Long-chain (LC) n-3 PUFA play an important role in the human diet as they have been implicated in the prevention of CVD(Reference Harris1), chronic inflammatory disorders(Reference Calder2) and mental health conditions(Reference Assisi, Banzi and Buonocore3). This group of fatty acids comprises EPA (20 : 5n-3), docosapentaenoic acid (DPA; 22 : 5n-3) and DHA (22 : 6n-3), which are obtained primarily from fish and fish oil. However, it has been assumed that the physiological requirement for LC n-3 PUFA can be satisfied by the consumption of plant foods containing their precursor, the shorter-chain (18 : 3n-3) fatty acid, α-linolenic acid. However, dietary recommendations have recently been revised in Australia(4) to reflect the importance of including LC n-3 PUFA in addition to α-linolenic acid in our diet. This change in policy acknowledges that conversion of α-linolenic acid through to the LC n-3 PUFA in humans is limited(Reference Arterburn, Hall and Oken5–Reference Plourde and Cunnane7) and therefore dietary intakes of both α-linolenic acid and LC n-3 PUFA are desirable.
While the primary source of LC n-3 PUFA is seafood, meat and other foods also contribute to LC n-3 PUFA intake(Reference Howe, Meyer, Record and Baghurst8, Reference Meyer, Tsivis, Howe, Tapsell and Calvert9). Indeed in Australia and other societies where meat consumption greatly exceeds the consumption of fish, the former may represent a major source of dietary LC n-3 PUFA intake(Reference Howe, Meyer, Record and Baghurst8, Reference Howe, Buckley and Meyer10). However, alternative sources may be produced by enriching processed foods with LC n-3 PUFA(Reference Howe, Downing, Grenyer, Grigonis-Deane and Bryden11–Reference Murphy, Meyer and Mori13). Increasing the LC n-3 PUFA content of food may adversely affect its sensory qualities, due to the susceptibility of n-3 PUFA to oxidation(Reference Kouba, Enser, Whittington, Nute and Wood14, Reference Lyberg, Fasoli and Adlercreutz15). It is widely accepted that even minimal n-3 PUFA oxidation can result in a profoundly fishy odour and flavour, reducing consumer acceptability of the food.
Meat and eggs can be enriched with LC n-3 PUFA by feeding appropriate sources to single-stomached animals (pigs, chickens) which can efficiently take up and incorporate LC n-3 PUFA into TAG stores in adipose tissue and in skeletal muscle phospholipids. The latter are more resistant to oxidation than NEFA or TAG(Reference Lyberg, Fasoli and Adlercreutz15) and therefore less susceptible to tainting. Using a fortified tuna fishmeal as a source of LC n-3 PUFA, predominantly DHA, Howe and colleagues have refined the production of premium-quality DHA-rich chicken and pork(Reference Howe, Downing, Grenyer, Grigonis-Deane and Bryden11, Reference Howe16) and we are now seeking to demonstrate the potential health benefits of regularly consuming these alternative sources of LC n-3 PUFA.
The aim of the present study was to determine whether regular consumption of n-3-enriched pork for 12 weeks could deliver demonstrable health benefits. Fish oil supplementation has been shown repeatedly to increase the content of LC n-3 PUFA in erythrocytes and reduce fasting blood TAG and inhibit serum thromboxane production, all of which are recognised biomarkers of cardiovascular health status(Reference Grimsgaard, Bonaa, Hansen and Nordoy17–Reference Howe, Clifton and James21). The levels of LC n-3 PUFA in the fish oil supplements used to demonstrate these benefits have generally exceeded that which could be acquired from regular consumption of foods; for example, we recently reported that >1 g LC n-3 PUFA per d was required to lower TAG(Reference Milte, Coates, Buckley, Hill and Howe22) while Howe et al. (Reference Howe, Clifton and James21) reported reduction of thromboxane production with higher doses of fish oil. However, studies with n-3-enriched eggs have reported anti-platelet and TAG-lowering effects with modest LC n-3 PUFA intakes(Reference Lewis, Seburg and Flanagan23). Hence we sought to demonstrate such benefits with DHA-rich pork products.
Experimental methods
Participants
Healthy men and women aged 18–65 years who enjoyed eating pork were recruited to participate in a dietary intervention trial. Subjects were excluded if they ate fish or seafood more than once per week, were taking fish-oil capsules, or taking blood-thinning or lipid-modifying medication. All subjects provided informed written consent and all procedures were approved by the Human Research Ethics Committees of both the University of Adelaide and the University of South Australia.
Preparation of n-3-enriched pork
Twenty-four female pigs (Large white × Landrace) were randomly allocated to one of two dietary treatments fed ad libitum for 6 weeks before slaughter containing either a standard finisher diet (control) or an identical diet supplemented with 15 % PorcOmega® (Bartlett Grain, Sydney, NSW, Australia) as reported previously(Reference Sioutis, Coates, Buckley, Murphy, Channon and Howe24). Regular pork and n-3-enriched pork were trimmed of all visible fat, and processed into five different varieties (steak, stir-fry, diced, mince, and sausage). Pork was then packaged into 200 g servings by a local butcher (Standom Smallgoods, Hendon, SA, Australia) and stored at − 20°C until the day of consumption.
Dietary trial
Thirty-three participants were enrolled in a 12-week, parallel, double-blind, placebo-controlled, dietary intervention trial and randomised to consume either regular pork (control group) or n-3-enriched pork (n-3 group). Twenty-nine subjects completed the 12-week intervention study while three subjects withdrew due to personal reasons unrelated to the study and one subject was excluded from analysis due to a high LC n-3 PUFA intake at baseline. Block randomisation ensured groups were matched for baseline BMI, age, and sex (Table 1). Each week, participants consumed a 200 g serving of each of the five different varieties of pork (1000 g/week) and were instructed to limit all fish and seafood consumption to no more than one serving per fortnight. Subjects were instructed to substitute the pork varieties into their regular diet to maintain energy intake and also to keep a log of consumption indicating which day each variety had been consumed to monitor compliance. Apart from introducing the pork into their diets, volunteers were requested not to change their usual dietary or physical activity habits.
Table 1 Baseline characteristics of subjects*
(Mean values with their standard errors)
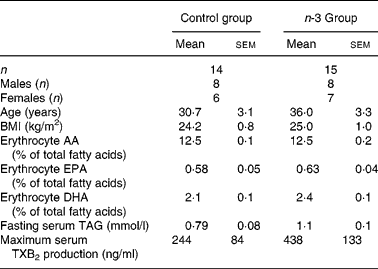
AA, arachidonic acid; TXB2, thromboxane B2.
* There were no significant differences in any parameters.
Blood collection and anthropometry
Subjects attended the Nutritional Physiology Research Centre at the University of South Australia (May–August 2006) on three occasions at baseline (3 d over 1 week) and then on a fortnightly basis to have fasting blood samples collected via venepuncture, to have anthropometric measures taken and to collect pork products and recipe ideas.
Following a 10 h fast, blood was collected from an antecubital vein of the forearm into vacutainer tubes containing serum-separating gel (serum TAG analysis) or EDTA (erythrocyte membrane fatty acid analysis), and kept on ice until centrifuged (4000 rpm; 10 min; 4°C). For thromboxane B2 (TXB2) analysis, blood was collected into vacutainer tubes containing no additive and then incubated at 37°C for 60 min to maximally stimulate the release of thromboxane before centrifugation (4000 rpm; 10 min; 4°C). Serum TAG levels were collected fortnightly while erythrocyte membrane fatty acids and TXB2 levels were measured every 4 weeks (weeks 0, 4, 8, 12). All samples were stored at − 80°C until completion of the study so samples could be analysed in a single batch.
Height was measured to the nearest 0·1 cm with volunteers barefoot using a wall-mounted stadiometer (SECA; Vogel & Halke, Hamburg, Germany). Weight was measured to the nearest 0·1 kg with participants wearing light clothing using the TANITA Ultimate Scale 2000 (Tanita Corporation, Tokyo, Japan). BMI was then calculated as weight (kg) divided by height (m2).
Laboratory methods
Erythrocyte membrane fatty acid composition
EDTA blood was centrifuged (4000 rpm; 10 min; 4°C) and the supernatant fraction discarded. Erythrocytes were washed with 0·9 % isotonic saline and isolated from solution by a second centrifugation. Packed erythrocytes were frozen (24 h; − 20°C), followed by storage at − 80°C until later analysis. Samples were later thawed and mixed with 10 ml 2-amino-2-hydroxymethyl-propane-1,3-diol–EDTA (10:1, v/v) to cause lysis. Ultracentrifugation (48 000 rpm; 30 min; 4°C) in a Beckman Optima LE-80K Preparative Ultracentrifuge (Beckman Instruments, Fullerton, CA, USA) formed an erythrocyte pellet, which was dissolved into 300 μl water and 2 ml methanol–toluene (4:1, v/v). and the fatty acids were transesterified according to the method of Lepage & Roy(Reference Lepage and Roy25). The upper toluene phase containing the fatty acid methyl esters was removed and analysed by flame ionisation gas chromatography (model GC-20A; Shimadzu, Kyoto, Japan). Individual fatty acids were identified by comparison with known standards (NuChek Prep Inc., Elysian, MN, USA).
Serum TAG analysis
Serum samples were thawed and TAG concentrations determined spectrophotometrically using a Konelab 20XTi automated sample analyser (Thermo Electron, Melbourne, Vic, Australia) by standard enzymic methods. Analysis required Infinity Triglyceride reagent TR22421, Trace DMA Triglyceride internal standard TR22923, and lipid controls TR40001 and TR41001 (Thermo Electron).
Serum thromboxane B2
Samples were thawed and analysed on a commercially available TXB2 competitive express enzyme immunoassay kit (Cayman Chemical Company, Ann Arbor, MI, USA). The concentration of TXB2 in serum was calculated spectrophotometrically against a standard. TXB2 is the stable metabolite of thromboxane A2 (TXA2) and represents production of the latter.
Data analysis
Data analyses were conducted using SPSS software (version 15; SPSS, Inc., Chicago, IL, USA). Random-effects mixed-model analysis was performed to determine changes over time in the two treatment groups. This analysis makes efficient use of all available data(Reference Cnaan, Laird and Slasor26). Linear regression was used to correlate the changes in serum TAG and TXB2 levels with the changes in erythrocyte membrane fatty acids. All data are presented as mean values with their standard errors. Statistical significance was set at P ≤ 0·05.
Results
Baseline characteristics of the subjects allocated to the n-3 group and the control group are shown in Table 1. The groups were matched on age, sex and BMI and there were no significant differences in any of the measured biomarkers. There were no significant changes in weight or BMI over the 12 weeks (data not shown).
Self-reported records of pork consumption showed that compliance was high in both groups with a mean of 100 % in the control group and 98 % in the n-3 group. The composition of the pork has been previously described in detail(Reference Sioutis, Coates, Buckley, Murphy, Channon and Howe24). All of the meat provided was lean and contained less than 5 % saturated fat. The LC n-3 PUFA levels in the n-3-enriched pork were 3·1 times higher in steak, 2·9 times higher in stir-fry, 3·9 times higher in diced, 6·1 times higher in mice and 4·8 times higher in sausage than in regular pork. The participants consumed 200 g portions of each of the five cuts per week so that subjects in the n-3 group received 1·3 g LC n-3 PUFA per week from the pork, equating to an average daily intake of 185 mg/d compared with 41 mg/d for those eating the control pork. Consumption of the n-3 and control pork contributed similarly to arachidonic acid (AA) intake (86 (sem 20) and 82 (sem 10) mg/d, respectively).
Fig. 1 shows the resultant changes in selected LC PUFA in erythrocyte membranes (expressed as percentage total fatty acids) at 4-weekly intervals over the 12-week study. Regular consumption of pork resulted in increased AA levels but the increase was attenuated in the group consuming the LC n-3 PUFA-enriched pork (12·5 (sem 0·2) to 12·6 (sem 0·2) %) compared with the controls (12·5 (sem 0·1) to 13·0 (sem 0·1) %) (significant group × time interaction; P < 0·01). There was a significant group × time interaction for DHA content (P < 0·001). After the 12-week dietary intervention erythrocyte membrane DHA levels increased in the n-3 group by 0·6 %, compared with a decrease of 0·2 % in the control group. There were no significant changes in EPA over the intervention in either group. However, there was a significant group × time effect for the DHA:AA ratio (n-3 group 17·1 (sem 3·5) % v. control group − 8·1 (sem 2·0) %; P < 0·0001) and a significant group × time interaction of the LC n-3 PUFA:AA ratio (P < 0·001) with a 9 (sem 3) % increase in the n-3 group and a 6 (sem 1) % decrease in the control group. There was also a group × time effect (P < 0·0001) for the combined levels of EPA and DHA with an increase seen after 12 weeks in the n-3 group from 4·5 (sem 0·3) % to 5·1 (sem 0·2) % and a decrease in the control group from 4·6 (sem 0·2) % to 4·4 (sem 0·2) %.

Fig. 1 Changes in fatty acid content of erythrocytes (% total fatty acids) from subjects in eating control pork (–○–) or n-3-enriched pork (- -●- -) over 12 weeks. Values are means, with standard errors represented by vertical bars. (a) Arachidonic acid (AA); (b) EPA; (c) DHA. For AA, there was a significant group × time interaction (P < 0·01); for DHA there was a significant group × time interaction (P < 0·001).
Changes in fasting serum TAG levels are shown in Fig. 2. After 12 weeks, there was a significant reduction in serum TAG in the n-3 group compared with the change seen in the control group ( − 0·3 (sem 0·1) v. 0·0 (sem 0·1) mmol/l, respectively; P < 0·05). No relationship was detected between the change in erythrocyte membrane DHA content and the change in serum TAG.
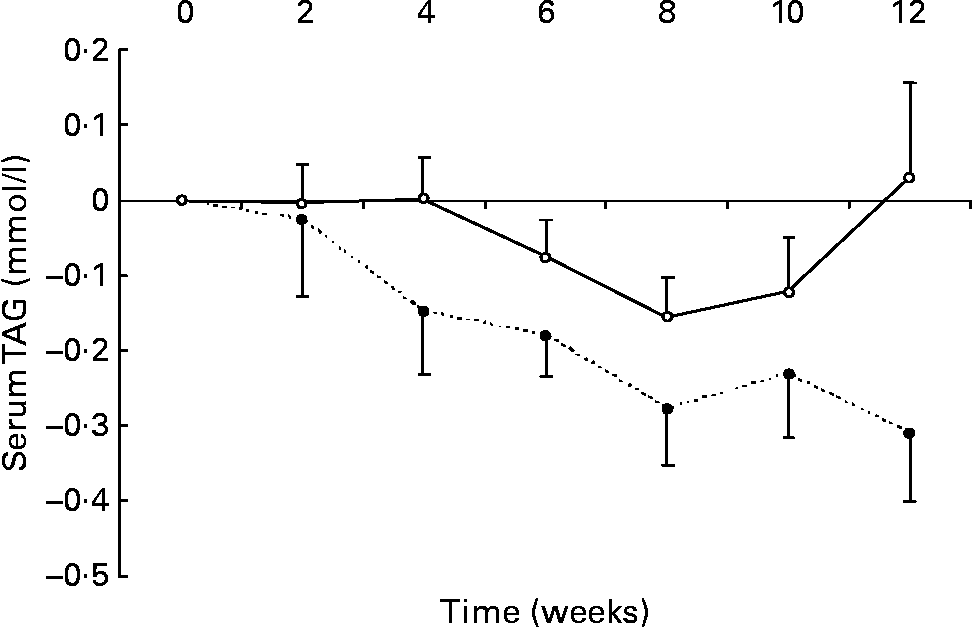
Fig. 2 Changes in serum TAG in healthy subjects eating regular pork (n 14) (–○–) or n-3-enriched pork (n 15) (- -●- -) for 12 weeks. Values are means, with standard errors represented by vertical bars. There was a significant group × time interaction (P = 0·02).
There was a significant group × time interaction for maximally stimulated serum TXB2 production such that there was a smaller increase in the n-3 group (438 (sem 133) to 550 (sem 91) ng/ml) than in the control group (244 (sem 84) to 674 (sem 141) ng/ml) over the 12 weeks (P < 0·01) (Fig. 3). At 8 weeks there was a significant inverse correlation between the change in thromboxane and the change in DHA erythrocyte content (r − 0·453; P < 0·05).
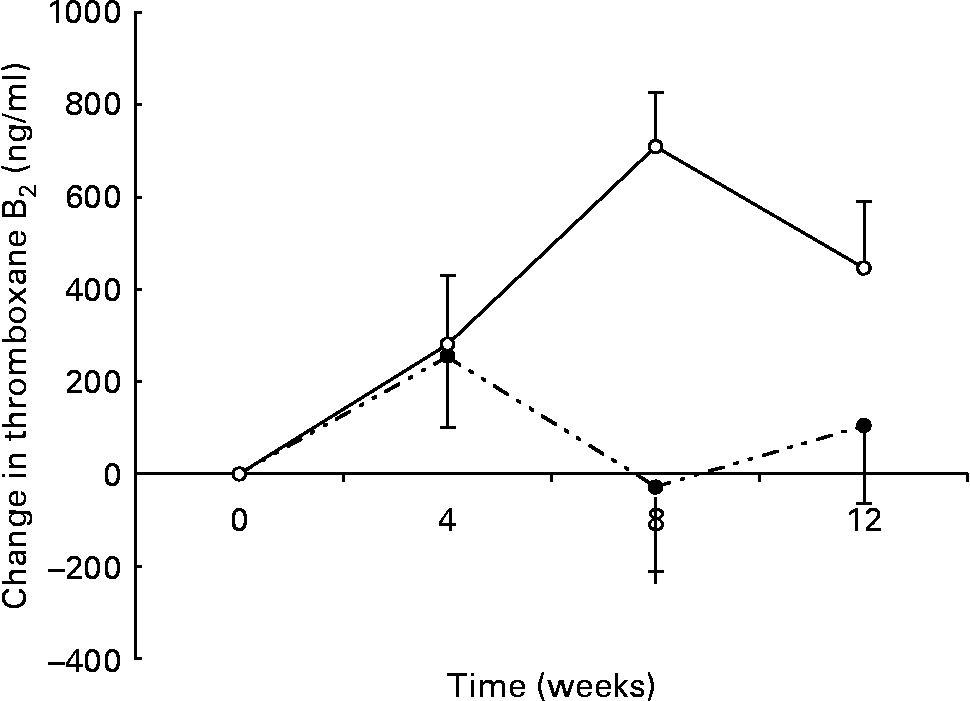
Fig. 3 Changes in serum thromboxane in healthy subjects eating regular pork (n 14) (–○–) or n-3-enriched pork (n 15) (- -●- -) for 12 weeks. Values are means, with standard errors represented by vertical bars. Random-effects mixed-model analysis revealed significant differences in the slopes of the treatment groups (group × time effect; P < 0·01).
Discussion
The present study has shown for the first time that regular consumption of n-3-enriched pork can significantly elevate the n-3 content of erythrocytes, an independent negative risk factor for CVD, and can decrease serum TAG and thromboxane production in healthy subjects. Health authorities in Australia(4) and elsewhere(27) are recommending intakes of LC n-3 PUFA of approximately 500 mg/d for optimal health. The current dietary intakes of LC n-3 PUFA (EPA+DPA+DHA) in Australia (140 mg/d for men and 90 mg/d for women) are well below the levels recommended for the prevention of CVD risk factors (610 mg/d for men, 430 mg/d for women)(4). Whilst fish and seafood are the primary sources of these beneficial fatty acids, there are various reasons why people do not eat these foods, including fish availability, affordability, taste preference, and the concern for environmental toxins(Reference Gebauer, Psota, Harris and Kris-Etherton28).
The n-3-enriched pork used in the present study delivered between 58 and 226 mg/100 g serve such that when volunteers consumed a variety of cuts they received 1·3 g LC n-3 PUFA per week. There are several n-3-enriched common foods in the Australian marketplace which deliver between 33 mg and 150 mg/serve through microencapsulation. One of the limitations of this technique is the amount of enrichment that can be achieved before tainting is detected. One of the benefits of using the fishmeal product PorcOmega® to enrich pork is that the LC n-3 PUFA become incorporated into membranes and are stabilised such that we have previously shown that sensory profiles of pork are not affected when PorcOmega® is incorporated up to 15 % in the finisher diet(Reference Howe, Downing, Grenyer, Grigonis-Deane and Bryden11). Consumer demand for n-3-enriched food products will continue to drive the need for research and development of other common foods as novel food sources of EPA and DHA. If the marketplace can provide a greater range of foods enriched with LC n-3 PUFA then it will assist to increase population intakes of EPA+DHA to levels corresponding with health benefits, primarily cardiovascular. Previous studies have shown that n-3-enriched foods can increase the percentage of LC n-3 PUFA incorporated into erythrocyte membranes. Payet et al. (Reference Payet, Esmail, Polichetti, Le Brun, Adjemout, Donnarel, Portugal and Pieroni29) demonstrated a significant increase (2·7 %) in erythrocyte membrane DHA of elderly patients after 3 months of regular consumption of n-3-fortified egg-yolk powder. Similarly Murphy et al. (Reference Murphy, Meyer and Mori13) tested a range of novel foods which together delivered 1·0 g EPA+DHA per d and found that the increased incorporation of LC n-3 PUFA was associated with improvements in some markers of cardiovascular health over 6 months.
Harris & von Schacky(Reference Harris and von Schacky30) have defined an Omega-3 Index as the sum of EPA+DHA (% total fatty acids) contained within phospholipids of erythrocyte membranes. Evidence suggests that the LC n-3 PUFA content of erythrocytes is a strong and independent risk factor for mortality from CHD. An index ≤ 4 % is associated with the highest risk of mortality, whilst an index ≥ 8 % represents the greatest cardioprotection(Reference Harris and von Schacky30). In their study, Harris & von Shacky report that this highest level of protection can be achieved with as little at 0·5 g EPA+DHA per d over 20 weeks in healthy subjects. In the present study, consumption of n-3-enriched pork for 12 weeks was able to increase the EPA+DHA content of erythrocytes to 5·1 v. 4·4 % with regular pork. According to the Omega-3 Index this should afford a greater level of cardiovascular protection for those subjects in the n-3 group. Future studies should investigate whether longer-term consumption of the enriched pork continues to increase the LC n-3 PUFA content of erythrocytes.
Dietary EPA and DHA supplementation has consistently been shown to have favourable effects on circulating TAG levels and TXA2 production, both of which are biomarkers of CVD risk. Dietary supplementation with fish oils containing varying amounts of EPA and DHA have repeatedly demonstrated a TAG-lowering effect in both normolipidaemic(Reference Grimsgaard, Bonaa, Hansen and Nordoy17) and hypertriacylglycerolaemic participants(Reference Mori, Burke, Puddey, Watts, O'Neal, Best and Beilin18, Reference Nestel, Shige, Pomeroy, Cehun, Abbey and Raederstorff19). Early studies with EPA-rich fish oil suggested that EPA was the LC n-3 PUFA primarily responsible for conferring cardiovascular protection(Reference Miller, Heath, Choraria, Shephard, Gajendragadkar, Harcus, Batson, Smith and Saynor31). More recently, however, DHA has been implicated as equally if not more effective for cardioprotection(Reference Mori, Burke, Puddey, Watts, O'Neal, Best and Beilin18, Reference Howe, Clifton and James21, Reference McLennan, Howe, Abeywardena, Muggli, Raederstorff, Mano, Rayner and Head32).
The American Heart Association recommends 1000 mg EPA+DHA daily for cardioprotection and up to 4 g/d for management of hypertriacylglycerolaemia or for patients with coronary artery disease(Reference Kris-Etherton, Harris and Appel33). However, more modest intakes can achieve a reduction in TAG. We recently reported that 3 g tuna fish oil per d (containing 780 mg DHA and 180 mg EPA) was effective for lowering TAG(Reference Milte, Coates, Buckley, Hill and Howe22). Moreover, Geppert et al. (Reference Geppert, Kraft, Demmelmair and Koletzko34) demonstrated a reduction in circulating TAG levels with 940 mg LC n-3 PUFA per d in healthy subjects and Visioli et al. (Reference Visioli, Rise, Plasmati, Pazzucconi, Sirtori and Galli35) reported a reduction in TAG with as little as 300 mg EPA+DHA per d in milk. In the present study a supplementary intake of approximately 185 mg LC n-3 PUFA per d (predominantly DHA), delivered primarily as phospholipids in meat, reduced TAG by 0·3 (sem 0·1) mmol/l. While the importance of such a reduction in terms of protection against CVD risk is unclear, particularly given that TAG were not elevated in the population studied, the present findings indicate that a low intake of LC n-3 PUFA delivered in the form of enriched foods (i.e. pork) can reduce circulating TAG levels. Future prospective studies should investigate the long-term impact of sustained low-level intakes of LC n-3 PUFA, achieved through the consumption of enriched foods.
The anti-thrombotic effects of fish oil are explained via an inhibition of platelet TXA2 and changes to clotting mechanisms. Early in vivo human studies reported significant reductions in TXA2 production following fish oil treatment(Reference Weber20). More recently Woodman et al. (Reference Woodman, Mori, Burke, Puddey, Barden, Watts and Beilin36) compared high doses of DHA and EPA supplementation (4 g/d) on TXA2 production and found reduced platelet aggregation as indicated by a reduction in thromboxane levels with DHA only whilst others have shown equal effects of EPA and DHA(Reference Howe, Clifton and James21). DHA may be more potent than EPA in reducing the formation of TXA2(Reference Akiba, Murata, Kitatani and Sato37) by inhibiting thromboxane synthase(Reference Abeywardena, McLennan and Charnock38) and there is also evidence that DHA and EPA act as antagonists at the TXA2 receptor in human platelets(Reference Swann, Venton and Le Breton39). In the present study, there was an increase in TXB2 (TXA2 production) on eating regular pork, which may be attributable to the increased incorporation of AA (direct precursor of TXA2) into cell membranes. Consumption of n-3-enriched pork attenuated this increase in TXA2 over time. The change in TXA2 production at 8 weeks was inversely related to the change in DHA in erythrocytes. This is consistent with a study by Mann et al. (Reference Mann, Sinclair, Pille, Johnson, Warrick, Reder and Lorenz40) showing that meat consumption did not alter either TXB2 or PGI2 production but a diet rich in fish reduced platelet TXB2 production without affecting PGI2 levels.
The results of the present study indicate that it is possible to deliver LC n-3 PUFA, particularly DHA, through regular consumption of n-3-enriched pork products. Such products offer useful alternatives to individuals with a low fish intake and who may not derive adequate amounts of these essential fatty acids in their diet. This single dietary modification has the potential to impact favourably on CVD risk factors, even in a healthy population.
Acknowledgements
The present study was supported by an Australian Research Council linkage grant (LP0561211) in partnership with Bartlett Grain Pty. Ltd. and Australian Pork Ltd. The authors thank Amanda Jager and Keren Kneebone for expert technical contributions and John Petkov for statistical advice.
None of the authors had a financial or personal conflict of interest.
S. S. contributed to study design and conception, recruitment, data collection, data entry, data analysis, data interpretation and manuscript preparation. A. M. C. contributed to study design and conception, data collection, data analysis, data interpretation and manuscript preparation. J. D. B. contributed to study design and conception, data analysis, data interpretation, sourcing funding support and manuscript preparation. P. R. C. H. contributed to study design and conception, sourcing funding support, data interpretation, manuscript preparation and project management.






