Flavonoids are polyphenolic compounds with a C6–C3–C6 backbone and are an integral part of the human diet, these compounds are widespread in plant-derived foods such as vegetables, fruits, seeds and tea. Flavonoids are generally present in food as the O-glycosides and can be divided into flavonols, flavones, flavanones, catechins (flavanols), anthocyanidins, isoflavones, dihydroflavonols and chalcones according to their different chemical structures(Reference Lwashina1). Within the subgroups of flavonols and flavones, the flavonol quercetin is the most frequently occurring compound in foods. Also common are kaempferol, isorhamnetin and the flavones apigenin and luteolin.
Recently, increasing attention has been paid to flavonoids because of their association with a wide range of different biological activities such as anti-bacterial, anti-inflammatory, anti-thrombotic, vasodilatory and anti-carcinogenic(Reference Middleton, Kandaswami and Theoharides2). However, their investigation in epidemiological studies is hampered by difficulties in exposure assessment. A number of epidemiological studies have been carried out on the health effects of some flavonoids (flavonols, flavones, flavanols and isoflavones) and lignans(Reference Arts and Hollman3), which largely relied on the accurate estimation of polyphenol intake or exposure. So far, the restricted amount of epidemiological evidence on the roles of flavonoids in human disease prevention has produced conflicting results. On the correlation of flavonoids (mainly flavonols) with CVD risk, some prospective studies in human subjects have shown an inverse association(Reference Geleijnse, Launer and Van der Kuip4, Reference Erdman, Carson and Kwik-Uribe5), whereas others have shown no association(Reference Knekt, Isotupa and Rissanen6, Reference Sesso, Gaziano and Liu7). Studies related to cancer have also produced contradictory results(Reference He, Sun and Pan8, Reference Goldbohm, Hertog, Brants, Armado, Andersson, Bardócz and Serra9). Difficulties in assessing the exact exposure level are among the most important reasons for this inconsistency.
Accurate assessment of the relationship between the ingestion of flavonoid compounds and human health requires a food composition database that provides quantitative information on specific compounds in specific foods to support clinical and epidemiological studies(Reference Beecher10). However, limited availability of food composition data and bias inherent in dietary assessment methods made the estimation of dietary flavonoid intake difficulty, which can confound the ability to infer epidemiological relationships regarding health and disease. To obtain further insight into the health effects of flavonoids, reliable biomarkers for the flavonoid intake are needed. The most common approaches to studying biomarkers are the measurement of the original compound or its metabolites in blood and urine(Reference Crews, Alink and Andersen11). In fact, these methods have been successfully tested in studies in which the human diet has been supplemented with high-flavonoid foods(Reference Erlund, Meririnne and Alfthan12, Reference Ito, Gonthier and Manach13) or isolated compounds(Reference Erlund, Kosonen and Alfthan14). However, few data are available concerning plasma flavonoid concentrations in subjects following their habitual diets. In the search for biomarkers applicable to epidemiological studies, a combination of a single biomarker measurement together with long-term dietary intake estimates is urgently needed.
Therefore, in the present study, we chose five flavonoids, quercetin, isorhamnetin, kaempferol, apigenin and luteolin, the most important representatives of the flavonols and flavones. The average dietary intake of the five flavonoids was estimated by means of 7-d dietary records in combination with a database providing the flavonoid content of food, which had been previously established by the method of Cao et al.(Reference Cao, Zhao and Wu15). Flavonoids in plasma were quantified by means of HPLC. We compared the flavonoid intake with the corresponding fasting plasma concentration. Furthermore, we determined plasma apigenin concentration after celery leaf consumption in the human dietary intervention study.
Materials and methods
Materials
All chemicals were analytical grade or chromatographic grade. The standards for kaempferol, quercetin, isorhamnetin, apigenin, luteolin and fisetin were obtained from Sigma (St Louis, MO, USA). Celery was purchased in a local supermarket.
Subjects and dietary assessment
Ninety-two students from the university campus were recruited. All subjects completed the study. The mean age of the subjects was 24·16 years with a range of 20–28 years, and the mean BMI was 21·31 kg/m2 with a range of 16·02–32·46 kg/m2. Inclusion criteria were as follows: being healthy (not using medicines, such as aspirin, anti-hypertension drugs and especially antibiotics); not suffering from metabolic diseases; not being pregnant or breastfeeding; not following a special diet. All subjects gave written informed consent after having been informed about the study aims and procedures. The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and the study protocol was approved by the ethical committee of Harbin Medical University.
Data on the flavonoid (quercetin, isorhamnetin, kaempferol, apigenin and luteolin) content in food were taken from our earlier study, the results of which are described in detail elsewhere (manuscript submitted). We used the method of Cao et al.(Reference Cao, Zhao and Wu15) to determine the content of selected flavonoids (quercetin, isorhamnetin, kaempferol, apigenin and luteolin) in 100 edible vegetables and fruits in Harbin, China. Thus, we have established a flavonoid (quercetin, isorhamnetin, kaempferol, apigenin and luteolin) database for Harbin, China. Regarding food outside our database, we referred to the data in the United States Department of Agriculture database. For the present evaluation, the food content of quercetin, isorhamnetin, kaempferol, apigenin and luteolin was investigated.
Nutritional data for the participants were collected by means of 7-d dietary records (7-d DR) using a picture booklet for the estimation of portion sizes. The participants were given instructions by an experienced nutritionist at the start of the protocol period. During the protocol phase, all subjects were recontacted at least once by telephone. The flavonoid intake was obtained by multiplying the food content of flavonoids by the daily consumption of food items. Individual flavonoid intake data were calculated as the mean over the 7-d period (7-d DR). Data were examined for possible recording errors by checking for very high and very low intakes.
Collection and preparation of blood samples
Fasting blood samples were obtained from each participant at the end of the protocol period. Venous blood samples were drawn into tubes containing EDTA and centrifuged (4000 rpm, 15 min). Plasma samples were obtained and stored at − 80°C until analysis.
A previously described methodology(Reference Zhao, Wu and Zhao16) was applied to the hydrolysis and extraction procedure of the selected flavonoid compounds. To a volume of 1 ml plasma, 110 μl sodium acetate (0·78 mol/l) and 100 μl ascorbic acid (0·1 mol/l) were added. About 100 μl fisetin (2 μg/ml) was added as an internal standard. After adding 232·5 U sulphatase and vortex mixing for 1 min, the mixture was incubated for 30 min at 37°C. The incubation was terminated by adding 20 μl phosphoric acid (85 %). The flavonoids were extracted using a Bond Elut C18 solid-phase extraction column (Oasis HLB, Waters Corp., Milford, MA, USA). The flavonoids of matrix interference were eluted with 1 ml methanol–2 % acetic acid aqueous solution (v/v, 5/95). The flavonoid compounds were eluted with 2 ml methanol and dried under vacuum. The residue was then resolved in 100 μl methanol/0·1 % phosphoric acid aqueous solution (3/2, v/v).
HPLC analysis
The plasma concentrations of quercetin, kaempferol, isorhamnetin, apigenin and luteolin were measured using a HPLC (Waters 600)(Reference Zhao, Wu and Zhao16). The HPLC system included a Waters 2996 diode array detector and a Waters 2465 electrochemical detector. For HPLC analysis, 30 μl of the final solution were injected onto a Hypersil C18 ODS column (250 mm × 4·6 mm, particle size 5 μm, Thermo Corporation) maintained at 40°C. The mobile phase consisted of two solvents (A/B, v/v, 3/2): A (distilled water adjusted to pH 2·25 with phosphoric acid) and B (methanol). The flow rate was 1 ml/min. We used isocratic elution. Chromatograms were monitored by the electrochemical detector at a voltage of 1100 mV. Direct-current voltage amperometric mode was used. For onward determination, UV spectra were produced using the diode array detector. The acquisition and processing of chromatography data were achieved using Waters' Empower software, and the substances were identified by comparison with the retention time of the flavonoid standards and by analysis of the spectra.
Mean recovery of flavonoid standards added to plasma (n 3) amounted to 104·25 % (quercetin), 97·31 % (kaempferol), 95·93 % (isorhamnetin), 97·26 % (luteolin) and 93·49 % (apigenin). The method showed good reproducibility with coefficients of variation of 6·84 % (quercetin), 6·01 % (kaempferol), 5·92 % (isorhamnetin), 5·77 % (luteolin) and 7·98 % (apigenin).
The dietary intervention study
Supplementary to the 7-d dietary investigation, we randomly chose twenty students (ten males and ten females) from the above subjects as the subsample for the dietary intervention study. These subjects were in the age range of 24–27 years, and the mean BMI was 20·81 kg/m2. The subjects were requested to adhere to an apigenin- and luteolin-free diet (because luteolin is the main metabolite of apigenin(Reference Nielsen, Breinholt and Justesen17)) for 3 d before the experimental day. Early in the morning on the experimental day, a fasting blood sample was collected and the subjects then consumed celery leaf at a dose of 2 g/kg bw. Afterwards, blood samples were collected at 4, 6, 7, 8, 9, 10, 11 and 28 h after celery leaf consumption, of these blood samples, the one collected at 28 h also served as the fasting blood sample (on the day after the experimental day). And at the same time, we gave the subjects bread for three meals on the experimental day in order to meet their basic metabolic needs. The blood samples were prepared and analysed using the same method as described above.
Statistical analysis
All analyses were performed with the statistics software SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA). Statistical significance was set at P < 0·05. All of the statistical tests were two tailed. The results are presented as means, standard deviation (sd), minimum (Min) and maximum (Max) and percentiles (10 % and 90 %). Deviation of the flavonoid intake estimates and plasma concentrations from normal distribution were tested by the Kolmogorov–Smirnov test. Non-parametric tests were used in the statistical analyses of the data. Spearman correlation was performed to analyse the associations of the dietary intakes with plasma concentrations. Regression analysis was applied for normally distributed data.
Results
The dietary flavonoid intakes (7-d DR) are shown in Table 1. The mean intake of the flavonols quercetin, kaempferol and isorhamnetin was 13·58, 14·97 and 12·31 mg/d, respectively. The average intake of the flavones apigenin and luteolin was 4·23 and 8·08 mg/d, respectively. A large range was found for the estimated individual mean flavonoid intake. The mean intake of quercetin, kaempferol, isorhamnetin, apigenin and luteolin ranged from 0–50·74, 0–70·48, 0–40·76, 0–17·95 and 0–33·13 mg/d, respectively. The single flavonoid intake in some subjects was zero. For all five flavonoids, the 10th percentile was above zero. For all subjects, the main sources of flavonols intake were apple (13 %), potato (8 %) and celery (7 %). The dietary flavones were mainly provided by celery (9 %) and eggplant (6 %).
Table 1 The mean 7-d dietary intake (mg/d) derived from 7-d dietary records and fasting plasma concentrations (nmol/l) of quercetin (Qu), kaempferol (Ka), isorhamnetin (Is), apigenin (Ap) and luteolin (Lu) in ninety-two students
(Mean, median, standard deviations, minimum and maximum values)

Fls, flavonols; Fes, flavones; Fds, flavonoids.
* Below the limit of detection (results below the limit of detection have been denoted by the value zero).
The plasma flavonoids were determined after enzymatic hydrolysis, and only aglycones were detectable in each of the plasma samples. The mean plasma concentrations of quercetin, kaempferol, isorhamnetin, apigenin and luteolin are shown in Table 1. The average values of quercetin, kaempferol, isorhamnetin, apigenin and luteolin were 80·23, 57·86, 39·94, 10·62 and 99·90 nmol/l, respectively. It should be noted that the plasma flavonoid concentrations below the limit of detection have been denoted as zero when calculating their mean values. On average, plasma concentrations below the limit of detection were measured for quercetin in eighteen participants (20 %), for kaempferol in thirty-six participants (39 %), for isorhamnetin in twenty participants (22 %), for apigenin in twenty-five participants (27 %) and for luteolin in thirty-two participants (35 %). In contrast, the maximum values suggested that comparably high plasma concentrations could be found for all of the five flavonoids.
For all five plasma flavonoids, spearman correlation analyses revealed statistically significant relationships between single and total flavonoid intakes and their corresponding fasting plasma concentrations (Table 2). For the results of the 7-d DR, correlation coefficients ranged from 0·33 (isorhamnetin) to 0·51 (quercetin; P < 0·05). Correlation coefficients for quercetin intake v. the sum of plasma flavonol concentrations, isorhamnetin intake v. the sum of plasma flavonol concentrations, apigenin intake v. the sum of plasma flavonoid concentrations and luteolin intake v. the sum of plasma flavonoid concentrations were 0·52 (P = 0·000), 0·39 (P = 0·000), 0·55 (P = 0·000) and 0·56 (P = 0·000), respectively. We found a weak correlation between the dietary intake of isorhamnetin and the fasting plasma flavonol concentrations.
Table 2 Correlation between the fasting plasma flavonoid concentrations (nmol/l) and the mean 7-d dietary flavonoid intake (mg/d) derived from 7-d dietary records obtained in ninety-two students
(Spearman correlation coefficients)
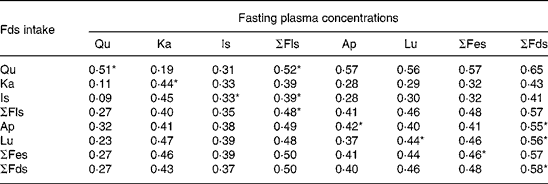
Qu, quercetin; Ka, kaempferol; Is, isorhamnetin; Ap, apigenin; Lu, luteolin; Fls, flavonols; Fes, flavones; Fds, flavonoids.
* P < 0·05 (two tailed).
With respect to the sum of flavonols and flavones, spearman correlation coefficients were within the range given for the individual flavonoid compounds and for the sum of flavonoids, excluding the range given for the single compounds. For the sum of flavonols, the relationship between the dietary intake and the plasma concentration is shown in Fig. 1, including the results of the regression analysis. The dietary intake of total flavonols showed a significantly positive correlation with the corresponding plasma concentration (r 0·48, P = 0·000), which indicated that individual variation in dietary flavonol intake contributed to the variation in the plasma flavonol concentrations. Similarly, for the sum of flavonoids, the association of the dietary intake with the plasma concentration is shown in Fig. 1. The dietary intake of total flavonoids was also positively associated with the corresponding plasma concentration (r 0·58, P = 0·000).

Fig. 1 Relationship between the dietary intake and the corresponding plasma concentration in ninety-two students, for the sum of flavonols (quercetin, kaempferol and isorhamnetin) (r 2 0·24, P = 0·000) and for the sum of flavonoids (quercetin, kaempferol, isorhamnetin, apigenin and luteolin) (r 2 0·36, P = 0·000; all data here fit a normal distribution).
In the intervention study, before the consumption of celery leaf, apigenin was not detected in fasting plasma samples of subjects. After eating celery leaf, the plasma apigenin concentration showed an upward trend (Table 3) and reached a maximum concentration at 7·70 ± 0·82 h. The area under the blood concentration time curve from 0 to 28 h was 89·02 (sd 40·57) min × μmol/l. For all participants, the plasma apigenin concentration fell within 28 h to below the limit of detection (2·32 nmol/l). A large range of variation (0–396·25 nmol/l) was found among the subjects for plasma apigenin concentrations determined between 0 and 28 h.
Table 3 Plasma apigenin concentration (nmol/l) in twenty students by time (h) after eating celery leaf
(Mean, median, standard deviations, minimum and maximum values)
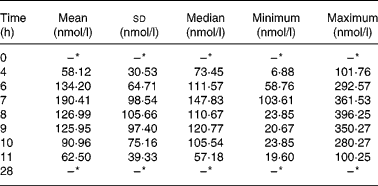
* Below the limit of detection.
Discussion
The HPLC analysis in the present study allowed the simultaneous quantification of five different flavonoids in plasma samples originating from subjects adhering to their habitual diet. Plasma samples collected at the end of the protocol period reflected the 7-d food registration, which may result in a reduction in the number of samples required and increase the strength of each analysis as it reflects an average consumption of flavonoids over 7 d instead of in a single day only, thus reducing any reporting errors or incomplete plasma collection.
In the present study, the range of the mean dietary flavonoid intake of the subjects was wide. The mean flavonol intake (28·55 mg/d for quercetin and kaempferol) was much higher than that reported previously (12·5 mg/d for quercetin and kaempferol(Reference Linseisen, Radtke and Wolfram18), 22·6 mg/d for flavonols and flavones(Reference Radtke, Linseisen and Wolfram19)). This suggests that the subjects consumed a high level of flavonoid-rich foodstuff. The inter-individual variation in intake of flavonols and flavones was comparably high, which reflected the variation in the subjects' diet. The dietary flavonoid intake strongly depends on food choice habits and the consumption of food, and the latter is influenced by the method of analysis of the flavonoids and factors affecting flavonoid content in food such as light, cultivar, growing location, environmental conditions, degree of ripeness and so on. Although data, such as the United States Department of Agriculture database for flavonoids, enable and facilitate the assessment of daily flavonoid intake(Reference Scalbert and Williamson20–Reference Peterson and Dwyer22), different populations in different countries consume different types of plant foods. Therefore, our previous laboratory detection of flavonoids using the method of Cao et al.(Reference Cao, Zhao and Wu15) provides the basis for the evaluation of the dietary flavonoid intake presented here. And it can be assumed that the calculated values are fairly good assessment of the true intake and comparable to other recent studies.
After polyphenol consumption, plasma concentrations varied widely according to the nature of the polyphenol and the food source. They were of the order of 0·3–0·75 μmol/l after consumption of 80–100 mg quercetin equivalent administered in the form of apples, onions or meals rich in plant products(Reference Graefe, Wittig and Mueller23–Reference Manach, Morand and Crespy25). Isoflavones are certainly the best absorbed flavonoids: plasma concentrations of 1·4–4 μmol/l were obtained between 6 and 8 h in adults who consumed relatively low quantities of soya derivatives supplying 50 mg isoflavones(Reference Xu, Wang and Murphy26–Reference Shelnutt, Cimino and Wiggins28). The situation for the diversity of flavonoids is similar at the absorption site, although the information has increased considerably in recent years(Reference Ader, Block and Pietzsch29). It is evident that the bioavailability of quercetin, and most likely of other flavonoids as well, is greatly affected by the type and binding site of the sugar moieties(Reference Hollman, Bijsman and van Gameren30). Flavonoid absorption rates are subject to considerable inter-individual variability(Reference Erlund, Meririnne and Alfthan31), which is illustrated in the present study by the large variation observed in plasma flavonoid concentrations.
A wide range of variation has been shown in the reported data on flavonoid concentrations in individual fasting plasma samples. To our knowledge, data on plasma quercetin concentration are mainly derived from studies in which rather large amounts of foods rich in quercetin were consumed. In the present study, fasting plasma concentrations of quercetin were found to be between 0 and 254·65 nmol/l as compared with the values of 0–142 nmol/l reported previously(Reference Manach, Morand and Crespy25, Reference Manach, Morand and Crespy32). The mean fasting plasma concentration of quercetin (80·23 nmol/l) was higher than that for kaempferol, isorhamnetin and apigenin, but lower than the value of luteolin, most likely as a consequence of the lower dietary quercetin intake. Although quercetin is widely distributed in plant species, it is generally present only in low concentrations, except for specific plant foods with very high quercetin content such as onions, cruciferous vegetables and some berries(Reference Kumpulainen, Lehtonen, Mattila, Kumpulainen and Salonen33). Endogenous metabolism resulting in configurational changes in the flavonoid aglycones has been reported, and the major proportion of the excreted flavonoids probably results from flavonoids absorbed from the diet. Isorhamnetin, however, may also result from endogenous metabolism of quercetin by catechol-O-methyltransferase(Reference Zhu, Ezell and Liehr34).
A potential limitation of the present study was that the samples of about one-fifth to one-third of the subjects contained non-detectable concentrations of individual flavonoids. However, this did not limit our ability to detect significant correlations between the intake and plasma concentrations. In the present study, statistically significant correlations between the mean intake (7-d DR) and the fasting plasma concentrations of quercetin (r 0·51), kaempferol (r 0·44) and the sum of flavonoids (r 0·58) were observed for the normal dietary intake. It has already reported in supplementation studies that the difference in the intake of quercetin and kaempferol can be distinguished by their concentrations in plasma and urine(Reference Nielsen, Kall and Justesen35). Linseisen et al. (Reference Radtke, Linseisen and Wolfram19) analysed the relationship between the mean flavonoid intake (7-d DR) and fasting plasma concentrations, and found statistically significant correlations between the mean intake and fasting plasma concentrations of quercetin (r 0·30) and kaempferol (r 0·46). Noroozi et al. (Reference Noroozi, Burns and Crozier36) quantified the relationship between the dietary quercetin intake and the plasma and urine concentrations in a study supplementing the usual diet with meals containing high levels of flavonols, and found statistically significant correlations between dietary quercetin intake and the quercetin concentration in plasma (r 0·74) and urine (r 0·81). Nielsen et al. (Reference Nielsen, Freese and Kleemola37) have reported significant correlations between the mean intake of fruits, berries and vegetables and the urinary concentration of flavonoids (r 0·308), and between the excretion of kaempferol and intake of tea (r 0·485). The difference in the degree of significance probably results from considerable individual variation in response to intake and other dietary factors influencing digestion and absorption.
In the intervention study, it was found that the plasma apigenin concentration of all subjects decreased to below the detection limit at 28 h after eating celery leaf. Meyer et al. (Reference Meyer, Bolarinwa and Wolfram38) have observed that after the ingestion of a bolus, the plasma apigenin concentration rose and fell within 28 h to below the limit of detection (2·3 nmol/l). Manach et al. (Reference Manach, Morand and Crespy25) reported that quercetin concentrations fell to basal levels at 20 h in the volunteers after the consumption of a complex meal rich in plant products. The present result demonstrates that the plasma concentrations of apigenin and quercetin only reflect short-term intake rather than the lifelong diet. Further investigation is required to determine whether after the ingestion of kaempferol, isorhamnetin and luteolin, their plasma concentrations fall to below the limit of detection within 28 h. Moreover, considering the dietary intake on the day before the collection of blood samples, the fasting plasma flavonoid concentrations were better markers of short-term intake.
The present study suggests that five different dietary flavonoids were measurable in plasma from subjects consuming their habitual diet, and that the sum of flavonoids extracted in plasma is associated with the dietary intake. Plasma flavonoids may therefore be a valid biomarker of flavonoid intake. This is supported by the positive correlation between the dietary flavonoid intake and the corresponding plasma concentration. Even though the present data suggest that the plasma total flavonols and flavonoids may be the best markers for the corresponding intake, individual flavonoids may serve as indicators of specific flavonoid intake. The strength of the correlation between the dietary flavonoid intake and the corresponding plasma concentration seems comparable to other nutritional biomarkers. Plasma carotenoids have often shown only a weak correlation with the intake of fruits and vegetables(Reference Zino, Skeaff and Williams39). The impact of other parameters (besides diet) affecting plasma concentrations, such as the bioavailability and the metabolic handling of the compounds, needs to be defined more precisely.
In conclusion, fasting plasma flavonoid concentrations are significantly correlated with their corresponding dietary intake, and fasting plasma flavonoid concentrations seem to be potential biomarkers of their dietary intake. Single fasting plasma flavonoid values truly reflect the short-time dietary intake. However, more information on the dose dependency of flavonoid excretion is needed before the validity of plasma flavonoids as biomarkers of intake can be established. The validity of plasma flavonoids as biomarkers of the ordinary dietary intake should be further investigated in intervention and cohort studies.
Acknowledgements
Financial support from the Education Department of Heilongjiang Province (11541157) is gratefully acknowledged. We would like to thank all the subjects for their participation. All authors have contributed to the study and accept responsibility for the content of the paper. There are no conflicts of interest. X. J. Z. designed and supervised the study. J. C. analysed the study data and contributed to writing the manuscript. J. C., W. C. and Y. Z. supervised the community survey and the plasma flavonoid assays.






