With growing evidence that postprandial blood glucose responses have a direct role to play in the disease complications associated with glucose intolerance(Reference Guigliano, Ceriello and Esposito1), there is an increasing demand for food values that will facilitate food choices for blood glucose control, by being expressed as grams per serving or grams per reference amount customarily consumed(Reference Monro and Mishra2, Reference Miller-Jones3). Available carbohydrate values are not an accurate guide to the potential of a food to raise blood glucose levels because they are determined on samples that have been finely ground to ensure complete release of carbohydrate. In contrast, the glycaemic impact of a food, which in the present context means the blood glucose-raising potential of the glycaemic carbohydrate released in digestion, depends on the degree to which food structure survives mastication and on other factors that affect the rate and extent of carbohydrate digestion. To overcome the limitations of available carbohydrate in glycaemia management, the glycaemic index (GI) was introduced as an adjunct to available carbohydrate values(Reference Jenkins, Wolever and Taylor4). Although usually referred to as the ‘glycaemic index of a food’, GI is derived from the measured glycaemic effect of a food, calculated to an available carbohydrate basis, and is expressed as a percentage of the effect of glucose equal in weight to the carbohydrate. So, it becomes an imputed carbohydrate-based and not a food-based index.
GI was designed specifically to compare foods of equal carbohydrate content after they had been classified into food exchange categories of similar composition for intensive diabetes management(Reference Jenkins, Wolever and Taylor4). It is more difficult and less appropriate to use GI for accurate glycaemic control under the everyday conditions of food purchasing, cooking and eating, in which most foods, even within marketed food categories, do not have the same carbohydrate content, and are not presented or consumed in carbohydrate-based portion sizes. And because GI is a fixed carbohydrate-based index, it does change with the amount of food consumed, so it cannot indicate the effects of different food intakes on glycaemic impact(Reference Monro5).
The glycaemic load, calculated as the product of GI and the amount of carbohydrate consumed in a food(Reference Salmeron, Manson and Stampfer6), is an intake-responsive measure of the relative glycaemic impact (RGI) of an entire food. Glycaemic load has been expressed as glucose equivalents (GE)(Reference Livesey, Taylor and Hulshof7), but in terms of glycaemic effect, it is not generally the true GE of a food(Reference Monro and Shaw8) because it has been calculated from GI, and so it is based on glycaemic responsiveness per gram of glucose at the 50 g glucose reference intake, not at a glucose intake equivalent in effect to the 50 g carbohydrate food portion(9). And because the glucose dose–glycaemic response curve is non-linear, the response per gram of glucose at an intake of 50 g glucose may be much less than that at an intake of 50 g carbohydrate in food, especially with low GI foods. The effects of non-linearity on the determination of the GE of a food in terms of glycaemic potency and the need for values that reflect the effects of customarily consumed food portions have been addressed by measuring RGI of a standard glucose dose–glycaemic response curve, using relevant portion sizes and expressing the results as grams of glycaemic GE (GGE)(Reference Wallace, Monro and Brown10).
However, all the clinical procedures for measuring the glycaemic potency of foods have drawbacks in common: they are very costly to perform, they face the logistical and ethical demands typical of clinical trials and, importantly, they suffer from intra- and inter-subject variation, which means that large subject numbers are required to adequately power tests of differences between foods(Reference Vega-Lopez, Griffith and Ausman11, Reference Wolever, Vorster and Björk12).
One possible way around the difficulties of clinical trials is to measure glycaemic impact in vitro using a method that mimics the digestive actions of the gut. In this paper, we have applied a method for determining the GGE content of foods, and tested its performance against the results of clinical trials. The elements of the in vitro digestion are similar to those in other methods that simulate gut processes(Reference Woolnough, Monro and Brennan13), but to obtain values of true glucose equivalence that are relevant, the present trial differed from others in a number of respects: the clinical arm used portions similar to those customarily consumed, the in vivo GGE values were directly determined off a glucose standard curve, and the in vitro GGE values included the contribution of non-glucose glycaemic sugars. Most importantly, initial results in the study indicated that an adjustment was required for the effects of dose dependence of rates of glucose disposal (GD) that would occur in response to the different GGE intakes in the portions of food consumed. The effects of making such an allowance for homeostasis then became an important aspect of the analysis, and they are presented here.
Method
Food sampling
Foods were purchased from single outlets, but the same sample was used in the in vitro and in vivo analyses.
In vitro digestion
Reagents
All the reagents that were used, HCl, Na2HCO3, ethanol, sodium acetate 3-hydrate, maleic acid, d(+)-glucose, NaOH and sodium azide, were of high purity. Enzymes that were used were pepsin EC 3.4.23.1 from porcine stomach mucosa (Sigma, P 7000; 800–2500 U/ml; St Louis, MO, USA), pancreatin (Sigma, P7545; 8 × USP specifications), amyloglucosidase EC 3.2.1.3. from Aspergillus niger (Megazyme, E-AMGDF; 3260 U/ml) and invertase (BDH concentrate; 39 020 3D).
The 3,5-dinitrosalicylic acid reagent consisted of 10 g 3,5-dinitrosalicylic acid dissolved in a solution of 16 g NaOH plus 300 g Na–K tartarate in 1 litre, and it was allowed to stand for 2 d before use.
Sample treatment
All soft or crisp foods such as bakery products (Group A), breakfast cereals (Group B), and crackers, cakes and bars (Group C) were rubbed through a 4 mm square wire mesh, which provided a crumbled sample. Sundry cereals (Group D), snack foods (Group E), fruits (Group G), vegetables (Group H), meat (Group I), combination foods – savoury (Group J), and combination foods – sweet and meals (Group K) were processed through a Kenwood chef model A720 electric mincer with a 9 mm aperture plate, which subjected the samples to a shearing plus cutting action and produced a mechanically ‘chewed’ sample. Groups A–C were crumbled to avoid compaction, which occurred in some soft bakery products. Dairy-based foods (Group F) were analysed as is.
In vitro digestion
Food samples were digested in 70 ml open specimen pots inserted to their full depth in an aluminium heating block, placed on a 15 place magnetic stirrer, and covered with an insulating sheet. The digestion consisted of a simulated gastric digestion followed by small intestinal digestion, with timed sampling during the small intestinal phase. Briefly, 30 ml of water and 0·8 ml of 1 m-HCl were added to the sample to attain a pH of 2·5 ( ± 0·2), with pH adjustment if necessary, 1 ml of 10 % pepsin that was dissolved in 0·05 m-HCl was added, and the mixture was stirred slowly (130 rpm) for 30 min at 37°C to accomplish gastric digestion. The small intestinal phase was initiated by adding 2 ml of 1 m-NaHCO3 and 5 ml of 0·1 m-sodium maleate buffer, pH 6/0·02 % sodium azide/1 mm-CaCl2, followed by the addition of 0·1 ml amyloglucosidase and 5 ml of 2·5 % pancreatin in 0·1 m-maleate buffer, pH 6 in quick succession to start amylolysis, and the pots were quickly made to the 55 ml mark with distilled water. Digesta aliquots of 1·0 ml were removed before adding the amyloglucosidase–pancreatin (T = 0) and at 20, 60 and 120 min from the start of amylolysis, and were each added to 4 ml absolute ethanol in a tube and mixed.
Measuring sugars released during digestion
After at least 30 min, the tubes containing the timed samples in ethanol were centrifuged for 10 min at 2000 rpm (Centrifuge Omnifuge 2.0 RS Heraeus Sepatech, Osterode, Germany) to clarify. A 0·05 ml aliquot of ethanolic supernatant, or glucose standard (1 mg/ml), was added to 0·25 ml of acetate buffer, pH 5·2, containing 1 % invertase+1 % amyloglucosidase, and was incubated at 37°C for 10 min to complete depolymerisation to monosaccharides, which were measured as reducing sugars by a scaled-down dinitrosalicylic acid colorimetric method(Reference Englyst and Hudson14). Fructose was measured by the thiobarbituric acid method(Reference Blakeney and Mutton15), or values were taken from the New Zealand Food Composition Database(16). All the samples were measured in duplicate. Rapidly available carbohydrate was calculated as reducing sugar in the 20 min sampling of pancreatic digest.
Glycaemic sugars may also be measured as glucose, measured by a glucose oxidase assay, plus fructose.
Clinical glycaemic glucose equivalents determination
The study was conducted according to the guidelines laid down by the Declaration of Helsinki, and all procedures involving human subjects were approved by the Canterbury Ethics Committee. Written informed consent was obtained from all the subjects.
The in vivo trials were carried out over 4 years. Participants were all males aged between 20 and 64 years, and none had diabetes according to the WHO classification (fasting glucose>6·2 mmol/l). None suffered from any diagnosed gastrointestinal or hepatic conditions during or immediately before the measurement period.
Glucose references and test foods
Glucose was supplied as glucose beverages bottled as 75 or 50 g anhydrous glucose in 300 ml. The beverage containing 50 g glucose was diluted with soda water (to maintain electrolyte balance) in the ratio of 3:1 and 1:1 to make solutions containing 12·5 and 25 g glucose, respectively, so the final range of doses was 0 (soda water) 12·5, 25, 50 and 75 g glucose in 300 ml.
Procedure
The effect of each food on blood glucose concentrations was measured once in each individual. The foods tested are given in Table 1, along with the number of participants tested for each food. The number of individuals who were tested for each food varied from seven to twenty. On average, the glucose standard curves were measured at approximately three monthly intervals for each individual. However, for the first twenty-seven of the foods, marked with an * in Table 1, a single 25 or 50 g glucose reference was used, whichever was thought to give a response in the vicinity of that expected of the test food. Subsequently, glucose references of 0 (soda water), 12·5, 25, 50 and 75 g glucose were used to allow construction of glucose dose–glycaemic response standard curves. The order in which the glucose references and foods were measured was randomised and balanced so that the glucose references were spread evenly over the course of the testing, and the sequence for each individual was varied.
Table 1 Glycaemic impact as glycaemic glucose equivalents (GGE) per serving of foods determined in vivo and in vitro
(Mean values with their standard errors)

GI, glycaemic index.
* Fifty or twenty-five grams of glucose references used in initial measurement of in vivo GGE values, but values subsequently adjusted to their equivalent values on the glucose dose–glycaemic response standard curve using equation 1.
† Fifty grams of glucose references used in initial measurement of in vivo GGE values, but values were subsequently adjusted to their equivalent values on the glucose dose–glycaemic response standard curve using equation 1. All other GGE in vivo values were obtained directly from the standard curve.
All the tests were carried out at the Lipid and Diabetes Research Group following a standard protocol. Participants were asked to eat a meal containing carbohydrates, to refrain from drinking alcohol the night before each test and from physical exercise on the morning of the test and to report to the clinic after having fasted. Capillary blood samples were taken using a lancet. A drop of blood was collected into a HemoCue® cuvette, and blood glucose concentration was measured using a Hemocue® Glucose 201 Analyser (Helsingborg, Sweden)(Reference Stork, Kemperman and Erkelens17). The average of two fasting blood glucose concentrations, determined 5 min apart, was used as a baseline measure. Test foods and glucose references were consumed within 15 min, and capillary blood samples were taken at 15, 30, 45, 60, 90 and 120 min after the person began consuming the test food or glucose reference. If the blood glucose concentration had not returned to within 0·2 mmol/l of the baseline concentration at 2 h, further blood samples were taken at 150 and 180 min after the start time. Participants were asked to remain seated for the duration of the tests with the exception of visits to the toilet.
Analysis of results
Two different in vivo methods were used. Initially, the method used one glucose reference of either 25 or 50 g amount, and this method was used for twenty-seven foods (marked with an * in Table 1). As a result of progress in our research, a more accurate method was then developed for measuring GGE from a standard curve(Reference Wallace, Monro and Brown10), and the remaining sixty-three foods were tested by this method.
Calculation of incremental area under the blood glucose response curve
For all the foods, the incremental area under the blood glucose response curve (iAUC) to a maximum of 180 min was calculated geometrically using the method described by FAO/WHO for each of the test foods for each participant(18). Areas where the curve dropped below baseline were excluded.
Calculation of slopes and intercepts for glucose curves
The iAUC for each of the five glucose reference intakes at each time point were calculated as described earlier. The iAUC and the glucose intakes (0, 12·5, 25, 50 and 75 g) were then log transformed, and the slope and intercept were determined.
Calculation of the in vivo glycaemic glucose equivalents values of foods
The curves describing the relationship between reference glucose intakes and iAUC were used to estimate the glycaemic response for all foods measured with glucose curves. This was done by using the derived slope and intercepts from the individual's glucose response curve to estimate the GGE from the individual iAUC for each food. The GGE for each food at the specified portion size was then taken as the average GGE over the individuals for whom it was measured.
For the foods where a single 25 or 50 g glucose reference rather than a glucose standard curve was used, the GGE of the test food was calculated as GGE/portion size by dividing the iUACtest food by the iAUC average glucose, and by multiplying the result by the amount of glucose in the reference drink. Each glucose reference was measured at least three times. The average GGE/serve for each food by each method was taken as the average of all the individuals.
GGE values calculated from a single 25 or 50 g glucose reference value (marked with an * in Table 1) were adjusted to the value that would have been obtained directly from a glucose reference curve, by multiplying the ratio of the single reference value and the corresponding value on a glucose dose–glycaemic response curve, using the equation:
This equation was based on the results of six published studies of the relationship between glucose dose and glycaemic response normalised to a response of 50 GGE at an intake of 50 g glucose(Reference Monro and Shaw8).
Calculation of in vitro glycaemic glucose equivalents values
GGE values were based on rapidly available carbohydrate (20 min digestion) measured as total GE. The GE values were converted to in vitro GGE values by reducing the GE values by the proportion of fructose in the rapidly available carbohydrate multiplied by 0·78. Multiplying by 0·78 allows for the fact that fructose has 0·22 of the glycaemic potency of glucose (GI fructose = 22)(Reference Foster-Powell, Holt and Brand-Miller19), that is, it contributes 0·22 GGE/g. Thus, where the proportion of fructose is P f and the total amount of GE measured is GE, the in vitro GGE value is obtained as
If free glucose and fructose are measured enzymatically rather than as total GE and fructose, as in the present study, the in vitro GGE value is calculated as glucose+0·22 fructose.
No allowance was made for the presence of lactose (GI = 80) in the foods given in Table 1.
Allowance for apparent glucose disposal
The theoretical balance between GGE released in vitro from a portion of food after a given duration of digestion and GD that would have occurred in vivo in the same time in response to the GGE loading by the food portion was determined using the relationship: GD rate = − 0·000104GGE2+0·0169GGE g/min(Reference Monro, Mishra and Venn20) determined from previous clinical trials(Reference Venn, Wallace and Monro21). As the in vitro GGE value was determined after 20 min digestion, theoretical GD at 20 min was
So, the net GGE contribution, being the difference between GGE release from the food and GGE disposal (Table 2), was
Table 2 Steps in transforming in vitro glucose equivalents (GE) values to net glycaemic GE (GGE) values: GGE is calculated from GE by allowing for the relative glycaemic potency of fructose, and then net GGE is calculated from GGE by subtracting apparent glucose disposal at 20 min*

Analysis of glycaemic glucose equivalents in vitro–glycaemic glucose equivalents in vivo relationship
All GGE calculations, means, standard deviations and coefficients of variation were calculated in a Microsoft Excel spreadsheet. Prediction of in vivo GGE by in vitro GGE with and without adjusting for GD was tested by regression analysis weighted by the precision of the in vivo measures using the Genstat statistical package(22). A method comparison analysis was conducted, also in Excel, using the Bland & Altman(Reference Bland and Altman23) procedure, which involved plotting the means of the in vitro and in vivo determinations against the difference between the two. The Bland–Altman analyses were applied to the individual food groupings and then in turn to the relationship of GGE in vitro with GE, GGE and net GGE (GGE after adjusting for theoretical GD). Because of the heterogeneity of foods within most groups and the small number of foods in some groups, all foods were taken together to show the effects of converting GE to GGE and GGE to net GGE.
Results
The GGE values determined from clinical blood glucose response measurements (in vivo) and by in vitro digestive analysis of GGE as GE corrected for the low GI of fructose present (equation 1) are shown in Table 1, with their standard errors and CV. The corresponding GE values on which they were based are given in Table 2. The precision of measurement was much greater for the in vitro measurements than for the in vivo measurements within all groups; standard errors for in vivo measures ranged from 0·6 of the in vitro seto 72 times (median 4·8 times as large). The CV, being based on the variability of individual observations rather than on the mean, differ more markedly; however, the size of the CV (about 5 % for the in vitro procedure and about 70 % for the in vivo procedure) is not unusual for these types of measurement.
For each of the food groups shown and for all the foods taken together, the equations for predicting the in vivo GGE values from the in vitro measurements of net GGE and their R 2 values are given in Tables 3 and 4. Five groups (snack foods, fruits, crackers, vegetables and breads) had significant positive relationships between GGE in vivo measures and net GGE in vitro measures. For most, the slope of the equation was significantly less than one, indicating that the in vitro results for the foods in the group covered a wider range than the in vivo ones; the bread group was the one exception, with a slope close to one. Given that the five groups showing the positive in vitro–in vivo relationship account for fifty-seven of the eighty-three samples, it is perhaps not surprising that there is a significant (although not as strong) correlation between in vivo and in vitro measures for all the foods taken together.
Table 3 Regression equations predicting glycaemic glucose equivalents (GGE) in vivo (y; Table 1) from net GGE in vitro (x; Table 2) for food groupings and all the foods taken together
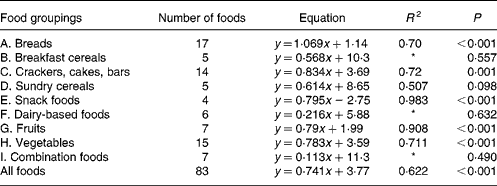
* Not statistically significant.
Table 4 Equations from Bland–Altman method comparison applied to foods within the food groups; the mean (x) of net GGE in vitro (Table 2) and GGE in vivo (Table 1) is plotted against the difference (Table 2) between net GGE in vitro and GGE in vivo.
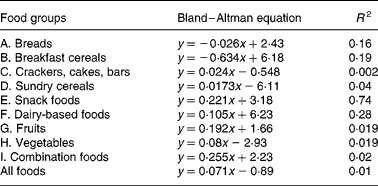
Bland and Altman plot(Reference Brighenti, Pellegrini and Casiraghi24) method comparison of GGE in vivo values and GGE in vitro values before correction for GD had indicated that the in vitro measures were generally higher than the corresponding in vivo values, and this discrepancy increased with the GGE quantity in the food (Fig. 1). In light of the increasing disparity between in vitro and in vivo GGE values with increasing GGE intake, we undertook the research, based on clinical measurements(22), which identified equations relating apparent GD, which represents the overall homeostatic adjustment to blood glucose loading, to GGE intake(Reference Venn, Wallace and Monro21). Applying the GD equation to the unadjusted GGE values allowed a GD value (GD, Table 2) to be calculated for the GGE dose contributed by each food (GGE, Table 2). Subtracting GD from GGE provided GD-adjusted values termed ‘net GGE’ (Table 2). As the values given in Table 2 are derived from the values given in Table 1, the measures of precision have not been repeated in Table 2.

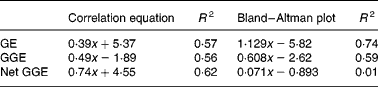
Fig. 1 Bland–Altman method comparisons (mean of in vitro net glycaemic glucose equivalents (GGE) and in vivo GGE v. difference between in vitro net GGE and in vivo GGE) showing improving correspondence between in vitro and in vivo measurements by allowing, firstly, for the relative glycaemic potency of fructose (glucose equivalents (GE) to GGE conversion), and secondly, for glucose disposal (GGE conversion to net GGE, by subtracting glucose disposal at 20 min.). (a) Data from GGE in vivo (Table 1) and net GGE in vitro (Table 2). (b) Data based on net GGE values and glucose disposal baselines in Monro et al. (Reference Monro, Mishra and Venn20). (a) GE: y = 1·129x − 5·82; R 2 0·74; GGE: y = 0·608x − 2·62; R 2 0·59; net GGE: y = 0·0705x − 0·8931; R 2 0·0105. (b) GE: y = 0·491x − 1·89; R 2 0·56; GGE: y = 0·29x − 0·32; R 2 0·50; net GGE: y = 0·048x − 1·862; R 2 0·030.
Comparison of the in vivo GGE values with the in vitro values for GE, GGE and net GGE showed an improved correspondence between the in vivo and in vitro methods as one converted successively from GE to GGE to net GGE (Fig. 1 and Table 5). Subtracting apparent GD from GGE to obtain net GGE provided, on average, an almost perfect overall correspondence between the in vivo and in vitro mean GGE determinations, although the individual data points remained scattered. The disparity, GGE in vitro minus GGE in vivo, was >5·0 g for forty-six foods (44>+5 g, 2 < − 5 g), while for net GGE in vitro (GD allowed for) minus GGE in vivo, the number of foods showing a>5 g disparity was reduced to twenty-five in total (10 < − 5 g, 15>5 g). The in vitro GGE minus in vivo GGE disparity was >10 g for twenty-three foods (no differences < 10 g), whereas the net GGE in vitro minus GGE in vivo disparity was >10 g for only eight foods (4>+4 g, 4 < − 4 g; Table 2).
Table 5 Changes in relationship between in vivo and in vitro values for glucose equivalents (GE), glycaemic glucose equivalents (GGE) and net GGE for all that foods based on values given in Table 2
Discussion
The present study investigated the in vitro measurement of the RGI of customarily consumed food portions. It confirmed with a large sample of eighty-three foods that use of a correction for GD, derived in a previous detailed study of responses to fifteen food intakes(Reference Monro, Mishra and Venn20), improves the validity with which in vitro determinations of the RGI of customarily consumed amounts of foods may predict relative glycaemic effects in vivo. Although the procedure was similar in principle to many other in vitro methods, e.g. Brighenti et al. (Reference Brighenti, Pellegrini and Casiraghi24), Englyst et al. (Reference Englyst, Englyst and Hudson25) and Goni et al. (Reference Goni, Garcia-Alonso and Saura-Calixto26) there are two particular aspects of the present study that deserve comment.
Firstly, the term ‘RGI’ was chosen to represent a rapidly acting food property, not a food effect. It is the rapidly imposed dietary loading of glycaemic carbohydrate by a food during digestion, measured relatively as GE by using a glucose reference and adjusted by the relative glycaemic potency (GI) of the constituent monosaccharides, that converts the GE to GGE. RGI therefore states the relative potential of a food quantity to increase blood glucose concentrations expressed as the amount of glucose that would theoretically have the same effect. The term impact is appropriate because the GGE determination was based on rapidly available carbohydrate measured in a time-limited digestion, and not on the exhaustive digestion used in usual available carbohydrate determination for food labelling. The terminology for both RGI and GGE is, therefore, scientific and accurate.
Secondly, the analysis was conducted in terms of usual food intakes, similar to or the same as the ‘reference amounts customarily consumed per eating occasion’ (US Food and Drug Administration, 2002), now preferred in USA nutrition labelling and dietetics(27). The data produced were, therefore, relevant to usual food consumption patterns. By comparing foods in portions of realistic size rather than in equicarbohydrate portions, as used in GI determination, the effects of homeostasis in the clinical results that were obtained and their consequent effect on the relationship between in vitro and in vivo GGE determinations were deduced. The work reported here has demonstrated the need to build an allowance for the emergent effects of homeostasis into the in vitro analysis.
There was a large amount of scatter in the data of the present study. The results of the Bland–Altman analyses (Fig. 1) showed that the approach taken to correcting for homeostasis was valuable, even if not totally sufficient for all the foods. However, two more recent studies that have examined the effect of allowing for apparent GD support the present findings with improved in vitro–in vivo correlations. The study in which the equations for the GD baselines were derived(Reference Monro, Mishra and Venn20), based on a sample of fifteen food intakes, produced an in vivo and in vitro relationship of

where y is the in vitro net GGE–in vivo GGE and x is the mean of net GGE in vitro and GGE in vivo, and the subsequent study of twenty-four British foods (results yet to be published) yielded relationships of

In the present study, the GGE values determined in vitro were able to give a statistically significant prediction of GGE values from human blood glucose responses for all the foods taken together and for five out of eleven of the individual food groupings (A, C, E, G and H; Table 3). Those food groupings that did not give a significant correlation generally contained few foods, and may yield significant predictive equations after adding further values. Although the present work has shown the benefit of allowing for homeostasis when establishing an in vitro–in vivo relationship, a very close correspondence between the in vitro and in vivo GGE values is always difficult to achieve because of the enormous variability in clinical blood glucose response measurements(Reference Vega-Lopez, Griffith and Ausman11, Reference Wolever, Vorster and Björk12, Reference Venn and Green28).
A number of factors that could act in vivo and not in vitro (Reference Louis-Sylvestre29, Reference Brand-Miller and Holt30) may have contributed to the modest correlations between in vitro and in vivo results, and they may have differed between and within the food groups due to the heterogeneity of food types in each group. Further research may yield additional factors, such as a quantity-dependent factor for delay in gastric emptying, which may further improve in vitro prediction of relative in vivo responses for certain food groups. For instance, delayed gastric emptying may have been a factor in foods containing a relatively high proportion of fat and of relatively large portion size. Combination foods – savoury (Food grouping J), which showed the greatest dependence of in vitro overprediction of in vivo GGE with increasing mean GGE dose (Bland–Altman slope 0·255, Table 4), consisted of 250–270 g portions and contained relatively high fat contents.
Given the large clinical variability in blood glucose responses seen here, it perhaps makes good sense, for the purposes of comparing foods, to measure RGI independently of the unstable physiological factors that affect measurements of glycaemic responses such as those used in standard GI determination. It is well established that relative rates of digestion are major determinants of glycaemic effects that can be measured with good precision in vitro (Reference Englyst, Englyst and Hudson25), the intrinsic glycaemic potency of major food sugars (their glycaemic indexes) has been replicated in numerous studies(Reference Foster-Powell, Holt and Brand-Miller19), and the glucose dose–glycaemic response relationship is very consistent when expressed on a glucose equivalence basis(Reference Monro and Shaw8). Now, dose-dependent rates of GD, as GGE/min, have been determined(Reference Monro, Mishra and Venn20), and in so far as they are reflected in the glucose dose–glycaemic response relationship, they are also likely to be consistent intrinsic human responses to blood glucose loading. Therefore, most of the elements necessary to obtain a valid indication of the RGI of amounts of foods customarily consumed, free of physiological fluctuations, are available. Thus, in vitro analysis may soon be able to provide predictions of RGI accurately enough to be used routinely in place of in vivo analysis. The in vitro analysis is, after all, a modified available carbohydrate determination in which the standard factors are included to introduce nutritional relevance.
There is a tendency to reject the use of in vitro measures of glycaemic impact when they cannot accurately predict clinical responses, and an assumption is usually made that the in vitro measures are unsuitable in guiding food choices for glycaemic control(Reference Brand-Miller and Holt30). However, one may conversely argue that glycaemic response is too inconsistent and too state-dependent, for its measurement under one set of clinical conditions (those of GI determination), with portion sizes that are not customarily consumed, to be any more valid than in vitro values as food guides in a community setting. Whether imprecise RGI values from such clinical measurements of glycaemic response are any more effective in dietary management of glycaemia than precise values from in vitro digestive analyses which have included factors for physiological relevance is yet to be established.
Acknowledgements
J. A. M. wrote the paper with comments from co-authors. J. A. M. and S. M. conducted the in vitro analyses. J. A. M. conceived the use of a GD baseline to obtain net glycaemic loading. A. W., S. E. and A. W. conducted the clinical measurements. R. S. S. advised D. H. carried out the statistical analysis. The research was supported by the New Zealand Foundation for Research, Science and Technology as part of contract C02X0401 (Foods for Energy Balance). None of the authors has any personal or financial conflict of interest.








