The increasing prevalence of obesity and obesity-related disorders such as type 2 diabetes has become the greatest health problem of the present and coming decades(Reference Zimmet, Alberti and Shaw1). According to the physiological state where abnormalities in glucose (GLUC) metabolism are present but below the cut-off point for the diagnosis of type 2 diabetes, individuals can be grouped into those who suffer from (1) impaired fasting GLUC or (2) impaired GLUC tolerance (IGT). Individuals with isolated IGT show moderate to severe muscle insulin resistance and suffer from a defect in both the early- and late-phase insulin secretory response to an oral GLUC load. Patients with IGT have a 2- to 5-fold greater risk of developing CVD, compared with age-matched normoglycaemic controls(2). Each year, about 10 % of the subjects with impaired fasting GLUC and IGT progress to develop type 2 diabetes(Reference Knowler, Barrett-Connor and Fowler3). Lifestyle intervention, directed towards a healthy diet, i.e. a reduction in saturated fat intake and an increase in low-glycaemic carbohydrate (CHO) intake, and an increase in habitual physical activity level, has proven effective in preventing or delaying the onset of type 2 diabetes in subjects with IGT(Reference Unwin, Shaw and Zimmet4, Reference Roumen, Corpeleijn and Feskens5). Interventions to reduce the glycaemic index (GI) and glycaemic load of the daily diet have received much interest in nutritional research(Reference Brand-Miller6, Reference Livesey7). So far, numerous studies have reported that diets low in GI or glycaemic load can have beneficial effects on weight loss and/or reduce the risk of developing chronic metabolic disease in human subjects(Reference Brand-Miller6, Reference Ludwig8–Reference Larsen, Dalskov and van Baak10). Whereas some have suggested that diets high in CHO may have an adverse effect on TAG concentrations and HDL-cholesterol(Reference Chong, Fielding and Frayn11), others have failed to confirm those findings. The apparent discrepancy between studies is probably attributed to differences in the duration of the intervention, sex and the use of different types of sugars between studies(Reference Swanson, Laine and Thomas12–Reference Bossetti, Kocher and Moranz14).
It has been hypothesised that low-GI foods may affect body-weight control and insulin sensitivity by promoting satiety and stimulating fat oxidation at the expense of CHO oxidation(Reference Brand-Miller, Holt and Pawlak15). This increased fat oxidation may reduce fat storage in adipose and non-adipose tissues, thereby promoting insulin sensitivity and an improved metabolic profile. Indeed, animal studies have shown that a reduced GI can shift substrate use in favour of fat oxidation, independent of diet-induced changes in body composition or energy intake(Reference Pawlak, Kushner and Ludwig16–Reference Isken, Klaus and Petzke18). We recently showed that a reduced glycaemic response after a mixed meal containing trehalose (TRE) or isomaltulose (IMU) may improve fat oxidation rates at the expense of CHO oxidation in overweight subjects(Reference van Can, Ijzerman and van Loon19, Reference van Can, Ijzerman and van Loon20). Similar findings(Reference Stevenson, Thelwall and Thomas21) have also been observed during exercise conditions.
So far, it is not known whether these beneficial effects on fat oxidation also extend to impaired GLUC-tolerant subjects who show profound disturbances in the capacity to utilise fat as a substrate source during basal fasting conditions as well as in the capacity to switch between CHO and fat oxidation during postprandial conditions(Reference Corpeleijn, Mensink and Kooi22). The fact that disturbances in fatty acid uptake and oxidation are already present in the pre-diabetic state suggests a key role in the progression towards type 2 diabetes(Reference Mensink, Blaak and van Baak23). Consequently, more work is warranted to assess the impact of low-GI CHO on postprandial substrate use in an obese group with IGT. Therefore, we examined the metabolic response to the ingestion of two slowly digestible CHO sources, TRE and IMU, respectively. TRE is a GLUC disaccharide with an α-1,1 glycoside linkage, whereas IMU is a disaccharide produced by an enzymatic conversion of sucrose (SUC).
We hypothesised that the ingestion of TRE and IMU will be accompanied by a lower glycaemic and/or insulinaemic response, an attenuated inhibition of postprandial lipolysis and fat oxidation rate and a lower plasma TAG response when compared with GLUC and SUC, respectively.
Methods
Subjects
A total of ten overweight men (n 6) and women (n 4), of which two were post-menopausal, with IGT were recruited for the present study. Subjects’ characteristics are presented in Table 1. Subjects with type 2 diabetes and/or overt cardiovascular complications, and those using medication for digestive disorders were excluded from the study. All subjects were screened with a standard 75 g oral glucose tolerance test after an overnight fast. IGT was diagnosed based on the WHO criteria. The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Medical Ethical Committee of the Maastricht University Medical Centre. All subjects gave written informed consent.
Table 1 Subjects’ characteristics
(Mean values and standard deviations)

HOMA-IR; homeostasis model assessment-insulin resistance; HbA1c, glycated Hb; ALAT, alanine transferase.
*To convert insulin from μU/ml to pmol/l, multiply by 6.
Study design
Each subject participated in four trials, separated by a 1-week washout period, in which the metabolic response was measured after ingestion of four different CHO drinks. CHO drinks were ingested after an overnight fast (breakfast drink) and in combination with a standardised mixed meal (lunch). The CHO drinks (GLUC, TRE, SUC and IMU) were provided in a single-blind, randomised order.
Protocol
At the beginning of the experimental day, after an overnight fast, a cannula was inserted into an antecubital vein. The CHO load consisted of 75 g CHO equivalents and was dissolved in 400 ml of water. The CHO drink was consumed after an overnight fast at breakfast (08.45 hours) or in combination with a mixed meal at lunch (12.30 hours) within a period of 15 min. Energy expenditure and substrate utilisation were measured, before and for 3 h after ingestion of the meal and/or drink using a ventilated hood system (Omnical)(Reference Adriaens, Schoffelen and Westerterp24). Gas analyses, recorded every minute, were performed by dual paramagnetic O2 analysers and dual IR CO2 analysers (type 1156, 1507, 1520; Servomex), similar to the analysis system described by Schoffelen et al. (Reference Schoffelen, Westerterp and Saris25). Blood samples were taken before consumption of the meal/drinks (t = − 5 min) and then at t = 30, 60, 90, 120, 150 and 180 min after CHO ingestion to determine circulating metabolites and hormone concentrations. Expired breath samples were collected each hour to determine 13CO2 enrichment. Energy expenditure and substrate use were calculated using the formulas of Weir(Reference Weir26) and Frayn(Reference Frayn27).
Lunch had a total energy content equivalent of 50 % of calculated 24 h resting energy expenditure based upon the formula of Harris & Benedict(Reference Harris and Benedict28). Lunch macronutrient composition represented 55 En% CHO, 30 En% fat and 15 En% protein; 25 En% of the total energy content of the meal was provided in the form of a beverage containing either TRE, IMU, GLUC or SUC.
Test products
Trehalose
TRE is a disaccharide of GLUC with an α-1,1 glycoside linkage. It is a non-reducing sugar that is naturally present in honey, bread, mushrooms and fermented drinks. For our experiment, 13C-enriched TRE was produced by enzymatic conversion using maize starch as the base material. In the human intestine, TRE is exclusively digested by epithelial trehalase into two d-GLUC molecules, which are subsequently absorbed and metabolised(Reference Richards, Krakowka and Dexter29, Reference Dahlqvist30). Apart from the trehalase action, it appears that ingestion, hydrolysis, absorption and metabolism of TRE are essentially identical to all other digestible disaccharides(Reference Richards, Krakowka and Dexter29).
Isomaltulose
IMU is a disaccharide produced by an enzymatic conversion of SUC, whereby the 1,2-glycosidic linkage between GLUC and fructose is rearranged to a 1,6-glycosidic linkage. For our experiment, 13C-enriched IMU was produced by enzymatic conversion using cane sugar as the base material. The sucrase–isomaltase complex located on the brush-border membrane of the small-intestinal epithelial cells hydrolyses both IMU and SUC. The resulting monosaccharides, GLUC and fructose, are taken up into the portal blood(Reference Lina, Jonker and Kozianowski31).
Biochemical analyses
At all time points, 8 ml blood were collected in pre-chilled tubes with 200 μl of 0·2 m-EDTA (Sigma). After collection, blood samples were centrifuged immediately at 4°C for 10 min at 1000 g and frozen at − 80°C until further analysis. Plasma was used for the enzymatic colorimetric quantification of NEFA (NEFA C kit; Wako Chemicals) and TAG (Sigma) on a COBAS FARA centrifugal spectrophotometer (Roche Diagnostica). Plasma GLUC concentration (ABX Diagnostics) was measured enzymatically on a COBAS MIRA automated spectrophotometer (Roche Diagnostica). Plasma insulin was measured with a double antibody RIA (Linco Research). Breath samples were analysed for 13C:12C ratio by GC–isotope ratio MS (Finnigan MAT 252; Finnigan), as described in van Can et al. (Reference van Can, Ijzerman and van Loon19, Reference van Can, Ijzerman and van Loon20).
Statistics
A computerised statistics program (SPSS 15 for Windows; SPSS, Inc.) was used to perform all calculations. All data are expressed as means with their standard errors. The total response of parameters after CHO ingestion was expressed as the incremental area under the curve (iAUC) and calculated by the trapezoid method. Response is defined in the Results section as iAUC, unless mentioned otherwise. Differences between responses to GLUC v. TRE and SUC v. IMU were analysed by means of Student's paired t test. Student's paired t test was used to compare differences in peak response between the different CHO. The four CHO were not compared with each other due to the fact that they are made out of different CHO sources. Therefore, TRE is compared with GLUC and IMU compared with SUC.
Results
Circulating metabolites
Glucose response
Ingestion of TRE resulted in lower peak GLUC concentrations when compared with GLUC both during breakfast drinks (P < 0·01) and lunch (P = 0·001) (Fig. 1(a)). This did, however, not result in a significant difference in glycaemic response, expressed as iAUC (Table 2). GLUC peaks were lower after ingestion of IMU compared with SUC during breakfast (P = 0·01) and lunch (P = 0·001) (Fig. 1(b)). There was a reduced incremental glycaemic response after the ingestion of IMU when combined with a mixed meal (P < 0·001; Table 3).
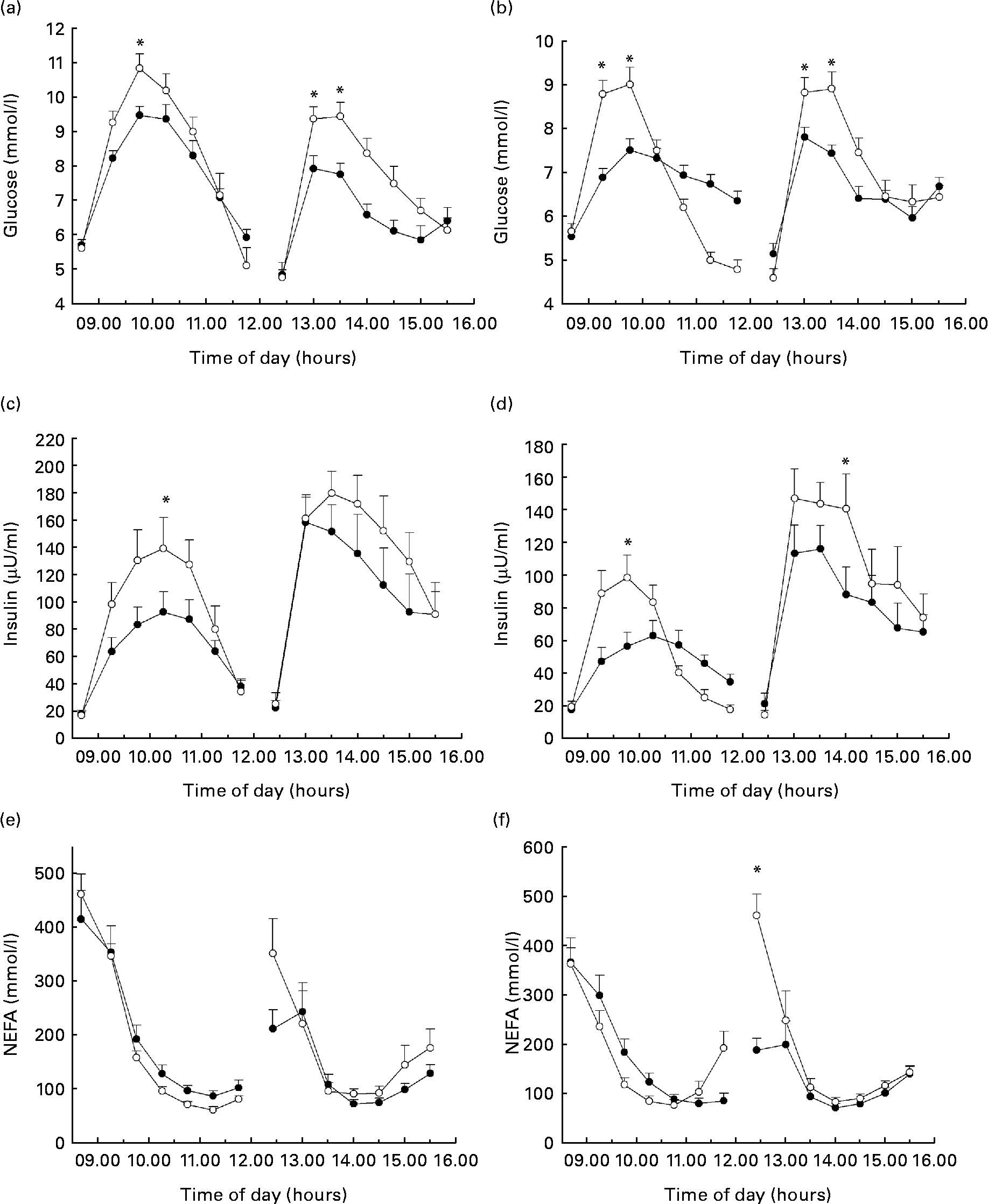
Fig. 1 Time course of the glycaemic response after the intake of (a) trehalose (TRE, ![]() ) v. glucose (GLUC,
) v. glucose (GLUC, ![]() ) and (b) isomaltulose (IMU,
) and (b) isomaltulose (IMU, ![]() ) v. sucrose (SUC,
) v. sucrose (SUC, ![]() ). Time course of the insulinaemic response after the intake of (c) TRE v. GLUC and (d) IMU v. SUC. To convert insulin from μU/ml to pmol/l, multiply by 6. Time course of NEFA concentrations after the intake of (e) TRE v. GLUC and (f) IMU v. SUC. Values are means, with standard errors of the mean represented by vertical bars (n 10). *Mean values were significantly different (P < 0·05).
). Time course of the insulinaemic response after the intake of (c) TRE v. GLUC and (d) IMU v. SUC. To convert insulin from μU/ml to pmol/l, multiply by 6. Time course of NEFA concentrations after the intake of (e) TRE v. GLUC and (f) IMU v. SUC. Values are means, with standard errors of the mean represented by vertical bars (n 10). *Mean values were significantly different (P < 0·05).
Table 2 Metabolic responses, expressed as change in area under the curve (iAUC), after ingestion of trehalose and glucose

Mean value was significantly different from that of glucose: ** P < 0·01.
† To convert insulin from μU/ml to pmol/l, multiply by 6.
Table 3 Metabolic responses, expressed as change in area under the curve (iAUC), after ingestion of isomaltulose and sucrose

Mean values were significantly different from those of sucrose: * P < 0·05, ** P < 0·01.
† To convert insulin from μU/ml to pmol/l, multiply by 6.
Insulin response
TRE resulted in lower peak insulin concentrations when compared with GLUC following breakfast (P = 0·003) and lunch (P = 0·025; Fig. 1(c)). The iAUC was lower after the ingestion of TRE compared with GLUC during breakfast (P = 0·009) but not when TRE was ingested with a mixed meal during lunch (Table 2). Insulin responses were reduced after the ingestion of IMU compared with SUC following breakfast (iAUC, P < 0·05) and lunch (iAUC, P = 0·001) (Fig. 1(d); Table 3).
NEFA response
As expected, plasma NEFA concentrations decreased after CHO ingestion. Ingestion of either TRE or GLUC resulted in a similar NEFA response pattern, also when ingested in combination with a mixed meal (Fig. 1(e)). There were no significant differences in the integrated decrement between TRE and GLUC (Table 2). Ingestion of IMU in combination with a mixed meal during lunch resulted in a less inhibition of the decline in plasma NEFA concentrations when compared with SUC (P < 0·0001; Fig. 1(f); Table 3).
TAG response
TAG concentrations increased after the ingestion of the different CHO drinks and when the drinks were ingested in combination with a mixed meal. There were no differences in incremental TAG AUC after the ingestion of TRE compared with GLUC during breakfast and lunch (Fig. 2(a); Table 2). There was a trend towards a lower iAUC when IMU was ingested in combination with a mixed meal (P = 0·06; Fig. 2(b); Table 3).

Fig. 2 Time course of TAG concentrations after the intake of (a) trehalose (TRE, ![]() ) v. glucose (GLUC,
) v. glucose (GLUC, ![]() ) and (b) isomaltulose (IMU,
) and (b) isomaltulose (IMU, ![]() ) v. sucrose (SUC,
) v. sucrose (SUC, ![]() ). Time course of fat oxidation after the intake of (c) TRE v. GLUC and (d) IMU v. SUC. Time course of carbohydrate (CHO) oxidation after the intake of (e) TRE v. GLUC and (f) IMU v. SUC. Values are means, with standard errors of the mean represented by vertical bars (n 10). *Mean values were significantly different (P < 0·05).
). Time course of fat oxidation after the intake of (c) TRE v. GLUC and (d) IMU v. SUC. Time course of carbohydrate (CHO) oxidation after the intake of (e) TRE v. GLUC and (f) IMU v. SUC. Values are means, with standard errors of the mean represented by vertical bars (n 10). *Mean values were significantly different (P < 0·05).
Thermogenesis and substrate oxidation
There were no differences in the thermogenic response between the CHO drinks during breakfast or when ingested with a mixed meal (Tables 2 and 3).
There were no differences in the iAUC of the respiratory quotient after TRE ingestion compared with GLUC during breakfast and lunch (Table 2). Intake of IMU did not result in differences in respiratory quotient response compared with SUC during breakfast, whereas IMU ingested in combination with a mixed meal resulted in a reduced respiratory quotient response compared with SUC (P = 0·034; Table 3).
There were no significant differences in the decrement in fat oxidation rates between TRE and GLUC during breakfast and lunch (Fig. 2(c); Table 2). Fat oxidation did not differ between IMU and SUC during breakfast; interestingly, fat oxidation was significantly less suppressed after IMU when compared with SUC following lunch (P < 0·05; Fig. 2(d); Table 3).
There were no significant differences in CHO oxidation between TRE and GLUC during breakfast and lunch (Fig. 2(e), Table 2). Intake of IMU did not result in significant differences following breakfast when compared with SUC, whereas the increment in CHO oxidation was lower after the ingestion of IMU when compared with SUC during lunch (P = 0·036; Fig. 2(f); Table 3).
No differences were observed in the minimal estimates of exogenous CHO oxidation rates between the experiments. The mean percentage of the enriched CHO oxidised, as calculated by the recovery of 13CO2 in the expired breath, was 11 % for TRE, 12 % for GLUC, 15 % for IMU and 19 % for SUC, respectively.
Discussion
Substrate utilisation
The main finding of the present study is that intake of IMU in combination with a mixed meal resulted in an attenuated rise in postprandial plasma GLUC and insulin concentrations and a lesser inhibition of circulating NEFA concentration and fat oxidation compared with SUC ingestion. The reduced inhibition of postprandial fat oxidation could be attributed to a greater supply of NEFA to the fat-oxidising tissue, secondary to a reduced insulin-mediated suppression of lipolysis(Reference Wolever and Mehling32). The present results seem consistent with other work, highlighting the stimulating effects of IMU ingestion on postprandial fat oxidation and/or lipid deposition when compared with SUC, in rats, healthy and overweight subjects(Reference van Can, Ijzerman and van Loon19, Reference Sato, Arai and Mizuno33, Reference Arai, Mizuno and Sakuma34). The present study shows that IMU ingestion in exchange for SUC has beneficial effects in subjects with IGT and, as such, may help to prevent the progression into type 2 diabetes.
The attenuated postprandial decline in fat oxidation induced by the ingestion of IMU may have implications for body-weight control. Flatt(Reference Flatt35) proposed that subjects who continue to oxidise CHO in the post-absorptive state deplete their endogenous glycogen stores, thereby stimulating food intake. Through this mechanism, inter-individual differences in substrate selection may play a key role in the development of obesity. A lower decrement in circulating NEFA and fat oxidation following the ingestion of more slowly digestible CHO may favour fat oxidation above storage, resulting in less fat accumulation in non-adipose tissues with a favourable effect on insulin sensitivity by preventing late hypoglycaemia and the accompanying increase in plasma NEFA concentrations(Reference Jenkins, Wolever and Ocana36). High NEFA concentrations may be linked with insulin resistance and CVD by increasing muscle ectopic fat promoting lipotoxicity, which may reduce insulin action(Reference Bays, Mandarino and DeFronzo37).
Glycaemic and insulinaemic responses
The attenuated glycaemic and insulinaemic responses following TRE and IMU ingestion are attributed to the slower rates at which TRE and IMU are digested and absorbed. Several studies have shown that the absorption rates of TRE and IMU are slower than GLUC and SUC, respectively(Reference Dahlqvist and Thomson38, Reference Dahlqvist, Auricchio and Semenza39). TRE as well as IMU are absorbed and tolerated well in human subjects(Reference Richards, Krakowka and Dexter29, Reference Lina, Jonker and Kozianowski31). Reduced GLUC and insulin concentrations after the intake of TRE or IMU have been observed in trained athletes, healthy subjects, as well as in overweight subjects(Reference van Can, Ijzerman and van Loon19, Reference van Can, Ijzerman and van Loon20, Reference Kawai, Okuda and Yamashita40, Reference Jentjens and Jeukendrup41). The present study is the first to show that intake of TRE and IMU attenuated the postprandial rise in plasma GLUC and insulin concentrations in subjects with IGT. Although there were no significant differences in the integrated glycaemic responses following the ingestion of different CHO after an overnight fast (breakfast), there was a clearly attenuated rise in peak plasma GLUC concentration after the ingestion of IMU compared with SUC and after the ingestion of TRE compared with GLUC (see Fig. 1).
Postprandial TAG concentration
High plasma TAG concentrations are considered to be risk factors for the development of CVD(Reference Sparks and Sparks42). Low-glycaemic, low-insulinaemic CHO sources may be used to attenuate the postprandial rise in TAG concentrations. However, data show no consensus regarding higher postprandial TAG concentrations following the ingestion of fructose(Reference Bouche, Rizkalla and Luo43, Reference Teff, Elliott and Tschop44). In the present study, we observed a trend towards reduced TAG concentrations with ingestion of IMU in combination with a mixed meal compared with SUC ingestion, whereas no such differences were observed for TRE. In contrast, in healthy, overweight subjects, TRE resulted in reduced TAG concentrations during breakfast(Reference van Can, Ijzerman and van Loon20). This discrepancy could be explained by the higher age of the subjects in the present study. Animal as well as human studies generally observed more pronounced effects in younger subjects(Reference Isken, Weickert and Tschop45, Reference van Dam, Visscher and Feskens46).
A limitation of the present study is that the number of subjects is rather small. We cannot rule out sex differences, although the cross-over design limits inter-individual variation. The set-up of the study provides a proof of principle on the impact of TRE and IMU in the breakfast setting and under more physiological conditions where the drink is consumed in combination with a mixed meal. Further studies are warranted to investigate the overall response and physiological significance of the observed differences.
In conclusion, ingestion of TRE and IMU results in an attenuated postprandial rise in plasma GLUC and insulin concentrations when compared with GLU and SUC, respectively. Co-ingestion of IMU with a mixed meal resulted in an attenuated decline in plasma NEFA concentrations and postprandial fat oxidation rate when compared with SUC, which may reduce ectopic fat accumulation and improve insulin sensitivity. Thus, exchanging SUC for IMU may be favourable to prevent metabolic disturbances, thereby potentially slowing down the progression to type 2 diabetes. More studies are needed to determine the long-term effects of exchanging rapid for more slowly digestible sugars on body-weight control and the prevention of type 2 diabetes in subjects with IGT.
Acknowledgements
This study was supported by an unrestricted research grant from Cargill R&D Center Europe, Vilvoorde, Belgium. The authors thank Jos Stegen for expert technical assistance. E. E. B. and F. B. designed the experiment. J. G. P. v. C. executed the experiment, collected the data and wrote the manuscript. L. J. C. v. L. and F. B. read the manuscript and contributed to the discussion. E. E. B. supervised the project, read the manuscript and contributed to the discussion. None of the authors had any financial or personal interest in any company or organisation sponsoring the research.







