Clinical studies on probiotics have traditionally focused mostly on gastrointestinal infections, and therefore the role of the gut mucosal immune system in understanding the mechanisms on how probiotics evoke their beneficial effects has been of greatest relevance. However, in recent years, clinical data on the role of probiotics in respiratory infections have accumulated(1). Therefore, the need to investigate the possible mechanisms of probiotics in the oropharyngeal mucosa has emerged.
For the probiotic to be able to affect both non-immune defence and the mucosal immune system, adhesion on the mucosal surfaces is a precondition. At the time the present trial was designed, only one non-randomised and non-controlled pilot study had examined the oropharyngeal adherence of a Lactobacillus strain administrated orally in a food product. The trial showed that in the six studied subjects, L. plantarum DSM 9843 was able to adhere to the surface of human tonsils for up to 4 h after intake in fermented oatmeal gruel in all subjects(Reference Stjernquist-Desatnik, Warfving and Johansson2). However, it was found that the strain remained for 8 h in only one of the volunteers. More recently, another small (n 19) non-randomised and non-controlled trial evaluated the ability of a probiotic to colonise in the nasopharynx(Reference Power, Burton and Chilcott3). It was found that after a 10 d intervention with Streptococcus salivarius K12 preparation, only one of the nasopharyngeal swab samples was culture-positive for the studied strain, and three of the seven adenoid samples taken were K12 culture-positive.
The present trial was set up to investigate whether extended oral consumption of a probiotic would lead to recovery of the probiotic strain in the oropharynx. For the probiotic, we chose L. rhamnosus GG (GG), as its adherence to intestinal gut mucosa has been demonstrated in clinical trials(Reference Alander, Satokari and Korpela4). In terms of adherence in the oral cavity, only indirect measures (saliva samples) have been used to study the effect of GG on the oral biofilm(Reference Haukioja, Yli-Knuuttila and Loimaranta5). The study indicated that individual variation in the adherence capacity exists and that the oral persistence of GG is only temporary. GG has also been studied in several clinical trials on respiratory tract infections, both as a single strain(Reference Hatakka, Savilahti and Ponka6–Reference Hojsak, Snovak and Abdovic8) and as a part of a multispecies probiotic combination(Reference Hatakka, Blomgren and Pohjavuori9–Reference Rautava, Salminen and Isolauri11).
We chose to study the recovery of GG in the palatine tonsil samples, as they are a major part of the Waldeyer's ring, the lymphoid tissue ring strategically located in the naso-oropharynx to perform regional immune functions. The Waldeyer's ring plays an important immune-inductive role, yet it has similar structures to lymph nodes and it may act as an effector organ in local systemic-type and mucosal-type adaptive immunity(Reference Brandtzaeg12). Because a relationship between bacterial colonisation of tonsillar fossa and post-tonsillectomy bleeding has been found(Reference Stephens, Georgalas and Kyi13), it is of importance to also evaluate the safety associated with possible adherence of GG in the tonsil tissue.
Subjects and methods
Study design
In the present randomised, double-blind and placebo-controlled intervention study, volunteers were randomly assigned to one of the three study groups: probiotic group receiving GG as a single strain (GG group), probiotic group receiving GG as a part of a multispecies combination (multispecies group) or placebo group. The study consisted of a 1-week run-in, a 3-week intervention and a 2-week follow-up period. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the local ethics committee of the Helsinki University Central Hospital. Written informed consent was obtained from all subjects.
Subjects
Patients aged 18–30 years, who were on the waiting list for tonsillectomy due to chronic or recurrent tonsillitis in the department of Otorhinolaryngology and Head and Neck Surgery at the Helsinki University Central Hospital, were contacted between October 2007 and November 2008 on their willingness to participate in the study. Patients with diagnosed chronic gastrointestinal diseases, chronic sinusitis, allergy causing airway symptoms, alcohol or drug abuse, milk allergy, regular use of probiotic-containing products or antibiotics, or consumption of drugs associated with intestinal diseases, immunosuppressive drugs or inhaled asthma or allergy medications were excluded from the present study. Subjects were not allowed to participate in any other clinical trials during the present study, and they had to agree to follow the protocol.
Study procedures
A pre-study visit with the study nurse was organised for all subjects before they were enrolled in the study. On this visit, subjects were given information about the purpose and requirements of the study and written informed consents were obtained. For background information, data on, for example, medications, smoking and prior probiotic consumption were collected. The list of probiotic-containing products available in the market was given to subjects, and they were advised not to consume any of the listed products during the run-in and intervention periods. Otherwise, they were instructed to continue their normal diet and lifestyle throughout the study.
Subjects were given a structured study diary for the intervention and follow-up periods. During the intervention period, subjects were requested to fill out the diary with information on consumption of the study product, presence of any respiratory or gastrointestinal symptoms as well as consumption of any medication or prohibited probiotic products daily. During the follow-up period, the diary included questions on the presence of any post-operative symptoms (bleeding, pain or temperature ≥ 37·5°C). After the follow-up period, subjects were requested to mail the diary to the study nurse.
Study products were given to subjects in capsule form on the pre-study visit. Subjects were asked to consume the study products for 3 weeks prior to the scheduled tonsillectomy. Subjects were advised not to consume capsules as such. They were given one pot of yoghurt per d (175 g each), to which they were to mix the content of two capsules daily at breakfast. The last pot of yoghurt was to be consumed on the day preceding the operation. After the intervention, subjects returned the leftover capsules, which were counted as a measure of compliance.
Study products and randomisation
The single-strain probiotic used in the present study was GG (LGG®, ATCC 53 103; Valio Limited). The probiotic multispecies combination (LGG® Extra; Valio Limited) consisted of four bacterial strains, namely, GG (ATCC 53 103), L. rhamnosus LC705 (DSM 7061), Propionibacterium freudenreichii subsp. shermanii JS (DSM 7067) and Bifidobacterium animalis subsp. lactis Bb12 (DSM 15 954). The placebo products contained only hemicellulose. Amounts of probiotic strains per capsule are presented in Table 1.
Table 1 Amounts of individual probiotic strains per capsule at the time of production

GG, group receiving L. rhamnosus GG as a single strain; cfu, colony-forming units; Multispecies, group receiving L. rhamnosus GG as a part of a multispecies combination.
All capsules given to study subjects were identical in appearance. They were packed in bottles and consecutively numbered. Study numbers were randomly assigned to one of the three treatment groups following a computer-generated random number list prepared by a statistician who had no clinical involvement in the trial. After the study nurse at the hospital obtained the subject's consent, she gave the subject the next available study number. The study nurse outside the clinic, who had no other clinical involvement in the trial, provided the study nurse at the hospital with the products that the study number was assigned for in the randomisation. Everyone else in the study staff, study nurse at the hospital, investigators and statisticians, were kept blinded to the allocation of the study products.
Faecal, tonsil and blood sample collection
Faecal samples were collected at the end of the run-in period and at the end of the intervention period. Tonsil samples were collected during the tonsillectomy at the end of the intervention period. Two tonsils collected from each subject were divided into ten parts, placed in Eppendorf tubes and freezed in liquid N2. All samples were stored at − 70°C until analysis. Venous blood samples were drawn 4 h after the operation.
Faecal, tonsil and blood sample analysis
In faecal samples, a strain-specific real-time quantitative PCR assay was used to quantify GG according to a published method(Reference Ahlroos and Tynkkynen14). The frozen faecal samples were thawed, suspended in blender bags in the ratio 1:10 with 50 mm-EDTA and homogenised for 2 min with a stomacher blender (Seward). The suspension was diluted in the ratio 1:10 using 50 mm-EDTA (final faecal dilution 1:100) and 1 ml of the dilution was centrifuged at 14 000 g for 2 min. The collected cells were resuspended in 480 μl of 50 mm-EDTA, for which 100 μl of 50 mg/ml lysozyme (Amresco) and 20 μl of 50 U/μl (0·08 kat/l) mutanolysine were added (Sigma) and the mixture was incubated at 37°C for 1 h. The mixture was centrifuged for 2 min at 14 000 g, the supernatant was discarded and the cell pellet was extracted with a Wizard® Genomic DNA Purification Kit (Promega), according to the manufacturer's instructions. The purified DNA was suspended in 200 μl of Tris–EDTA buffer. When the DNA of the bacterial pure culture was needed, cells from 1 ml of the culture were collected and the DNA was isolated using the same method. The strain-specific detection limit for GG was 3·0 × 105 genome copies/g for the wet weight of faecal samples. Values under the detection limit were imputed by a value of detection limit divided by 2. This imputation results in a value of 5·18 on the log10 scale. In tonsil samples, tonsil aliquots of approximately 0·5 g were immersed in 480 μl of 50 m-EDTA followed by cell lysis according to the assay described earlier. Because of the impurities caused by the blood contained in the material, the samples were repurified with the Wizard® Genomic DNA Purification Kit (Promega) before PCR. The DNA dilutions were made up to 100 μg/ml and 50 ng were used to detect GG in real-time PCR. Quantitative analyses were not possible to perform because of the differences in the amount of tonsil surface and thus differences in the potential adhesion area of GG in each sample. Genomic DNA from pure culture GG was used as a standard in quantitative PCR. The blood samples drawn after the tonsillectomy were analysed for bacterial growth by using an accredited method in a clinically certified laboratory, the Laboratory of Helsinki University Central Hospital, Finland.
Outcome measures
The primary outcome measure in the present study was the recovery of GG from the tonsil tissue. The secondary outcome measures were faecal recovery of GG and its comparison with tonsil recovery. In addition, safety data (symptoms during intervention and follow-up periods from study diaries and post-operative complications from hospital patient records) were analysed.
Statistical analyses
The data are expressed as means and standard deviation or as medians with interquartile ranges. The comparisons between groups were made by the Fisher–Freeman–Halton test for dichotomous and ordinal level outcomes. For continuous variables, the Kruskal–Wallis test followed by the Dwass–Steel–Chritchlow–Flinger test for pairwise comparison were applied. Exact P values were calculated when appropriate. The comparison between tonsil and faecal recovery of GG at the end of the intervention was determined by the κ statistic and the Jaccard similarity index (Chamberlain's positive agreement). The level of agreement is considered to be poor with κ < 0·20, fair with κ = 0·21–0·40, moderate with κ = 0·41–0·60, substantial with κ = 0·61–0·80 and very good with κ>0·80. The 95 % CI for the κ statistic were obtained by bias-corrected bootstrapping. The Jaccard index was defined as a proportion in which positive agreement (both faecal and tonsil samples are positive) is divided by all positive findings in either of the samples. The 95 % CI for the Jaccard index were calculated by using the jackknife equation.
Results
Characteristics of subjects
A total of sixty-one subjects were randomised for the present trial and data from fifty-seven subjects were available for the analysis of the primary outcome (Fig. 1). Subjects had no diagnosed diseases and did not consume any regular medications that would be of relevance in the present study (Table 2).

Fig. 1 Flow diagram of the trial according to the Consolidated Standards of Reporting Trials statement(Reference Moher, Schulz and Altman27).
Table 2 Baseline characteristics of subjects (Mean values and standard deviations; number of subjects and percentages)

GG, group receiving Lactobacillus rhamnosus GG as a single strain; Multispecies, group receiving L. rhamnosus GG as a part of a multispecies combination.
Compliance
Of the fifty-seven subjects, four failed to return the leftover capsules, and therefore the capsules of fifty-three subjects were available for counting. Of those fifty-three subjects, forty-two (79 %) consumed at least 95 % of the advised amount of capsules during the intervention period. In terms of prohibited probiotic products, ten (10 %) out of forty subjects reported consumption of some of the listed probiotic products during the intervention, but all were one-time consumptions.
Recovery of Lactobacillus rhamnosus GG in tonsil and faecal samples
GG was recovered in the tonsil tissue of eight (40 %) of the twenty subjects in the GG group, seven (42 %) of the seventeen subjects in the multispecies group and six (30 %) of the twenty subjects in the placebo group (P value between the groups 0·79).
As secondary outcome measures, faecal recovery and its comparison with tonsil recovery of GG were assessed. Baseline faecal samples were collected from all the study subjects who completed the study (n 57). Of these fifty-seven subjects, six were unable to provide a faecal sample at the end of the intervention, which means that data from fifty-one subjects were available for analysis of the recovery of GG in faecal samples and its comparison with the tonsil recovery at the end of the intervention.
Of the forty-one subjects who had a positive faecal recovery of GG at the end of the intervention, seventeen (41 %) also demonstrated positive recovery of GG in the tonsil tissue. GG was not recovered from the tonsil tissue of any of the ten subjects with negative recovery of GG in faeces. Thus, the observed agreement was 53 % (twenty-seven out of fifty-one subjects). The κ statistic was 0·22 (95 % CI 0·10, 0·40) and the Jaccard index 0·41 (95 % CI 0·26, 0·57). Results on faecal and tonsil recovery of GG are presented in Table 3.
Table 3 Recovery of Lactobacillus rhamnosus GG after a 3-week GG intervention in faecal and tonsil samples (n 51)

GG, group receiving L. rhamnosus GG as a single strain; Multispecies, group receiving L. rhamnosus GG as a part of a multispecies combination.
In faecal samples, the concentration of GG increased in subjects from both probiotic groups during the 3-week intervention period, but remained unchanged in subjects in the placebo group (Fig. 2). The groups did not differ in terms of the faecal GG concentration at baseline (P= 0·38). At the end of the intervention, the difference between the groups in faecal concentration of GG was statistically significant (P< 0·001).
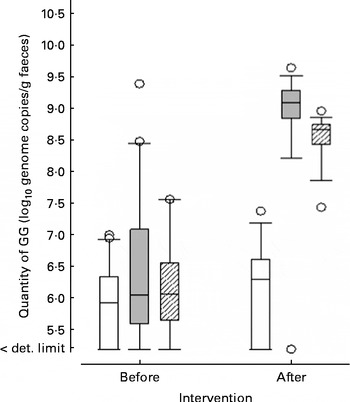
Fig. 2 The quantities (log10 genome copies/g) of Lactobacillus rhamnosus GG in the faecal samples taken before and after the 3-week intervention period in the control, single-strain probiotic (GG) and multispecies probiotic (multispecies) groups. Boxes show interquartile range with the median (square); whiskers are the 10th and 90th percentiles; and dots represent outliers. □, Placebo; ![]() , GG;
, GG; ![]() , multispecies. det. limit, Detection limit.
, multispecies. det. limit, Detection limit.
Safety
Data from forty (70 %) of the seventy-five study subjects were available for analysis of the post-operative symptoms as the rest of the subjects failed to return the study diary. Of these forty subjects, during the 3-week intervention period, ten (25 %) had respiratory symptoms and four (10 %) had gastrointestinal symptoms at least on day 1. There was no statistically significant difference between the groups in respiratory symptoms (3/13, 3/12 and 4/15 in the GG, multispecies and placebo groups, respectively; P= 0·99), nor in gastrointestinal symptoms (0/13, 1/12 and 3/15 in the GG, multispecies and placebo groups, respectively; P= 0·30); however, a statistically significant difference between the groups was found in the total number of subjects having a temperature ≥ 37·5°C post-operatively (2/13, 4/12 and 0/15 in the GG, multispecies and placebo groups, respectively; P= 0·036). During the 2-week follow-up period, thirty-nine subjects (98 %) experienced pain in the throat (12/13, 12/12 and 15/15 in the GG, multispecies and placebo groups, respectively; P= 0·62).
Looking again at the larger group of fifty-seven subjects, post-tonsillectomy bleeding requiring interventions in the hospital occurred in fourteen (25 %) subjects. There was no significant difference between the groups in terms of post-operative bleeding (5/20, 6/17 and 3/20 in the GG, multispecies and placebo groups, respectively; P= 0·32). Of these subjects, two experienced primary bleeding ( < 24 h after surgery) and thirteen secondary bleeding (>24 h after surgery), one subject experienced both. All venous blood cultures drawn after the tonsillectomy were negative for bacterial growth.
Discussion
In the present study, we found that GG was recovered in the tonsil tissue of 41 % of subjects who received GG as a single-strain probiotic or as a part of a multispecies probiotic combination. However, GG was also found in the tonsil tissue of 30 % of the subjects in the placebo group. In these subjects, GG was also recovered in the faecal sample taken at the beginning and at the end of the intervention, which indicates more persistent adherence of the probiotic.
Previous pilot studies on different probiotic strains have shown that the ability of the strains to be recovered in the naso-oropharynx might differ from one individual to another(Reference Stjernquist-Desatnik, Warfving and Johansson2, Reference Power, Burton and Chilcott3). This is consistent with the present findings, which showed that the recovery of the probiotic in the tonsil tissue was not achieved in all subjects receiving GG. In the present study, the lack of recovery of GG in the tonsil tissue in 59 % of subjects receiving the strain is not likely to be due to inadequate consumption of the study products, as the compliance was confirmed by analyses of the probiotic in the faecal samples as well as by counting the leftover capsules.
Previous studies have shown that tonsillar tissue contains pathogenic bacteria(Reference Jeong, Lee and Ryu15) and that during tonsillectomy transient bacteraemia may occur in as many as 25–27 % of patients via a breach in the oropharyngeal mucosa(Reference Kaygusuz, Gok and Yalcin16, Reference Yildirim, Okur and Ciragil17). In the present study, no bacteraemia occurred in the venous blood samples drawn 4 h after the tonsillectomy. Therefore, the present trial confirms the previous findings on the safe use of GG(Reference Kukkonen, Savilahti and Haahtela10, Reference Salminen, Tynkkynen and Rautelin18, Reference Manzoni, Lista and Gallo19). In the present study, all the six subjects having temperature were in the probiotic study groups. This might be a coincidence due to the small number of subjects, but this observation could also be linked to the fact that probiotic consumption has previously been shown to activate the host's immune response(Reference Walker20, Reference Oelschlaeger21).
We are aware of several limitations to the present study. First of all, our subjects represented only a small age group of adults, as all the subjects were aged between 18 and 30 years. Therefore, the results cannot be generalised to, for example, children, because there might be differences in the naso-oropharyngeal microbiota, which has been shown at least for pathogens(Reference Gaffney, Freeman and Walsh22, Reference Loganathan, Arumainathan and Raman23). In addition the studied subjects do not represent a healthy population as they were all due to undergo tonsillectomy because of recurrent or chronic tonsillitis, which can also lead to changes in the tonsillar microbiota(Reference Jeong, Lee and Ryu15). In theory, both these factors could also affect the adherence of a probiotic in the tonsillar tissue. Also, the study product dosage and possible protocol deviations present limitations in the present study. The study product was given to subjects in capsules, which they were advised to stir into a pot of yoghurt before consumption. Even though we measured compliance by counting the leftover capsules and by analysing GG in faecal samples, we cannot rule out the possibility that subjects might have consumed the capsules as such. This would have made it impossible for the probiotic to adhere to tonsil tissue. Also, we found several positive faecal and tonsil recoveries of the probiotic in the placebo group at the end of the intervention. We did control the use of products containing probiotics by giving the subjects a list of products to avoid during the run-in and intervention periods and advising them to report any deviations from this guidance. Only one subject with a positive faecal but negative tonsil recovery in the placebo group reported one-time use of a GG-containing product. It is possible that the colonisation in subjects with positive faecal recovery in the placebo group might have persisted throughout the 3-week study period preceding the end-of-intervention tonsil and faecal sample collection. This hypothesis is consistent with a study by Saxelin et al. (Reference Saxelin, Lassig and Karjalainen24), which found that in 28 % of subjects, GG was recovered from the faecal sample at the end of the 3-week follow-up period. A similar recovery rate of GG has been demonstrated in other clinical trials also. In a study by Kumpu et al. (Reference Kumpu, Kekkonen and Kautiainen25), GG was recovered from the faecal sample of 34 % of subjects after the 2–3-week run-in period. Previously, a mean baseline level of 5·18 log10 colony-forming units of GG per g was reported following a 4-week run-in period(Reference Kekkonen, Ahlroos and Suomalainen26). Despite similar recovery rates found in other trials, we do acknowledge that the longer run-in period in the present study might have decreased the number of subjects in the placebo group with GG recovered.
To summarise, GG can be recovered from tonsil tissue when administrated as a single-strain probiotic or as a part of a multispecies probiotic combination. However, it seems that individual variation exists in the adherence of the probiotic to the tonsil tissue. We encourage future studies that would combine tonsil sample collection with longer intervention trials including respiratory symptom data collection. These studies would offer an opportunity to assess if the adherence of the probiotic in the oropharynx is linked to the clinical effect on respiratory symptoms. Future clinical trials on probiotics should also include a run-in period of at least 3 weeks in order to avoid high persistence of the probiotic affecting the results.
Acknowledgements
First of all, we would like to thank the volunteers who made the present study possible. We would also like to thank the study nurses Tiina Puttonen and Maarit Tuomisto for their invaluable assistance in the practical arrangements of the study, and Jaana Oksanen for her help with the faecal and tonsil sample analysis. The present study was funded by Valio Limited, and supported by grants from the Päivikki and Sakari Sohlberg Foundation, as well as the special governmental subsidy for health sciences research in Finland. R. K. and A. P. initiated, designed, coordinated and supervised the study. K. H. and R. A. K. designed and coordinated the study. S. T. was responsible for the GG analyses and S. J. for the statistical analyses in the study. M. K. and E. S. participated in the coordination of the study, interpreted the results and wrote the first version of the manuscript. All authors read and approved the final version of the manuscript. Conflicts of interest are as follows: M. K., S. T., K. H. and R. A. K. are employees of Valio Limited. R. K. was employed by Valio at the time of the intervention.







