End-jejunostomy syndrome is classified as a type of short bowel syndrome (SBS); it is caused by resection or exclusion of the ileum and colon. It is a rare condition with a prevalence in Europe estimated to be between 0·4 and 30·0 per million for SBS(Reference Jeppesen1), and 17 % of this number is accounted for by the jejunostomy type(Reference Amiot, Messing and Corcos2). The prevalence of the end-jejunostomy type of SBS is higher in countries where surgeons are less inclined to bowel reconstructive surgery and where the survival of home parenteral nutrition (HPN) patients is higher. Massive intestinal resection induces major functional and metabolic changes, not only within the gastrointestinal (GI) system but also extends consequences to other organs and tissues. The severity of those disturbances depends primarily on the length of the remaining intestine but is also influenced by coexisting medical conditions and the types of treatment applied(Reference Pironi, Arends and Bozzetti3). ESPEN guidelines on chronic intestinal failure in adults (recommendation 42) suggest limiting the oral intake of low-Na, hypotonic (e.g. water, tea, coffee or alcohol) and hypertonic (e.g. fruit juices and colas) solutions in order to reduce output in patients with net-secretion and a high output jejunostomy (grade of evidence-low, strength of recommendation-weak), but does not address acceptable limits of stoma output. Use of H2-receptor antagonists, proton pump inhibitors, loperamide, teduglutide or short-term use of octreotide is suggested to reduce stoma output exceeding 2 litres/d. Guidelines published by Nightingale & Woodward on behalf of the British Society of Gastroenterology(Reference Nightingale and Woodward4) describe in detail medical metabolic problems related to jejunostomy and recommend pharmacotherapy, modification or reduction of oral feeding. These authors note that losses of <1200 ml are easier to manage, but that marked Na and water depletion and severe thirst might require keeping the patient ‘nil by mouth’; they do not, however, indicate an acceptable limit for the stoma output.
The anatomical alterations in end-jejunostomy patients are more severe than those in the other types of SBS (jejunoileal and jejunocolic anastomosis), which make this group of patients prone to the most critical malabsorptive complications and presents the highest risk of dependence on permanent parenteral nutrition (PN). Long-term complications of ileal resection include hepatic steatosis, gallstones, renal failure and intestinal failure-associated liver disease(Reference Pironi, Arends and Bozzetti3,Reference Buchman5–Reference Tappenden8) .
There are different approaches to the treatment of high output end-jejunostomy syndrome with regard to oral intake. Some centres argue that it is unethical to forbid a patient to take oral nutrition and that any restriction of oral intake is unacceptable, so the patient should be allowed to eat ad libitum. Others claim that it is more efficient to limit oral intake in an attempt to reduce stomal output and, in consequence, to diminish nutrient and water losses. The principal aim of either approach should be to prepare the patient for any feasible reconstructive surgery as soon as possible.
The aim of the study was to compare the results of two nutritional approaches: unrestricted and restricted oral intake in patients with end jejunostomy and SBS commencing HPN. Outcomes included biochemical markers of liver and renal status and the time to reconstructive bowel surgery, in each case with correlation to stoma output. The first approach was based on unrestricted oral food and fluid intake, and the second included oral intake restrictions in order to keep stomal output below 1000 ml/d.
Materials and methods
This retrospective study comprised new patients with a high output jejunostomy (>1500 ml/d) included in a home parenteral nutrition (HPN) programme in the years 2015 and 2016. Twenty patients were involved in the study. The aetiologies of the end-jejunostomy were as follows: Crohn’s disease (five patients), intestinal ischaemia (three patients), intestinal obstruction (three patients), trauma (two patients), ulcerative colitis (one patient) and other postoperative complications (six patients). Once stabilised, patients were divided into two groups according to their preference:
-
Group A – ten patients with oral intake restricted to keep stoma output under 1000 ml and
-
Group B – ten patients without any oral intake restrictions.
All patients then received HPN infused every day over 16–18 h.
The characteristics of the two groups are presented in Table 1.
Table 1. Characteristics of groups (Numbers of patients; median values and interquartile ranges (IQR))
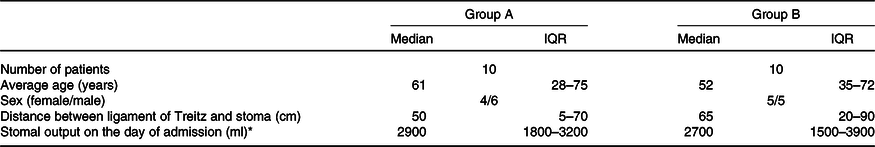
* First admission to home parenteral nutrition unit.
The following parameters were evaluated over a 6-month period: stoma output, self-estimation of condition, body weight gain, plasma concentrations of bilirubin and creatinine, number of hospitalisations prior to surgery, number of daily emptyings of ostomy bag, feelings of hunger and thirst in the daytime, and time to gain optimal status for reconstructive surgery.
At the time of recruitment, patients had spent 10–26 d in the ward to stabilise their metabolic status, cure any infections (e.g. CRBSI), achieve long-term central venous catheter implantation, reduce the stomal losses (pharmacological, dietetic), optimise the PN mixture and educate the patient and/or family member.
The education programme includes the preparation and connection of a nutritional mixture, knowledge about complications and necessary procedures if suspected (cessation of infusion, contact with the PN centre 24 h/d and 7 d/week, admission 24 h/d), diet and techniques to limit losses from the stoma, and also includes consultation with a psychologist to assist with the acceptance of a difficult life situation and the importance of complying with medical recommendations.
The educational programme was conducted by a physician, a nurse, a psychologist and HPN patients from the national support group. The patient was discharged home only if his/her education satisfied all educators. The recommendations on adjustment of oral diet were the same in both groups and included a limitation of solid foods, restricting fluids to small amounts of isotonic fluids or fluids mixed with thickening powder. Pharmacological recommendations were uniform and included proton pump inhibitors, loperamide and racecadotril if needed. Each patient was advised to regulate the oral intake so that the secretion from the stoma did not exceed 1000 ml/d.
The composition of the nutritional mixture was adjusted as follows:
-
Energy from glucose and lipids – increased to the level at which the patient would not feel hunger, usually in the range of 84–126 kJ/kg (20–30 kcal/kg) body weight,
-
Amino acids 1 g/kg body weight,
-
Electrolytes – to keep plasma levels within reference values,
-
Vitamins and trace elements – average daily dose,
-
Water – to keep diuresis in the range of 1500–2000 ml/d.
Monitoring visits were scheduled 4 weeks from discharge from hospital and then every 2–8 weeks depending on patients’ metabolic stability and well-being. A standard evaluation comprised subjective assessment, recording of symptoms, stomal output, diuresis in the 48 h prior to the visit, morphometric evaluation and appropriate blood tests. The PN admixture was adjusted according to these parameters. Typically, patients increase physical activity at home and require a 10–20 % increase in energy from macronutrients to suppress hunger and weakness in the afternoon. During each visit of both groups, the physician encouraged the patient to limit stomal output.
Because the data were collected retrospectively, the statistical power was estimated post hoc. The non-parametric Mann–Whitney U test was used for the analysis. Power calculations were based on a sample size of ten participants in each group (allocation 1:1) with two-sided significance level of 0·0125 (Bonferroni correction was used for multiple comparisons). The estimated statistical power of the analysis was 0·75 for primary outcomes (time-to-surgery). Also, the estimated statistical power of the analyses for secondary outcomes was 0·99 (creatinine in sixth month on HPN), 0·46 (bilirubin in sixth month on HPN) and 0·37 (number of hospitalisations prior to surgery). Statistical power was calculated using G*Power version 3.1.9.2 (Universität Kiel)(Reference Faul, Erdfelder and Lang9).
The Mann–Whitney U test was used to show differences for selected parameters in groups A and B. The following parameters were included: time-to-surgery (primary outcome), bilirubin in sixth month on HPN, creatinine in sixth month on HPN, and number of hospitalisations prior to surgery (secondary outcomes). The effect size for the observed difference was estimated with the help of Cohen’s d coefficient, whereby 0·2 is considered a ‘small’ effect size, 0·5 represents a ‘medium’ effect size and 0·8 – a ‘large’ effect size(Reference Fritz, Morris and Richler10). The verification of the null hypothesis was conducted for each analysis with the a priori assumption of the statistical significance at 0·0125. All analyses were performed with STATISTICA version 13.3 (TIBCO Software Inc.).
The institutional ethics committee was informed about this retrospective study, no objections were raised and consent was given.
Results
Patients in group B experienced lower quality of life, caused mostly by the need to empty the stoma bag at night – from two to five times per night (compared with no need for emptying the bag in group A). Impaired quality of sleep and the need for frequent getting up at night contributed to permanent weakness and depression. These patients also suffered from peeling off and leaking from the ostomy bag, and skin erosion around the stoma and complained about hunger and thirst during the day (with minimal occurrences in group A). The stomal output in the group with restricted oral intake had fallen on average by 1950 ml by 4 weeks after the discharge from the HPN unit, while in group B, it increased by 100 ml (Fig. 1).
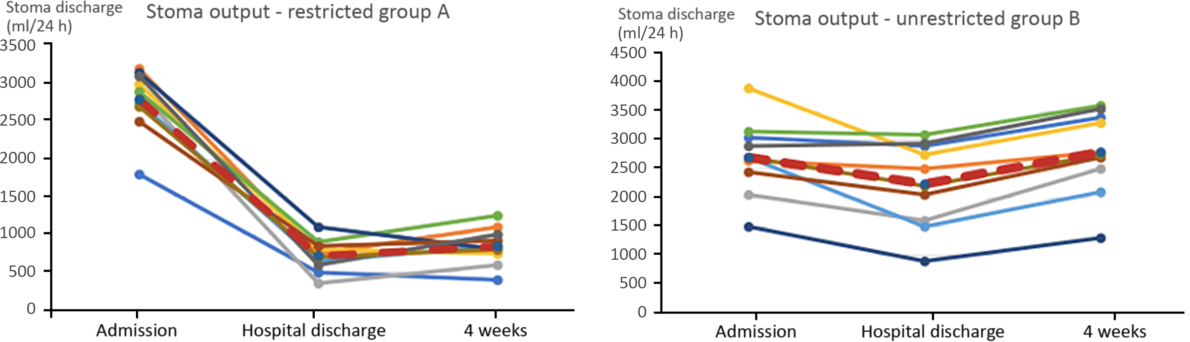
Fig. 1. Stoma output (ml) on admission, on discharge from hospital and after 4 weeks on home parenteral nutrition. ![]() , Average.
, Average.
The average volume of provided PN was accordingly higher in group B than in group A (4850 v. 3150 ml, respectively), but the average weight gain at 6 months was lower, as it reached 2·1 kg in group B and 5·5 kg in group A. Patients in group B developed more complications (mainly hepatic and renal dysfunction) and required more episodes of hospitalisation prior to surgery. Furthermore, one death was observed amongst the subjects on unrestricted oral intake, due to renal insufficiency followed by septic complications. The average time required for preparation to reconstructive surgery was 7·5 months in group A, and 22·1 months in group B. Other study results are presented in Tables 2 and 3. Correlations between stoma discharge at 4 weeks on HPN and serum creatinine or bilirubin at 6 months on HPN are presented in Figs. 2 and 3, respectively.
Table 2. Comparison between groups with regard to time-to-surgery, serum bilirubin and creatinine and number of hospitalisations prior to surgery (Median values and interquartile ranges (IQR))
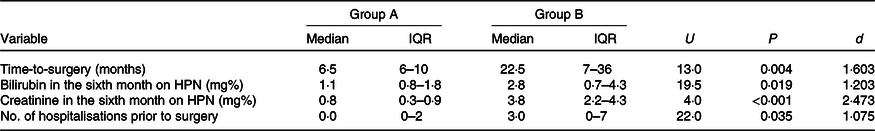
d, Cohen’s d coefficient; HPN, home parenteral nutrition.
Table 3. Comparison between groups with regard to the need for ostomy bag emptying at night, thirst, hunger and weakness
(Numbers, mean values and range)
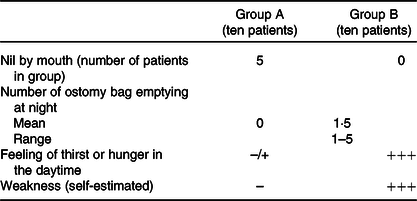
–/+, Rarely experienced; –, never reported; +++, experienced every day and reported by all patients.
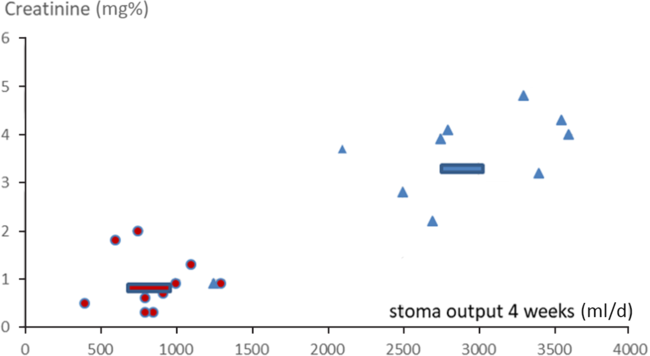
Fig. 2. Correlations between stoma discharge volumes at 4 weeks on home parenteral nutrition (HPN) and creatinine at 6 months on HPN (correlation coefficients group B 0·54, group A 0·0003). ![]() , Average restricted;
, Average restricted; ![]() , restricted;
, restricted; ![]() , average unrestricted;
, average unrestricted; ![]() , unrestricted.
, unrestricted.
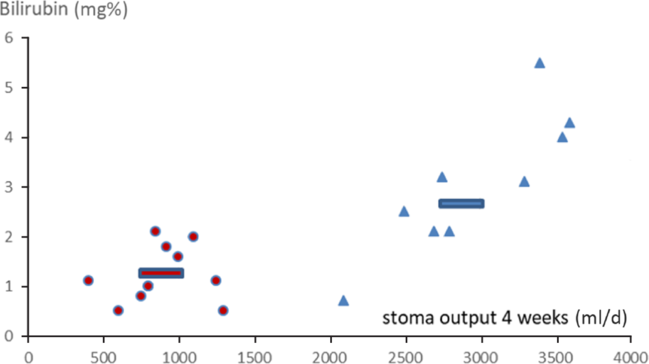
Fig. 3. Correlations between stoma discharge volumes at 4 weeks on home parenteral nutrition (HPN) and bilirubin at 6 months on HPN (correlation coefficients group B 0·77, group A 0·21). ![]() , Average restricted;
, Average restricted; ![]() , restricted;
, restricted; ![]() , average unrestricted;
, average unrestricted; ![]() , unrestricted.
, unrestricted.
Discussion
The argument put forward by the supporters of unrestricted oral intake is the positive impact of enteral feeding on intestinal adaptation after surgery. The presence of food components in the intestinal lumen affects enterohormone secretion and stimulates villous growth(Reference Ba’ath, Almond and King11,Reference McClure and Newell12) . Blood flow and the enteric nervous system are also stimulated. Nevertheless, possible advantages of unrestricted oral intake can probably be realised only when the fluid losses from the stoma do not exceed the volume of urine excretion, which is most rare among end-jejunostomy patients without oral restrictions. Another argument supporting unrestricted oral intake is that the patient’s quality of life is lower if he is not allowed to eat, mostly because of the accompanying feelings of hunger and thirst. The results of our study show the opposite results, with a better quality of life and significantly lower senses of thirst and hunger in patients with oral intake restrictions than in those with an unrestricted regimen. We conclude that this is because oral feeding induces more losses of nutrients than it effectively provides, being unable to satisfy the expectations of the meal’s intake, and leading to frustration in the patient. Data obtained from the study show that a PN mixture (adjusted to the individual patient) supplies adequate hydration and nutrition most effectively when excessive losses are avoided. If the patient has persisting thirst or hunger, adjustment of the PN mixture is sufficiently potent to control these negative feelings(Reference Bines, Taylor and Justice13,Reference Ławiński, Haraszczuk and Gradowska14) . Prolonging infusion of PN to 24 h using a portable pump system is useful as well. However, the numerical correction of fluid and energy balance by modified PN is evidently not sufficient to avoid the higher rates of complication seen in our patients on the unrestricted regimen.
Every day up to 8–10 litres of fluid, containing approximately 800 mmol of Na+, 700 mmol of Cl−, and 100 mmol of K+, passes through the intestinal lumen. The net fluid transport through the GI epithelium results primarily from the active transport of Na+, Cl− and
![]() ${\rm{HCO}}_3^ - $
(Reference Kiela and Ghishan15). In some individuals with an end-jejunostomy, effective water and salt absorption, together with reabsorption of intestinal and gastric secretions, becomes impossible to obtain(Reference Pironi, Arends and Bozzetti3,Reference Tappenden8,Reference Nightingale, Lennard-Jones and Walker16) . Increased gastric emptying and accelerated intestinal motility in these patients exacerbate this problem(Reference Nightingale, Kamm and Van der Sijp17) manifests ultimately as major stomal losses.
${\rm{HCO}}_3^ - $
(Reference Kiela and Ghishan15). In some individuals with an end-jejunostomy, effective water and salt absorption, together with reabsorption of intestinal and gastric secretions, becomes impossible to obtain(Reference Pironi, Arends and Bozzetti3,Reference Tappenden8,Reference Nightingale, Lennard-Jones and Walker16) . Increased gastric emptying and accelerated intestinal motility in these patients exacerbate this problem(Reference Nightingale, Kamm and Van der Sijp17) manifests ultimately as major stomal losses.
The complex interaction of stimulating and inhibitory enterohormones is severely compromised in end-jejunostomy patients. Because of the decrease in peptide tyrosine-tyrosine levels and lack of secretion of other inhibitory hormones, gastric emptying is accelerated and secretion increased(Reference Little, Doran and Meyer18,Reference Nightingale, Kamm and Van der Sijp19) . Reduced GLP-2 levels may also result in accelerated gastric emptying(Reference Nagell, Wettergren and Pedersen20). Moreover, in subjects with SBS, postprandial contractions have greater amplitude and velocity(Reference Scolapio, Camilleri and Fleming21). Because of these hormonal alterations and changes in motility, the passage time of nutrients is shortened, which leads to reduced absorption, deterioration in the patient’s nutritional status, and dehydration as the stomal output exceeds the fluid and nutrient intake. SBS also exacerbates Na, Ca, Mg and trace element deficiency, as they are all present in GI secretions(Reference Dijkstra, Kuipers and Smit22). In addition, hypersecretion of hydrochloric acid may cause acid-related disease (e.g. peptic ulcer)(Reference Tappenden8,Reference Williams, Evans and King23) , and the acid itself accelerates the GI transit time(Reference Fang, Ginsberg and Glassman24). Massive intestinal resection disconnects the inhibitory reflexes in the enteric nervous system, including cologastric and ileogastric reflexes, further contributing to accelerated motility(Reference Wood25). With an absence of ileum and colon in continuity, carbohydrate digestion is not only less efficient but that unabsorbed can also no longer provide the substrate for generation of NEFA (needed not least by any retained but excluded colon)(Reference Tappenden8,Reference DeSesso and Jacobson26–Reference Jeejeebhoy28) .
There are factors increasing the required energy input in some SBS patients, such as ongoing inflammation (bacterial overgrowth and translocation) and the consequences of complications of surgery(Reference DiBaise, Young and Vanderhoof29). The thermic effect of food also remains meaningful. It is defined as the amount of energy expenditure above the basal metabolic rate attributable to the processing of food for use and storage(Reference Denzer and Young30). Thus, the presence of food in the digestive tract forces the body to expend energy in digestion and absorption (e.g. enzyme synthesis, secretion, active absorption and motility)(Reference Barr and Wright31). Digestion and absorption of food and fluids are not a cost-free, passive process. It is inseparably related to important energy and protein expenditure, especially in the first part of the small intestine. The presence of food in the GI tract is considered to be the strongest secretory stimulus for the duodenum and jejunum. The presence of chyme also stimulates the production and secretion of saliva, gastric acid, pancreatic juice and bile salts. Monosaccharide absorption starts in the duodenum and the first 50 cm of jejunum via facilitated diffusion, but glucose and galactose are also absorbed actively, with the Na-dependent glucose transporter 1(Reference Harada and Inagaki32). Even facilitated absorption starts only when the osmolar load of the chyme becomes balanced by GI secretions. Energy (ATP), transport of ions (Na+, K+, Mg2+ and Cl–) and specific transporters are needed also for absorption of amino acids, peptides and fatty acids. The thermic effect of food equates to about 15–25 % of the energy content of ingested food and is a debt which must be paid, but will never be reimbursed, in the patient with a proximal jejunal stoma. Thus, excessive oral feeding promotes a negative energy balance. Attempts to compensate for this parenterally run the risk that the additional energy provision may overwhelm metabolic limits, burden the liver and lead to intestinal failure associated liver disease, as is suggested by the present study.
Proteins, in the form of enzymes and transporters needed for digestion and absorption, are crucial to the health of the individual, even leaving aside their structural importance and key roles in muscle. In health, there is a daily GI enzyme production of about 9 g of nitrogen as protein equivalent(Reference Nixon and Mawer33). Almost the full amount can be lost in a high stomal output. Complete parenteral compensation is difficult to achieve because of the metabolic limits for proteins, and for technical reasons of amino acid delivery and their stability in the admixture. In health, all of the ‘investment’ in enzymes has adequate recompense as peptides and numerous other dependent nutrients are regained during absorption. In contrast, in end-jejunostomy syndrome, with only a short part of the small bowel and no colon in continuity, digestion-related expenditure markedly exceeds its income. This phenomenon of major net loss from the stoma aggravated by uncompensated thermic effect of food is the main cause inhibiting weight gain in the patient awaiting reconstructive surgery. The uncompensated thermic effect of food mechanism may also contribute to the increased thirst and hunger in the unrestricted group: ‘the more you eat, the more you lose, the hungrier you are’.
Oral feeding stimulates the secretion of bile and bicarbonate via the secretin/CCK mechanism(Reference Rehfeld34). Bile losses will incorporate losses of: bile acids, phospholipids, cholesterol, fats, fatty acids, bilirubin and trace elements, and can contribute to hepatic deterioration – reflected in our study by an increased mean bilirubin in the unrestricted group (Fig. 3). Decreased levels of pancreatic bicarbonate secretion (
![]() ${\rm{HCO}}_3^ - $
) may contribute to acidosis, leading to an increased renal burden and greater risk of insufficiency. Creatinine levels readily rise, and patients require readmission to hospital (Table 2 and Fig. 2).
${\rm{HCO}}_3^ - $
) may contribute to acidosis, leading to an increased renal burden and greater risk of insufficiency. Creatinine levels readily rise, and patients require readmission to hospital (Table 2 and Fig. 2).
Optimal treatment of the patient with a high-output jejunostomy is a multidirectional task that requires specific knowledge and clinical experience(Reference Buchman5,Reference Ba’ath, Almond and King11,Reference Ławiński, Haraszczuk and Gradowska14,Reference Eça and Barbosa35) . Pharmacological agents (such as H2-receptor antagonists, racecadotril, proton pump inhibitors) are helpful to decrease gastric and consequently intestinal secretion(Reference Pironi, Arends and Bozzetti3,Reference Eça and Barbosa35,Reference Wall36) . Oral rehydration with the use of ORS (oral rehydration solutions), such as St. Mark’s solution (sodium chloride 3·5 g, sodium bicarbonate 2·5 g, glucose 20 g, water 1 litre) seems to be more effective than drinking water, as their higher Na content leads to less stimulation of GI secretion than from water. These solutions are believed to rehydrate quickly, as they are easily absorbed(Reference Atia and Buchman37,Reference Cutting and Langmuir38) .
We recognise some weak points of the study. (1) The study involved a small number of patients, but end-jejunostomy SBS is a rare disease. To the best of our knowledge, no other evidence exists in the literature specifically addressing the acceptable limit of stoma output. A multicentre study would be even more demanding and difficult to conduct. (2) The study is not randomised. Compelling patients to follow medical recommendations is inappropriate, and efforts to secure high rates of adherence are often very demanding. It may not be possible to check the patient’s compliance. A sincere declaration of patient compliance might therefore be more valuable than randomisation. A randomised study could be burdened with at least as much observational error as a retrospective study.
Conclusion
End-jejunostomy syndrome is a complex condition, requiring close cooperation of a multidisciplinary medical team, including surgeon, gastroenterologist, dietitian, psychologist and nurse trained in PN. Unrestricted oral intake provokes uncontrolled losses of energy and protein substrates resulting in inhibition of weight gain. Excessive losses of bile salts and energy overload burden the liver, whilst high losses of water and bicarbonate aggravate renal function. Restriction in oral intake seems to be valid and effective in the prevention of HPN-related complications and in shortening the time to surgery. Furthermore, in end-jejunostomy patients, oral intake increases stoma output, without reducing the hunger or thirst. At the same time, it tends to impair quality of life, leading to the conclusion that psychological support for these patients is crucial to reach nutritional goals. Psychological support and strict cooperation of the nutritional team with patients and families to help the patients restrict oral intake is crucial to reach nutritional goals.
Acknowledgements
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
J. S. designed the study; Z. Z., P. J., K. D., P. G. and K. M. collected the data; J. S., M. K. and A. F. interpreted the data; M. P. conducted the statistical analysis; Z. Z. drafted the manuscript and J. S. and A. F. revised the manuscript. J. S. had primary responsibility for the final content. All authors agreed on the final version of the manuscript.
On behalf of all authors, the corresponding author states that there is no conflict of interest.









