infant death in association with inadequate GWG, but a lower risk with excessive GWG, in comparison with GWG within the 2009 IOM guidelines(Reference Davis, Hofferth and Shenassa13). The two remaining studies did not observe any positive associations for neonatal or infant death(Reference Friedmann and Balayla14,Reference Langford, Joshu and Chang15) . In our study of nulliparous Chinese women, we also found increased risks of infant death and neonatal death for the lowest quintile, but not for the highest quintile, compared with the middle quintile of GWG rate in second/third trimester. Although the association of GWG rate with cause-specific death were not studied due to the relatively small number of cases, the primary causes of neonatal or infant death were asphyxia, premature birth, birth defects (such as congenital heart defects) and infection.
Our findings regarding birth weight outcomes were partially consistent with previous studies. A recent meta-analysis using total GWG showed a higher risk of SGA in association with inadequate GWG, and higher risks of macrosomia and LGA in association with excessive GWG, in comparison with GWG within the IOM guidelines(Reference Goldstein, Abell and Ranasinha1). Studies using the second/third-trimester GWG rate also reported an increased risk of SGA with inadequate GWG rate, and of LGA with excessive GWG rate, among American and Brazilian women(Reference Durie, Thornburg and Glantz7,Reference Drehmer, Duncan and Kac21) . Inadequate or excessive GWG rate may reflect exposure of the foetus to reduced or greater amounts of glucose and fatty acids during development, respectively(Reference Oken and Gillman22,Reference Taylor and Poston23) . In our study, we found higher risks of LGA and macrosomia for the highest quintile, but not of SGA for the lowest quintile among nulliparous Chinese women. Unexpectedly, we found an increased risk of LBW for the highest quintile of GWG rate. We wonder whether it reflects a true association, as it might be biased by gestational age at enrolment. For example, women enrolled at larger gestational age were more likely to have a higher GWG rate in second/third trimester and a higher rate of LBW. Although we had adjusted gestational age at enrolment as a continuous variable, a residual confounding might have still remained. Additionally, we did not find any positive association between GWG rate and LBW when the total GWG or the second/third-trimester GWG rates classified according to the 2009 IOM guidelines were used.
Results regarding the association between GWG and preterm birth reported to date are not consistent across studies. One meta-analysis indicated that total GWG below the IOM guideline was associated with a higher risk of preterm birth, while total GWG above the guideline was associated with a lower risk of preterm birth(Reference Goldstein, Abell and Ranasinha1). However, in our study, the lowest quintile of GWG rate was associated with a higher risk of early preterm birth, while the highest quintile was associated with overall preterm, mainly due to its association with late preterm. Our findings were supported by a cohort study in Peru indicating a U-shaped association between GWG rate and the risk of preterm birth(Reference Carnero, Mejia and Garcia5). One meta-analysis indicated that high total GWG was associated with a lower risk of preterm birth, while a high GWG rate was associated with a higher risk of preterm birth(Reference McDonald, Han and Mulla24). This discrepancy suggests that there may be some bias when using total GWG, because total GWG is inherently linked to gestational duration and, thus, to preterm birth. There are a number of possible biological mechanisms that may be involved in the association between GWG rate and preterm birth. A low GWG rate may be an indicator of micronutrient deficiency (such as vitamins C and E) and reduced expansion of plasma volume, and the two latter conditions likely lead to preterm birth(Reference Romero, Chaiworapongsa and Espinoza25,Reference Romero, Dey and Fisher26) . A high GWG rate is associated with altered placental microbiome profile, including decreased species richness and altered bacterially encoded metabolic pathways(Reference Antony, Ma and Mitchell27). A decreased species richness has been linked to insulin resistance, inflammatory phenotypes and infection and, thus, to preterm; altered bacterially encoded metabolic pathways, such as decreased abundance of folate biosynthesis and increased siderophore biosynthesis, were also linked to increased inflammation and worsened Fe status and, thus, to preterm(Reference Neilands28,Reference Aagaard, Ma and Antony29) .
Our study has some strengths. It was the first study to prospectively and comprehensively investigate the association of GWG rate in the second/third trimester with a range of pregnancy outcomes, including offspring mortality indicators, preterm birth and birth weight-related outcomes. Weekly GWG rate in the second/third trimester of pregnancy, rather than total GWG, was used as the exposure measure. GWG rate is likely more meaningful than total GWG in prenatal healthcare with respect to optimising weight gain during pregnancy. In addition, we adjusted for Hb and micronutrient status, which may be important confounders in the association between GWG and adverse pregnancy outcomes(Reference Siega-Riz, Adair and Hobel30–Reference Little, Brocard and Elliott32).
However, our study also had a number of limitations. First, data on potential confounders, such as diet and physical activity(Reference Stuebe, Oken and Gillman33,Reference Zhang and Ning34) , were not collected, so we could not exclude the potential confounding effects. Furthermore, our sample size was relatively small, which limited our ability to perform detailed subgroup analyses according to BMI categories. Furthermore, using early-pregnancy BMI as the approximation of preconception BMI might lead to some women being misclassified, although such misclassification would probably be small. In addition, about 90 % of women included in the study were farmers, which would likely restrict the generalisability of the results. Caution should be used when generalising the results to other ethnic populations, as our results were derived from a population mainly comprising Han Chinese women (99 %).
In conclusion, our study comprehensively investigated the association between GWG rate in second/third trimester and adverse pregnancy outcomes among Chinese nulliparous women. Very low and very high GWG rates were associated with increased risks of various adverse pregnancy outcomes, and very low GWG rates appeared to be particularly associated with mortality outcomes. Healthcare providers should integrate lifestyle and dietary interventions into routine healthcare during pregnancy to help women optimise their GWG(Reference Guelinckx, Devlieger and Mullie35,Reference Thangaratinam, Rogozinska and Jolly36) . Large-scale studies in Asian countries(Reference Hedderson, Gunderson and Ferrara37) with more representative populations are needed to examine whether the associations observed here persist across BMI categories.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (J. L., grant number 2016YFC1000401); the National Natural Science Foundation of China (J. L., grant number 81571517; Y. Z., grant number 81801542); and the Young Researcher Development Scheme of Peking University (Y. Z., grant number BMU20160571). The funders had no role in the conduct of this study.
J. L. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Y. Z., J. L.; acquisition, analysis or interpretation of data: all authors; drafting of the manuscript: Y. Z.; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: Y. Z.; administrative, technical or material support: all authors; study supervision: all authors.
There are no conflicts of interest.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0007114519001247








