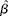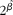Dietary Zn recommendations vary widely across Europe due to the heterogeneity of approaches used by expert panels(Reference Doets, de Wit and Dhonukshe-Rutten1). There is a need for a harmonised approach that is transparent and based on the best-quality data and methods available. Traditionally, the factorial approach is used in the determination of Zn requirements. This method seeks to estimate the Zn intake required to meet physiological requirements for growth, metabolism and tissue repair while replacing obligatory losses. An alternative approach is to examine the dose–response relationship between intake and biomarkers of status and also between intake and health outcomes. This information could then be integrated using a mathematical model to provide an insight into the level of Zn intake required for optimal health based on a range of parameters and indices of health that are known to be dependent upon dietary Zn intake(Reference Matthys, van 't Veer and de Groot2). To this end, the members of the EURopean micronutrient RECommendations Aligned (EURRECA) Network of Excellence have undertaken a series of systematic reviews of Zn intake–status relationships, according to rigorous protocols defined by consortium members and external experts(Reference Matthys, van 't Veer and de Groot2). We present the results of the systematic review and meta-analysis of the dose–response relationship between dietary Zn intake and Zn status using novel methodology developed by members of the EURRECA consortium.
The assessment of Zn status is notoriously problematic, as a sensitive, specific biomarker for Zn has not yet been identified(Reference King3). A systematic review and meta-analysis of biomarkers of Zn status was undertaken in 2009(Reference Lowe, Fekete and Decsi4). For many putative biomarkers (such as the Zn concentrations found in the cellular components of whole blood) there were insufficient data to arrive at a definitive conclusion regarding their efficacy as a biomarker of Zn status; however, plasma (or serum) Zn concentration was responsive to both Zn supplementation and Zn depletion and is the most widely reported biomarker for Zn. Hair and urine Zn concentrations were also considered to be potentially useful biomarkers in response to Zn supplementation.
The purpose of the present review was to systematically and quantitatively assess the dose–response relationships relevant to deriving Zn recommendations based on intervention studies, cohort (nested case–control) studies and cross-sectional studies. The specific questions to be addressed were: what is the effect of intake on indicators of exposure or body stores (i.e. biomarkers)? What factors affect this relationship?
The data used in the present meta-analysis were extracted from published studies (randomised controlled trials (RCT), prospective cohort studies, nested case–control studies and cross-sectional studies), performed in healthy adult and elderly populations, reporting the relationship between Zn status (plasma or serum Zn, hair or urine Zn concentration) and intake from supplements, fortified diets or natural food diets.
Methods
Search strategy
The present research was conducted within the framework of the EURRECA Network of Excellence that aims to identify the micronutrient requirements for optimal health in European populations (http://www.eurreca.org). This research was part of a wider review process to identify studies assessing the effect of Zn intake on different outcomes (biomarkers of Zn status and health outcomes). The wider searches were performed of literature published up to and including February 2010 using Ovid MEDLINE, Embase (Ovid) and the Cochrane Library (CENTRAL) using search terms for (‘study designs in humans’) AND (Zn) AND (intake OR status). Both indexing and text terms were used and languages included were restricted to those spoken in the EURRECA Network (English, Dutch, French, German, Hungarian, Italian, Norwegian, Polish, Spanish, Greek and Serbian). The full Ovid MEDLINE search strategy can be found in Table 1. Reference lists of retrieved articles and published literature reviews were also checked for relevant studies. Authors were contacted to request missing data or clarify methods or results. The search process is illustrated in Fig. 1.
Table 1 Ovid MEDLINE search strategy

pt, Publication type; ab, abstract; sh, subject heading; mp, multiple posting; exp, explode; adj3, words have to appear within three words of each other; ti, title.

Fig. 1 Study selection process for systematic review. RCT, randomised controlled trial (a colour version of this figure can be found online at http://www.journals.cambridge.org/bjn).
Criteria for the consideration of studies for the present review
Included studies were RCT, prospective cohort studies, nested case–control studies and cross-sectional studies in healthy human populations that supplied Zn supplementation (RCT) or measured dietary Zn intake with either a validated FFQ, a dietary history method, a 24 h recall method for at least 3 d, or a food record/diary for at least 3 d (observational studies). Studies had to be conducted in apparently healthy adult and elderly (human) populations aged ≥ 18 years and supplied Zn supplementation either as capsules or part of a fortified meal. If supplemental Zn was provided as a component of a fortified meal, studies were only considered acceptable if Zn was the only constituent that was different between treatment groups. Biomarkers of Zn status included plasma/serum, urine and hair Zn concentrations. Only studies that reported sufficient data or had sufficient data obtainable from the authors to estimate ![]() $\circ {>\beta }$ and se(
$\circ {>\beta }$ and se(![]() $\circ {>\beta }$) for the assumed linear relationship on the loge–loge scale were included. Studies were excluded if they were a group RCT (community trial), or were commentaries, reviews or duplicate publications from the same study. Studies were excluded if adults were hospitalised, had a chronic disease or if supplemental Zn was provided for less than 2 weeks.
$\circ {>\beta }$) for the assumed linear relationship on the loge–loge scale were included. Studies were excluded if they were a group RCT (community trial), or were commentaries, reviews or duplicate publications from the same study. Studies were excluded if adults were hospitalised, had a chronic disease or if supplemental Zn was provided for less than 2 weeks.
Selection of articles
Of 4719 identified articles in the wider search on Zn intake, status and priority health outcomes in all populations, 2557 were excluded based upon screening of the title and abstract. Two independent reviewers screened 10 % of the abstracts in duplicate and any discrepancies were discussed before screening the remaining references. Following subdivision into appropriate population groups, the full texts of the 1231 manuscripts were assessed to determine inclusion and exclusion by two independent reviewers and disagreements rectified through discussion. A total of 1147 studies were excluded because they did not meet the inclusion criteria. Of the remaining eighty-four studies, fifty-four were excluded as they related either Zn intake or status directly to a health endpoint, but they had not investigated the relationship between Zn intake and Zn related to biomarkers. A further seventeen studies were excluded from the meta-analysis because study participants were not healthy, insufficient data were reported, data were duplicated, or the dosage and duration were unclear. For the purpose of the present meta-analysis, ten RCT and three observational studies remained. The characteristics of the included studies are presented in Tables 2 and 3, respectively.
Table 2 Randomised controlled trials (n 10) reporting the effect of dietary zinc intake on serum/plasma zinc status in adults.

AAS, atomic absorption spectroscopy; ICP-MS, inductively coupled plasma MS.
Table 3 Observational studies (n 3) reporting the association between dietary zinc intake and serum/plasma zinc status in adults.

AAS, atomic absorption spectroscopy; ICP-MS, inductively coupled plasma MS.
Data extraction
For each of the identified papers, data were extracted independently by two reviewers into a standardised database. Extracted data included population characteristics, dose of Zn in intervention and placebo supplements, duration of the study, dietary intake of Zn, and mean concentration of Zn in plasma or serum at the end of the intervention period. Serum/plasma Zn concentrations were converted to μmol/l when applicable.
Data synthesis
Of the RCT, two that reported data for two Zn-treated groups and two control groups were treated as two independent estimates in the analysis(Reference Abdulla and Svensson5, Reference Boukaïba, Flament and Acher6). Where RCT provided outcome data for two or more Zn-treated groups, they were included as separate estimates in the meta-analysis(Reference Bogden, Oleske and Lavenhar7–Reference Sakagami, Ikeda and Tomita11). Where Zn status was measured at different time points within the same population only the final measure was used in the analysis(Reference Preziosi, Galan and Herbeth12, Reference Sullivan, Burnett and Cousins13). One observational study reported data from males and females and these were treated as two estimates in the meta-analysis(Reference Sánchez, Lopez-Jurado and Planells14). If dietary intake of Zn (in addition to the intervention) was not reported in the RCT, a value of 9·7 mg/d was imputed, which was the mean dietary intake level of the RCT that did report dietary Zn intake. As mean baseline serum/plasma Zn concentrations were infrequently reported in the RCT, the serum/plasma Zn concentrations in the control group were used as a proxy of the baseline serum/plasma Zn concentrations for our analyses.
Statistical analyses
A stratified random-effects meta-analysis was conducted using STATA (version 11; StataCorp LP), with one subgroup combining the evidence from RCT and the other subgroup combining the evidence from observational studies. As serum/plasma Zn levels have been reported to decline with age(Reference Rea15), a separate stratified random-effects meta-analysis compared Zn intake and status according to age in RCT ( < 55 years and ≥ 55 years). In addition, stratified meta-analyses were also conducted on dose of Zn ( < 35 mg/d and ≥ 35 mg/d) and trial duration (in weeks). It was not possible to perform a stratified meta-analysis for sex, because most studies included both men and women and data were not available at the individual level.
The transformations used to derive coherent single-study estimates from the available summary statistics per study have been described elsewhere(Reference Souverein, Dullemeijer and van 't Veer16). In short, an intake–status regression coefficient (![]() $\circ {>\beta }$) for each individual study was estimated from the mean serum/plasma Zn concentrations, based on the assumption of a linear relationship on the loge–loge scale (natural logarithm of intake v. natural logarithm of status). Algebraically deriving an estimate from each study of the regression coefficient (
$\circ {>\beta }$) for each individual study was estimated from the mean serum/plasma Zn concentrations, based on the assumption of a linear relationship on the loge–loge scale (natural logarithm of intake v. natural logarithm of status). Algebraically deriving an estimate from each study of the regression coefficient (![]() $\circ {>\beta }$) and its standard error (se(
$\circ {>\beta }$) and its standard error (se(![]() $\circ {>\beta }$)) enabled a comparison of the results from studies with heterogeneously reported associations and effects. The overall pooled
$\circ {>\beta }$)) enabled a comparison of the results from studies with heterogeneously reported associations and effects. The overall pooled ![]() $\circ {>\beta }$ and se(
$\circ {>\beta }$ and se(![]() $\circ {>\beta }$) were calculated using random-effects meta-analysis, which estimates the between-study variance using the method of DerSimonian & Laird(Reference DerSimonian and Laird17). This was then used to modify the weights used to calculate the summary estimate. Residual heterogeneity between studies was evaluated using the I 2 statistic. To evaluate potential sources of heterogeneity, the variables study duration, age, sex and Zn dose were added simultaneously to a meta-regression model as continuous variables. The statistical transformations to obtain
$\circ {>\beta }$) were calculated using random-effects meta-analysis, which estimates the between-study variance using the method of DerSimonian & Laird(Reference DerSimonian and Laird17). This was then used to modify the weights used to calculate the summary estimate. Residual heterogeneity between studies was evaluated using the I 2 statistic. To evaluate potential sources of heterogeneity, the variables study duration, age, sex and Zn dose were added simultaneously to a meta-regression model as continuous variables. The statistical transformations to obtain 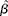 $\circ {>\beta }$ and se(
$\circ {>\beta }$ and se(![]() $\circ {>\beta }$) were performed using GenStat version 13-SP2 (VSN International Ltd) and the meta-analysis was performed using STATA (version 11.0; StataCorp LP), with statistical significance defined as P < 0·05.
$\circ {>\beta }$) were performed using GenStat version 13-SP2 (VSN International Ltd) and the meta-analysis was performed using STATA (version 11.0; StataCorp LP), with statistical significance defined as P < 0·05.
Assessment of risk of bias in included studies
In order to assess the quality of the included studies and the risk of bias, indicators of internal validity were collected during data extraction (Table 3). Based on the indicators two independent reviewers assessed the overall risk of bias and disagreements resolved by discussion. The criteria for judging these indicators were adapted from the Cochrane Handbook for Systematic Reviews(Reference Higgins and Green18).
Results
A total of twenty estimates of Zn intake and serum/plasma Zn status in ten RCT and four estimates in three observational studies were eligible for meta-analysis. All studies were published between 1979 and 2010. Although plasma/serum, urine and hair Zn concentrations were included as markers of status in the systematic review protocol, only plasma/serum Zn concentration was reported universally and sufficiently frequently to be used in the meta-analysis. Most studies included, but did not differentiate between, males and females, but three studies included only females(Reference Feillet-Coudray, Meunier and Bayle9, Reference Gibson, Heath and Limbaga19, Reference Chandyo, Strand and Mathisen20), two included only males(Reference Feillet-Coudray, Meunier and Rambeau8, Reference Sullivan, Burnett and Cousins13) and one provided both male and female data(Reference Sánchez, Lopez-Jurado and Planells14). Studies were conducted in Europe (n 7), North America (n 3), South Asia (n 1), East Asia (n 1) and Australasia (n 1) and ages of participants ranged from 18 to 106 years.
All but one RCT used a parallel design. Boukaïba et al. (Reference Boukaïba, Flament and Acher6) employed a cross-over RCT design. The RCT included 1285 participants in total with sample sizes ranging from five to 201. The median duration of the trials was 25 weeks (range 2–52 weeks). In nine studies Zn was supplemented alone at doses ranging from 15 to 135·3 mg/d and in one study Zn was provided within a multi-micronutrient supplement(Reference Preziosi, Galan and Herbeth12). Most studies (n 7) provided the Zn supplements in the form of zinc gluconate, but others used zinc sulfate(Reference Abdulla and Svensson5), zinc acetate(Reference Bogden, Oleske and Lavenhar7) or zinc carnosine(Reference Sakagami, Ikeda and Tomita11). Habitual Zn intakes ranged from 5·4 to 10·8 mg/d (where data were provided).
The observational studies included 1184 participants in total with sample sizes in the range of 170–500. Zn intake was measured using a combination of FFQ and 24 h recall, or 24 h recall alone and values ranged from 8·6 to 12·2 mg/d. The meta-analysis of available studies suggested that Zn supplementation was associated with increased serum/plasma Zn concentrations. The estimated effect for Zn supplementation on serum/plasma Zn concentrations from RCT and observational studies was 0·08 (95 % CI 0·05, 0·11; P < 0·0001; I 2 84·5 %) (Fig. 2). When datasets were grouped according to study design, only the RCT showed a significant effect size (0·09; 95 % CI 0·07, 0·120; P < 0·0001; I 2 79·1 %).

Fig. 2 Random-effects meta-analyses of randomised controlled trials and observational studies evaluating the pooled effect of dietary zinc on serum/plasma zinc in adults. β Values (♦) represent the regression coefficients for the linear association between loge-transformed zinc intake and loge-transformed serum/plasma zinc status (a colour version of this figure can be found online at http://www.journals.cambridge.org/bjn).
Since a base-e logarithmic transformation was applied to the Zn intake and serum/plasma Zn concentration before calculation of the study-specific ![]() $\circ {>\beta }$, the overall
$\circ {>\beta }$, the overall ![]() $\circ {>\beta }$ represents the difference in the loge-transformed predicted value of serum/plasma Zn status for each one-unit difference in the loge-transformed value in Zn intake. Therefore, an overall
$\circ {>\beta }$ represents the difference in the loge-transformed predicted value of serum/plasma Zn status for each one-unit difference in the loge-transformed value in Zn intake. Therefore, an overall ![]() $\circ {>\beta }$ of 0·08 means that for every doubling in Zn intake, the difference in Zn serum or plasma concentration is
$\circ {>\beta }$ of 0·08 means that for every doubling in Zn intake, the difference in Zn serum or plasma concentration is ![]() $2^{ \circ {>\beta }}$ (20·08 = 1·06), which is 6 %. This means that an individual with a Zn intake of 14 mg/d has a Zn serum/plasma concentration that is 6 % higher than an individual who has a Zn intake of 7 mg/d (Fig. 3).
$2^{ \circ {>\beta }}$ (20·08 = 1·06), which is 6 %. This means that an individual with a Zn intake of 14 mg/d has a Zn serum/plasma concentration that is 6 % higher than an individual who has a Zn intake of 7 mg/d (Fig. 3).

Fig. 3 Serum/plasma zinc concentration (μmol/l) as a function of dietary zinc intake (mg/d), estimated by random-effects meta-analyses of randomised controlled trials of adults: natural log-transformed data (a); untransformed data (b).
As plasma/serum Zn concentrations have been reported to decline with age(Reference Rea15), a separate subgroup analysis compared Zn intake and status according to age in RCT ( < 55 years and ≥ 55 years). Of the studies, two for which mean serum/plasma Zn values were given for adults whose ages spanned both age groups were excluded from this analysis(Reference Sakagami, Ikeda and Tomita11, Reference Preziosi, Galan and Herbeth12). A stronger effect size was found in adults aged under 55 years (0·14; 95 % CI 0·04, 0·24; P < 0·005; I 2 92·1 %) compared with adults aged 55 years and over (0·09; 95 % CI 0·07, 0·11; P < 0·0001; I 2 32·8 %), although care should be taken with interpreting this finding as the younger age group analysis is based on only three estimates in two studies. Stratifying the analysis for dose of Zn ( < 35 mg/d and ≥ 35 mg/d) revealed a stronger effect size for a Zn dose ≥ 35 mg/d (0·14; 95 % CI 0·08, 0·21; P < 0·0001; I 2 85·2 %) compared with < 35 mg/d (0·09; 95 % CI 0·07, 0·10; P < 0·005; I 2 27·6 %). Similar effect sizes were demonstrated for study duration (0–12 weeks: 0·13; 95 % CI 0·05, 0·20; I 2 92·4 %; >12 weeks: 0·10; 95 % CI 0·07, 0·12; I 2 75·8 %).
To evaluate potential sources of heterogeneity, the variables duration, age, sex and dose were added simultaneously to a meta-regression model as continuous variables. The analysis revealed that only Zn dose was a statistically significant determinant of the overall β. The model explained 50 % of between-study variance and the residual variation due to heterogeneity was reduced to 48·2 %.
Table 4 summarises the internal validity of the included studies, assessed as described in the Methods section. The risk of bias was high in five out of the ten papers(Reference Abdulla and Svensson5, Reference Boukaïba, Flament and Acher6, Reference Sakagami, Ikeda and Tomita11–Reference Sullivan, Burnett and Cousins13). Papers were given a high risk of bias rating due to insufficient information provided on sequence generation and/or allocation, drop-outs and funding bodies.
Table 4 Assessment of validity of included randomised controlled trials reporting zinc intake and serum/plasma zinc in adults

NR, not reported.
Discussion
The present review is unique in providing an estimate of the dose–response relationship of Zn intake and serum/plasma Zn concentrations in adults. A meta-analysis of twenty estimates in ten RCT and four estimates in three observational studies found that Zn supplementation produced a statistically significant increase in serum/plasma Zn concentrations and provided an estimate of the dose–response relationship between Zn intake and serum/plasma concentrations. An overall ![]() $\circ {>\beta }$ of 0·08 means that for every doubling in Zn intake, the difference in Zn serum or plasma concentration is 6 %. In other words, an adult with a Zn intake of 14 mg/d has a Zn serum/plasma concentration that is 6 % higher than an individual who has a Zn intake of 7 mg/d. This association was slightly stronger when considering only the RCT, as no observational studies found a significant association between Zn intake and plasma Zn concentrations. The intake–status regression coefficient for the observational studies is likely to be attenuated by random and intake-related errors in assessing dietary Zn intake(Reference Kipnis, Subar and Midthune21), whereas in RCT Zn intake can be considered as fixed at each level of dosage and random errors arise only through assessment of biomarkers.
$\circ {>\beta }$ of 0·08 means that for every doubling in Zn intake, the difference in Zn serum or plasma concentration is 6 %. In other words, an adult with a Zn intake of 14 mg/d has a Zn serum/plasma concentration that is 6 % higher than an individual who has a Zn intake of 7 mg/d. This association was slightly stronger when considering only the RCT, as no observational studies found a significant association between Zn intake and plasma Zn concentrations. The intake–status regression coefficient for the observational studies is likely to be attenuated by random and intake-related errors in assessing dietary Zn intake(Reference Kipnis, Subar and Midthune21), whereas in RCT Zn intake can be considered as fixed at each level of dosage and random errors arise only through assessment of biomarkers.
The studies included in the present meta-analysis were different in a number of aspects, such as using various designs, follow-up times, Zn doses and populations. Therefore, it is no surprise that, when combining these studies in a meta-analysis, large heterogeneity is observed between the studies (I 2 84·5 %; P = 0·0001). This between-study heterogeneity may be caused by methodological factors, such as differences in study population characteristics (age, socio-economic status) or differences in doses of provided Zn (amount, one or more doses per d, study duration). When considering some key variables (study duration, Zn dose, age and sex) in a meta-regression model, only dose explained some between-study heterogeneity. An individual participant data meta-analysis may have provided a more conclusive explanation of the between-study heterogeneity in the present meta-analysis. However, this type of analysis would involve the input of raw individual participant data provided by the original study investigators for reanalysis and combination in a pooled analysis and as such would be a major undertaking in terms of time, costs and collaboration. Moreover, an inability to include individual participant data from all relevant studies could introduce selection bias. The meta-analytic approach used in the present paper is not an attempt to accurately describe the biological relationship between actual Zn intake and Zn concentrations in blood under strict experimental conditions and on an individual level, but rather to simulate a dose–response relationship between Zn intake and status that is useful for surveillance studies with a public health point of view and, as such, deliberately incorporates the differences between dietary assessment methods, laboratory assessment methods and participant characteristics to ensure a broad external validity. Thus, the heterogeneity reflects the lack of standardisation of methods and the true heterogeneity between study populations and necessarily enters as uncertainty into the application of such data for public health purposes(Reference Moran, Skinner and Medina22).
To conduct the present meta-analysis some assumptions related to the availability of the required data or related to statistical issues had to be made. First, when two or more intervention groups were compared with the same control group (five RCT), independence of estimates was assumed. As a consequence, bias may have been introduced, by either increasing the estimates of the intervention effect (if the control group values were in fact lower), or decreasing the estimates of the intervention effect (if the control group values were higher). Second, the meta-analysis required transformations of the intake and biomarker data to a common scale, as the studies included in the present meta-analyses had different ways of reporting the relationship between Zn and serum/plasma Zn concentration. The different ways of reporting by transformation of both the intake and biomarker data were standardised to double loge scale, which allowed the derivation of a standardised estimate from each study of the regression coefficient and its standard error as a basis for comparing these heterogeneously reported results. A linear relationship on the double loge scale was also assumed. This transformation allowed the pooling of β values and enable these to be reported as a dose–response relationship between Zn intake and serum/plasma Zn concentrations(Reference Souverein, Dullemeijer and van 't Veer16).
The meta-analyses were conducted within the context of the EURRECA project as a means to provide additional evidence for underpinning reference values for Zn intake of populations. This dose–response relationship methodology may be used as either qualitative or quantitative evidence to substantiate the daily Zn intake dose necessary to achieve normal or optimal levels of biomarkers for Zn status. The dose–response relationship between Zn intake and plasma Zn concentration is of course subject to the debate around the usefulness of plasma/serum Zn concentration as a biomarker of Zn status, and its predictive value for relevant functional health outcomes, such as markers of immune function.
The relationship observed between serum/plasma Zn concentration and Zn intake may have been weakened by the limitation of this particular biomarker for Zn status. It is well established that plasma Zn concentration can fall in response to factors unrelated to Zn status or dietary Zn intake, such as infection, inflammation, exercise, stress or trauma(Reference King23). Conversely, tissue catabolism during starvation can release Zn into the circulation, causing a transient increase in circulating Zn levels. A total of six studies used non-fasted blood samples in their analyses(Reference Abdulla and Svensson5, Reference Bogden, Oleske and Lavenhar7, Reference Sakagami, Ikeda and Tomita11, Reference Sánchez, Lopez-Jurado and Planells14, Reference Chandyo, Strand and Mathisen20, Reference Prasad, Beck and Bao24). As postprandial plasma Zn concentrations have been reported to fall up to 19 %(Reference Lowe, Woodhouse and King25), the inclusion of these studies may have weakened the observed relationship between Zn intake and status. Whilst all studies included in the analysis were undertaken in individuals without chronic disease or severe protein–energy malnutrition, other factors such as stress, infection and inflammation may also have gone unreported. In addition, serum Zn concentration has been reported to decrease with age(Reference Rea15). Clearly, such confounders have a strong influence on the interpretation of plasma Zn concentrations. However, as more sensitive indices of Zn status have yet to be identified, plasma serum Zn remains by far the most commonly used biomarker of Zn status(Reference Lowe, Fekete and Decsi4).
In conclusion, the present review presents the application of a novel technique to analyse data from ten RCT and three observational studies reporting the relationship between Zn intake and serum/plasma Zn concentration. The present meta-analysis has provided an estimate of the dose–response relationship between Zn intake and serum/plasma Zn concentration in adult and elderly populations. Based on twenty-four estimates among 2469 participants, the results indicate that a doubling of Zn intake increases plasma/serum levels by 6 %. There is a high level of heterogeneity in the data obtained from the studies included in this meta-analysis. Analysis of the factors that may contribute to this, namely study duration, Zn dose, age and sex, indicated that Zn dose was able to explain 50 % of this heterogeneity. This novel method of analysing intake–biomarker relationships may be useful for the setting of future dietary Zn recommendations.
Acknowledgements
The present review was carried out within the EURRECA Network of Excellence (www.eurreca.org) which is financially supported by the Commission of the European Communities, specific Research, Technology and Development (RTD) Programme Quality of Life and Management of Living Resources, within the Sixth Framework Programme, contract no. 036196. This report does not necessarily reflect the Commission's views or its future policy in this area. The original conception of the systematic review was undertaken by the EURRECA Network and coordinated by partners based at Wageningen University (WU), the Netherlands and the University of East Anglia (UEA), UK: Susan Fairweather-Tait (UEA), Lisette de Groot (WU), Pieter van 't Veer (WU), Kate Ashton (UEA), Amélie Casgrain (UEA), Adriënne Cavelaars (WU), Rachel Collings (UEA), Rosalie Dhonukshe-Rutten (WU), Esmée Doets (WU), Linda Harvey (UEA) and Lee Hooper (UEA) designed and developed the review protocol and search strategy. The authors would also like to thank Joseph Saavedra, Nick Kenworthy, Sarah Richardson-Owen, Hannah Eichmann and Christine Cockburn for assistance with data extraction and Fiona Dykes for helpful discussions. N. M. L., M, W. M., A.-L.S., V. H. M. and M. N. collected and analysed the data; S. P. and L. S.-M. were also involved in the data analysis. O. W. S. and C. D. developed the statistical techniques and advised on their application to the present study. All authors were involved in writing the manuscript. There are no conflicts of interest for any of the authors.