Reliable information on the nutrient composition of foods, such as their Fe content, is essential to meet the needs of a wide variety of groups, including nutritionists, government agencies, health and agriculture professionals, policy makers and planners, food producers, retailers and consumers. Tables from McCance and Widdowson's The Composition of Foods, the UK Government's official source of food composition, provide the most recent available data on the Fe content of common foods in the UK, yet much of the nutrient analysis was carried out in the 1980s or earlier( 1 ).
Several studies based upon the comparison of data from different food composition tables have suggested that significant changes in the mineral content of food have occurred over time, with Fe contents declining in recent years in both the UK and USA( Reference White and Broadley 2 – Reference Davis, Epp and Riordan 5 ). However, these studies have had to assume that analytical data from different eras are similarly accurate while statistical comparisons have been basic, i.e. not allowing for multiple testing for example. Nonetheless, there are several lines of evidence that suggest that the nutrient content of certain foods could have altered in recent years. Mostly, such reports hypothesised that a decline in food nutrient content has occurred due to changes in agricultural practices, particularly depletion in available soil minerals( Reference Thomas 3 ).
Indeed, over the last century, food production has undergone a revolution, with changes in farming practice at the forefront. Before the Second World War, agricultural chemicals were rarely used; however, in modern agriculture, traditional organic fertilisers, such as manure, have been largely replaced by chemical fertilisers( Reference Hornick 6 ). Second, some foods, such as tomatoes, are now frequently grown hydroponically in nutrient solutions( Reference Kelly and Bateman 7 ). Third, evidence is emerging that the recent higher atmospheric CO2 levels may have an impact upon plant nutrient content at least in wheat and brown rice, by increasing the proportion of carbohydrate and thus leading to a relative reduction in the content of other nutrients such as Fe( Reference Loladze 8 ). Fourth, Fan et al. ( Reference Fan, Zhao and Fairweather-Tait 9 ) provide robust evidence from the contemporary analysis of archived wheat grain and soil samples, taken from the Broadbalk Wheat Experiment established in 1843 at Rothamsted, UK, that there has been a significant decrease in the content of Fe, and other minerals, in wheat over 160 years. However, they did not attribute this to a change in fertiliser usage, or a decrease in soil mineral content, but instead they suggested that the introduction of semi-dwarf cultivars in the mid to late 1960s, which are high-yielding crops, had a significant effect on the mineral content of wheat, including Fe( Reference Fan, Zhao and Fairweather-Tait 9 ). Whether this is true for other plant-based foods, and whether the modern practice of Fe fortification and restoration negates or even exceeds such declining effects, is not clear. There is no repository that allows for the direct comparison of the nutrient content of similar foods from different eras by contemporary analysis. However, one can at least address the issue of whether the values given for the Fe content of plant-based foods, in the current UK version of The Composition of Foods, is matched by data from re-analysis of the same foods more than 20 years later. In the present paper we report on the analysis of total Fe content in 146 commonly ingested plant-based foods and make a more thorough comparison of the Fe content of foods reported in the different versions of the UK The Composition of Foods since the 1930s.
Methods
Food samples
The foods analysed were purchased and carefully sampled in 2001–2 as part of an investigation into the silicon content of foods in the UK( Reference Powell, McNaughton and Jugdaohsingh 10 ). Briefly, foods were purchased from three different shops or supermarkets in South-East England, with the food varieties selected on the basis that they provided a fair representation of those commonly consumed by the British population. An equal amount was taken from each of the three samples of that food, and combined into a composite sample. Representative samples were taken, so in the case of lettuce, for example, both outer and inner leaves were used. The method commonly used to prepare a food in domestic practice was carried out if needed. For example, wholemeal pasta was boiled in water for 10 min. Composite samples were homogenised in a blender and then placed in a polypropylene tube and frozen, ready for peroxide and acid digestion before analysis.
Composite samples were digested using an acid-assisted microwave digestion system (Milestone Analytical UK Ltd) as described previously( Reference Powell, McNaughton and Jugdaohsingh 10 ). Briefly, 0·25 g of each sample was taken and placed in a microwave vessel with 10 ml 65 % (v/v) nitric acid (purity grade p.a. plus ( ≤ 0·1 mg/kg Fe); Fluka; Aldrich-Chemical Co.) and 1 ml of 30 % (v/v) H2O2 (AristaR; Merck Ltd), the vessel sealed and heated at 200°C for 15 min. In order to allow for possible Fe contamination from the environment, a baseline blank sample was similarly prepared with each digestion run (i.e. one blank for every nine samples). These vessels contained only the nitric acid and H2O2. Digested samples and blanks were diluted with approximately 33 ml ultra-high purity water (18 MΩ/cm; Elga Ltd) and total final volumes, assessed accurately by weight, were recorded before analysis.
Iron content
Total Fe content of composite samples and blanks was determined using an inductively coupled plasma–optical emission spectrometer (ICP-OES; Jobin-Yvon JY24 Instruments SA) with a V-groove nebuliser and Scott-type double-pass spray chamber at 259·940 nm. Fe concentration was determined by comparison with standards prepared from a stock ICP standard solution (1000 mg/l; Spectrol/Merck) with a matrix-matched diluent (i.e. containing nitric acid and H2O2). Blanks were randomly distributed throughout every sample batch. Fe levels in the samples are reported where the value for the sample (which is ‘sample minus mean batch blank’) exceeds the highest blank value of the batch. The mean values for the Fe content of all blanks were 5·4 (sd 1·8) μg/l. The minimally detected content of Fe in food was 0·12 mg Fe/100 g food, corresponding to a blank-subtracted Fe content of 6·82 μg/l in the analysed sample (i.e. post digestion and dilution). A certified reference material (Seronorm™ Trace Elements, lot NO0371; Alere Ltd) was processed as described above for the food samples and found to contain 1·87 (sd 0·02) mg Fe/kg by analysis, consistent with its certified level of 1·95 mg Fe/kg (range 1·71–2·18 mg Fe/kg).
Comparisons with UK literature values
Results are expressed in terms of Fe content/100 g while average portion sizes( Reference Mills and Patel 11 , Reference Davies and Dickerson 12 ) are indicated for each food. Data are compared with those from the 1980s and 1930s which, respectively, are published values from the 6th edition of McCance and Widdowson's The Composition of Foods ( 1 ) and McCance and Widdowson's The Chemical Composition of Foods ( Reference McCance and Widdowson 13 ). Using the Bland–Altman method, any bias was determined by comparing the mean of the differences with zero and their associated 95 % CI( Reference Bland and Altman 14 ).
We recognised that some foods, especially breakfast cereals, are fortified with Fe and that manufacturer's fortification practices change frequently. Thus analytical differences between the eras could represent (i) different fortification practices, (ii) genuine endogenous differences in mineral content or (iii) analytical error. To help control for the latter two we analysed for another, non-fortificant mineral, namely Mg, in four common breakfast cereals. ICP-OES was used as described above for Fe, using the same digested composite samples and blanks, and wavelengths for Mg analysis were 279·553 and 279·079 nm.
Comparison of iron intake in the UK adult population
The impact that our newly measured values may have on the mean daily Fe intake of the UK adult population was estimated using data from the National Diet and Nutrition Survey (NDNS) conducted in 2000–1( Reference Henderson, Gregory and Swan 15 , Reference Henderson, Irving and Gregory 16 ). Using intake data from adults aged 19–64 years, we replaced the Fe content established in the 1980s( 1 ) with our new data, also from 2001, and reported here for 128 plant-based foods (corresponding to 203 food codes). We did not undertake this for all subjects of the NDNS dataset, but, rather, for a subgroup (n 497) where we had a full set of matching data for breakfast cereal consumption (ten breakfast cereals corresponding to seventeen food codes) for the reasons explained below in the Results section. The means were compared using the mean of the paired difference with 95 % CI.
Results
We analysed 146 plant-based foods and 128 of those had detectable Fe, meaning an Fe content greater than or equal to 0·12 mg Fe/100 g food (Table 1). Historical values are also given in Table 1 and difference plots( Reference Bland and Altman 14 ), demonstrating variation between the years, are shown in Figs. 1–4.
Table 1 Iron content* of selected UK foods in 2001–2†, analysed by inductively coupled plasma–optical emission spectrometry (ICP-OES), and published values

na, Not available; nd, not detectable.
* Values shown are total Fe content and will include the natural Fe content plus any fortificant Fe where it has been used.
† Foods were purchased in 2001–2 but for clarity the study is referred to as the decade 2000s in the table.
‡ Mean content of four different varieties, namely apple mint, black mint, crispum and Moroccan mint, which had Fe content of 1·47, 1·40, 3·59 and 1·08 mg Fe/100 g food, respectively.

Fig. 1 Comparison of the iron content (mg iron/100 g food) of the same fruits between different decades: the analysis presented here (2000s), the latest published values (1980s)( 1 ) and the earliest published values (1930s)( Reference McCance and Widdowson 13 ). Comparisons were made using the Bland–Altman method( Reference Bland and Altman 14 ). Briefly, for each food, the difference in the iron content between the two decades being compared (decade 1 – decade 2) is plotted against the mean iron content of those two decades. The mean and 2 sd of the differences were calculated and are represented in each graph by the dotted lines. The continuous line represents the null difference. (a) 2000s v. 1980s (n 29); 2000s v. 1930s (n 20); (c) 1980s v. 1930s (n 20).

Fig. 2 Comparison of the iron content (mg iron/100 g food) of vegetables between different decades: the analysis presented here (2000s), the latest published values (1980s)( 1 ) and the earliest published values (1930s)( Reference McCance and Widdowson 13 ). Comparisons were made using the Bland–Altman method( Reference Bland and Altman 14 ). Briefly, for each food, the difference in the iron content between the two decades being compared (decade 1 – decade 2) is plotted against the mean iron content of those two decades. The mean and 2 sd of the differences were calculated and are represented in each graph by the dotted lines. The continuous line represents the null difference. Legumes and pulses were included only in the comparison between the 2000s and 1980s, as few data are available from the 1930s. (a) 2000s v. 1980s (n 47); 2000s v. 1930s (n 22); (c) 1980s v. 1930s (n 22).

Fig. 3 Comparison of the iron content (mg iron/100 g food) of cereal products (n 41) between the analysis presented here (2000s) and the latest published values (1980s)( 1 ). Iron content will include the natural iron content plus any fortificant iron where it has been used. Comparison was made using the Bland–Altman method( Reference Bland and Altman 14 ). Briefly, for each food, the difference in the iron content between the 2000s and 1980s is plotted against the mean iron content of those two decades. The mean and 2 sd of the differences were calculated and are represented in each graph by the dotted lines. The continuous line represents the null difference. One outlier, namely Bran Flakes, was removed (difference 35·0; mean 17·5) and was not included in the statistical analysis. The other breakfast cereals are highlighted in full circles for clarity. No comparison was made with the earliest published values (1930s)( Reference McCance and Widdowson 13 ) because of the small number of comparable foods and due to the major changes that occurred in cereal product manufacture, for example, fortification, since the 1930s.
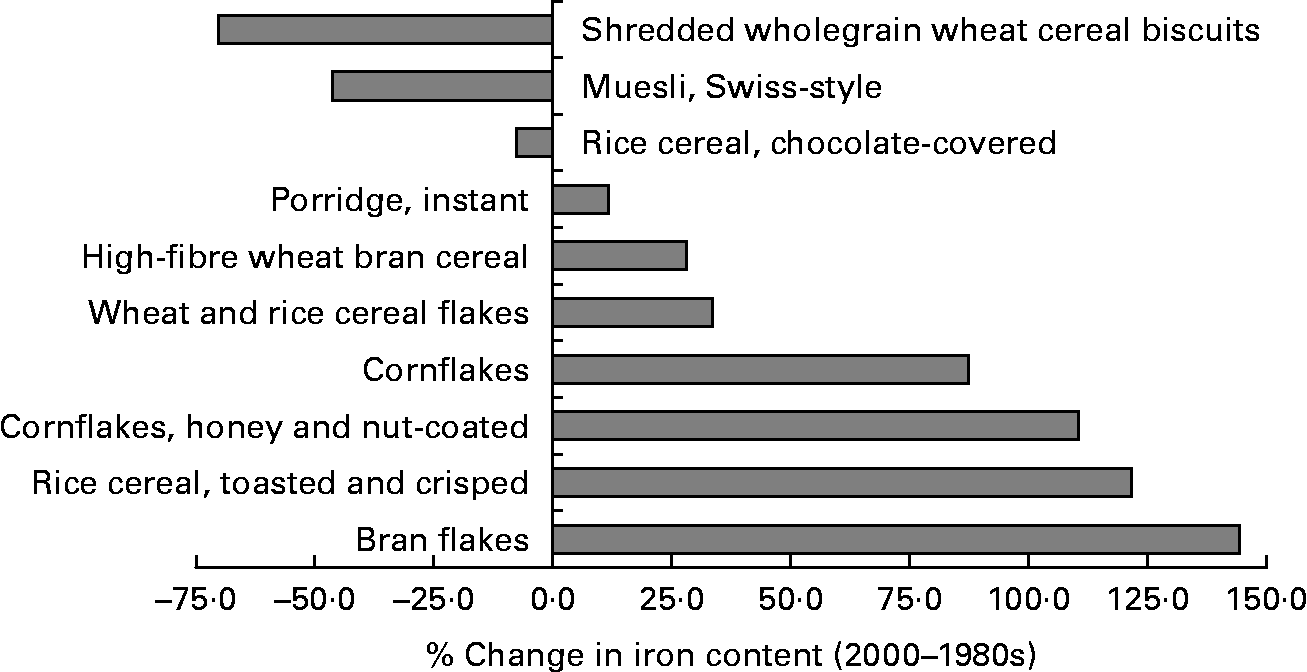
Fig. 4 Percentage changes in the iron content (mg iron/100 g food) of breakfast cereals between our analysis (2000s) and the latest published values (1980s)( 1 ). Iron content will include the natural iron content plus any fortificant iron where it has been used.
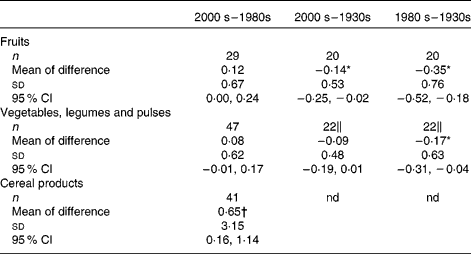
To determine whether significant differences existed between the reported Fe content of major food groups for each of the three decades we calculated means of differences and 95 % CI where matching analytical data were available. There were too few data for cereal and cereal products or legumes for the 1930s to allow for widespread comparisons, so in Table 2 we compared data on (a) fruit for the three decades, (b) vegetables including legumes and pulses for the last two decades, (c) vegetables alone for the comparison with the 1930s, and (d) cereals and cereal products for the last two decades. Overall, data from the 1930s were marginally but significantly higher than data from the later years, albeit not quite so for vegetables between the 2000s and 1930s. Between the 1980s and 2000s there was a tendency for analytical values to be higher for the latter decade, although this was only significant, with marked difference, for cereals and cereal products (Table 2). This may be explained by changes to fortification practices because Mg values generally matched between the 1980s and 2000s for the four cereals analysed, namely (all as mg/100 g prepared food for the 1980s v. 2000s analysis): 240 v. 220, 10 v. 13, 40 v. 49 and 120 v. 91 for, respectively, high-fibre wheat bran cereal, cornflakes, rice cereal toasted and crisped, and shredded wholegrain wheat cereal biscuits.
Table 2 Differences in the iron content (mg iron/100 g food)‡ of the plant-based food groups between the decades§ (Number of foods in the group, mean differences, standard deviations and 95 % confidence intervals)
nd, Not determined.
* Statistically significant decrease from the 1930s to the 1980s or 2000s (P < 0·05).
† Statistically significant increase from the 1980s to the 2000s (P < 0·05).
‡ Values shown are total Fe content and will include the natural Fe content plus any fortificant Fe where it has been used.
§ The decades compared were: the re-analysis presented in the present study (2000s), the latest literature values (1980s)( 1 ) and the earliest literature values (1930s)( Reference McCance and Widdowson 13 ).
∥ Vegetables only due to lack of data in 1930s on legumes and pulses.
Finally we questioned whether these differences in cereal Fe content between the 1980s and 2000s would make an impact upon reported dietary Fe intakes in the population. As shown in Fig. 3, fortified breakfast cereals had the highest Fe content as well as the greatest variation in Fe content between the 1980s and 2000s. Using the NDNS data we compared Fe intakes in those adults who ingested breakfast cereals for which we had a full set of matching analytical values for the two decades (i.e. 100 % of their breakfast cereal consumption was with breakfast cereals for which there were matching analytical data between the 1980s and 2000s). These were ten different breakfast cereals (Fig. 4) and 497 adult individuals matched this criterion. To assess intakes of Fe from the 1980s values the latest version of McCance and Widdowson ( 1 ) was used as shown in Table 1. To assess intakes of Fe from the year 2000s values, we still based the analysis on the latest version of McCance and Widdowson but substituted in all new Fe content values from the 2000s analyses where they were available. This was for 128 foods (Table 1), translating to 203 food codes, with, as noted above, ten of these being for breakfast cereals (seventeen food codes). For the 497 adult individuals described above their mean daily Fe intake, based upon 7 d weighed dietary records, was 12·2 (sd 4·2; 95 % CI 11·9, 12·6) mg Fe/d using the 1980s values for the Fe content of foods. However, using the 2000s values for the Fe content of foods, mean intake was 11·5 % higher at 13·6 (sd 7·2; 95 % CI 13·0, 14·2) mg Fe/d. The mean of the paired differences was 1·4 (95 % CI 1·0, 1·8) mg Fe/d. This subsample of 497 adults had Fe intake marginally higher than the entire sampled population of NDNS (n 1724), being 12·2 (95 % CI 11·9, 12·6) mg Fe/d v. 11·4 (95 % CI 11·2, 11·6) mg Fe/d, respectively, presumably as they were selected to be ‘breakfast cereal consumers’.
Discussion
Considering that the analytical data presented here span 70 years, are from different samples, used markedly different analytical techniques, and come from different laboratories, the similarities in the reported results are remarkable. Whether the marginally higher values of the 1930s reflect a genuine difference or a constant, small analytical bias is not clear. Reasonable arguments could be made either way: separating interferences from the true analytical signal would have been challenging in the 1930s. Conversely Fan et al. ( Reference Fan, Zhao and Fairweather-Tait 9 ) have reported from contemporary analysis of stored wheat samples that there has been a significant decrease in the Fe content of wheat in the 1960s as discussed above (see Introduction), which perhaps is true for other plant-based foods. However, whatever the reason(s), the differences are small (for example, 0·14 mg Fe/100 g for fruits overall and 0·09 mg Fe/100 g for vegetables overall for the 1930s v. 2000s in the context of a mean Fe content of 0·79 mg Fe/100 g and 0·76 mg Fe/100 g for those food groups, respectively). These differences are unlikely to convert to marked differences in total daily Fe intake from these sources and will be more than compensated for by the use of fortificant Fe in more recent years. In fact, between the 1980s and 2000s it is noteworthy how Fe fortification of breakfast cereals appears to have generally increased (Fig. 4).
Thus, when examining in greater detail the issue of food Fe content between different years or eras, there is, as yet, no real suggestion that, quantitatively, dietary Fe exposure is falling over time as some authors have suggested( Reference White and Broadley 2 – Reference Davis, Epp and Riordan 5 ). However, first, in our analysis we have been unable to compare Fe content of cereal and cereal products between the three different eras and this food group is the major source of dietary non-haem Fe. Second, there may be reasons to question whether, qualitatively, current dietary Fe intakes are satisfactory given this potentially increasing reliance upon fortificant Fe. For example, in 2003, Henderson et al. estimated that fortified breakfast cereals alone contributed about 20 % of daily Fe intake in the NDNS 19- to 64-year-old adults( Reference Henderson, Gregory and Swan 15 ). Fortificant Fe is used in a number of different chemical forms with variable bioavailability( Reference Zhu, Glahn and Nelson 17 , Reference Hurrell, Lynch and Bothwell 18 ). Current UK legislation does not consider this nor does it set a maximum value for fortification but, rather, an implied minimum value for bread and flour in terms of ‘restoration’ of the Fe that was removed during the milling process (the Fe content of flour should be at least 1·65 mg/100 g flour)( 19 ). Recently the observations that fortificant Fe may negatively affect the bacterial flora( Reference Zimmermann, Chassard and Rohner 20 ) and, at least in certain populations, affect colonic health( Reference Werner, Wagner and Martinez 21 , Reference Werner, Hoermannsperger and Schuemann 22 ), argue that better consideration should be given to (a) maximum dietary levels of fortificant Fe and (b) the chemical form of the fortificant Fe. Nonetheless, the present study only addresses the Fe content of plant-based foods. Both bioavailability, influenced, for example, by ascorbic acid-rich foods, and the addition of haem Fe to the omnivorous diet would alter the quantity of bioavailable Fe and the quality (i.e. chemical speciation and reactivity) of the Fe.
Finally, we note that when considering dietary Fe intakes, and especially fortificant Fe exposure, it would be advisable to have up-to-date values for the food Fe content.
Acknowledgements
This project made use of samples collected from an original UK Food Standards Agency (FSA)-funded project to determine silicon levels of plant-based foods in the UK diet( Reference Powell, McNaughton and Jugdaohsingh 10 ). Sample analyses were performed in the Gastrointestinal Laboratory, The Rayne Institute, St Thomas' Hospital, King's College London, UK. The authors would like to thank Mark Sykes, from the above-named institute, for his contribution in the sample preparation and analysis. The present study was supported by the Medical Research Council, Unit Programme no. U105960399. J. J. P. and R. J. designed the study, T. P. E. C. and R. J. conducted the study, C. W. T. contributed all content in relation to the NDNS and contributed to the preparation of the other data for the manuscript, S. F. A. B. analysed the data, performed statistical analysis with the assistance of A. O. and prepared the manuscript. S. F. A. B. and J. J. P. had primary responsibility for the final content. There are no conflicts of interest.








