Nutritional sufficiency of vitamin D (D) is of current interest, especially maternal sufficiency during pregnancy and the consequences of carryover effects onto the next generation. Our interest in maternal dietary D carryover effects on neonatal skeletal abnormalities began with an unprecedented outbreak and subsidence of kyphosis in our swine research herd at the University of Wisconsin Swine Research and Teaching Center (SRTC)( Reference Rortvedt and Crenshaw 1 ). Kyphosis is defined as an idiopathic disease characterised by outward spinal curvature. Following the initial outbreak, we determined, through multiple experiments, that the kyphosis was induced by an accidental omission of D from a custom mixed vitamin and mineral premix. For reproduction of the abnormality, D must be absent or marginal in maternal diets during gestation and lactation. The dietary constraints to induce the hypovitaminosis D kyphotic pig have been replicated in multiple controlled experiments in efforts to more completely understand and characterise the role of maternal dietary D in offspring bone development( Reference Rortvedt and Crenshaw 1 , Reference Amundson, Hernandez and Laporta 2 ).
Kyphosis was not the only apparent skeletal abnormality induced in the hypovitaminosis D kyphotic pig model, although classical symptoms of rickets, such as bowed limbs, were not visible. Other affected traits included an 11 % reduction in growth, a 25 % reduction in whole body bone mineral content, compromised femur mechanical strength properties and visual gross abnormalities in the growth plates of femurs and vertebrae at birth, weaning and the end of a 4-week nursery growth phase( Reference Amundson, Hernandez and Laporta 2 ). These results were unexpected as a deficiency of a fat soluble vitamin, such as D, was assumed to be difficult to induce over short durations (4-month gestation in sows) based on earlier work with rats( Reference Rosenstreich, Rich and Volwiler 3 , Reference Brouwer, van Beek and Ferwerda 4 ), pigs( Reference Johnson and Palmer 5 – Reference Burild, Frandsen and Poulsen 7 ) and humans( Reference Mawer, Backhouse and Holman 8 ). Additional support for physiologically relevant D tissue storage is based on the maintenance of human D status over seasonal cycles in populations at latitudes precluding UV exposure sufficient for D synthesis( Reference Blum, Dolnikowski and Seyoum 9 , Reference Didriksen, Burild and Jakobsen 10 ).
Maternal D status during pregnancy is of growing concern in human nutrition( Reference Liu and Hewison 11 , Reference Dror and Allen 12 ). Bone development was compromised in adolescents if mothers had poor D status, based on serum 25-OH-D3 concentrations( Reference Mahon, Harvey and Crozier 13 – Reference Javaid, Crozier and Harvey 15 ). A major limitation in understanding and identifying solutions to questions of maternal carryover implied in these and other human and animal studies involving D is the reliance and validity of serum 25-OH-D3 as a biomarker for D status( 16 ). The recommended daily allowance for human dietary D relies mostly on epidemiological studies which have inherent limitations( Reference Dror and Allen 12 ). A reproducible animal model such as the hypovitaminosis D kyphotic pig model offers a method to assess diet-induced factors which lead to bone abnormalities associated with maternal dietary D deficiencies. The swine model provides an opportunity to further understand the aetiology of offspring bone developmental abnormalities in response to maternal dietary D in both animals and humans.
Our previous results( Reference Rortvedt and Crenshaw 1 , Reference Amundson, Hernandez and Laporta 2 ) provided evidence for maternal dietary D carryover effects. Gross skeletal abnormalities have been partially characterised. The objective of the current experiment was to assess molecular changes in homoeostatic pathways associated with D and bone metabolism in pigs produced by sows fed maternal diets with a range of D supplementation. In addition, serum D, tissue D storage concentrations and molecular traits associated with skeletal development were characterised. Characterisation of molecular changes in homoeostatic pathways will help further clarify the maternal carryover effects that lead to the manifestation of kyphosis and associated bone abnormalities in young pigs.
Methods
Ethics statement
All animal procedures were approved (Protocol A00865) by the College of Agricultural and Life Sciences Animal Care and Use Committee, University of Wisconsin-Madison. Animals selected for tissue collections were humanely euthanised by electrical stunning followed by exsanguination.
Experimental design and maternal diets
An overview of the experimental design that involved gestation, lactation and nursery phases is outlined in Fig. 1. Using three farrowing groups (trials) to facilitate tissue collections, cross-bred (PIC Large White×Landrace) first parity sows (n 37) were bred via artificial insemination with semen collected from an individual Line 19 boar (PIC Inc.). All sows, the boar, and pigs were produced and housed at the SRTC throughout the trial. The SRTC is an environmentally-regulated, totally enclosed facility, which allows no animal exposure to UVB rays in the range of 280 –315 nm from natural or supplemental lighting. Fluorescent lights were controlled to provide 16 h light–8 h dark during gestation and 12 h light–12 h dark during farrowing and nursery phases.

Fig. 1 Timeline of dietary treatments fed to sows during gestation and lactation and diets fed to nursery pigs. Maternal diets were formulated to supply either 0 (−D); 8·125 (+D); or 43·750 (++D) µg vitamin D3/kg diet in complete diets fed from breeding through the lactation phase. Nursery diets were formulated to supply either 0 µg vitamin D3/kg (−D) or 7·0 µg vitamin D3/kg (+D) and either 75 and 95 % (LCaP) or 150 and 120 % (HCaP) of the calcium and phosphorus requirements, respectively, for 10–20 kg pigs. Pigs were fed an adjustment diet during the first week of the nursery, which was consistent with routine diets fed to the herd (whey, maize, soyabean meal and oat groats) except the diet contained no supplemental vitamin D.
At breeding, sows were randomly assigned (n 12, 12 and 13/treatment, respectively) to one of three dietary treatments that varied in D supplementation to provide either 0 (−D), 8·125 (+D) or 43·750 (++D) µg D3/kg diet (where 1 IU is defined as the biological activity of 0·025 µg cholecalciferol) (online Supplementary Table S1). These diets represent a negative control (−D), the standard amount of D routinely fed to sows for multiple years in the SRTC herd (+D) and a diet with D at concentrations consistent with amounts routinely fed to sow herds in the commercial swine industry (++D). Sows had free access to water and were fed their assigned dietary treatments throughout gestation (2·2 kg/d) and lactation (continuous access to feed) phases. Sows were allowed to complete parturition naturally.
Piglets were housed with the sow from birth through the lactation period and allowed uncontrolled access to the sow for milk consumption. Pigs were weaned after a 24·7 (sem 0·06)-d lactation period (3 weeks) and randomly assigned within maternal treatment groups to one of four nursery dietary treatments that varied in D, Ca and P concentrations. Using a 2×2 factorial design, nursery diets (online Supplementary Table S2) were formulated to supply either 0 (−D) or 7·0 (+D) µg D3/kg, each with 75 and 95 % (LCaP) or 150 and 120 % (HCaP) of the Ca and P requirements, respectively. Requirements were based on concentrations suggested for 10–20 kg pigs( 17 ). Pigs were fed an adjustment diet with no supplemental D for 1 week after weaning, before being fed treatment diets for 3 weeks for a total nursery phase length of 4 weeks. The 1-week adjustment diet included whey protein and was fed to allow pigs to transition from weaning to solid feed. Final observations and tissue samples were collected from a subset of pigs at 52·4 (sem 0·17) d of age (7 weeks). During the nursery phase pigs were housed in pens (1·4×2·4 m; n 2–6 pigs/pen) and allowed continuous access to their assigned dietary treatments and water throughout the nursery phase.
Pig body weights were recorded at birth (0 week), 3 weeks and weekly during the 4-week nursery phase. At 13 weeks of age remaining pigs were observed for visual evidence of kyphosis even though dietary treatments were discontinued at 7 weeks. Pigs were fed standard SRTC diets which included supplemental D (7·0 µg/kg diet) from 7–13 weeks. In earlier work with the hypovitaminosis D kyphotic model( Reference Rortvedt and Crenshaw 1 ), kyphosis was grossly evident at 13 weeks, but was difficult to visually appraise in pigs at younger ages.
Kyphosis prevalence
Observations for gross, visual symptoms of kyphosis in pigs were recorded at 13 weeks. A subjective score of 1 (no curvature), 2 (marginal curvature) or 3 (obvious curvature) was assigned to each pig by two observers based on visual evidence of spinal column curvature in the live animal. The final assessments of the prevalence of kyphosis were based only on animals which were assigned a score of 3 by both observers.
Serum analysis
Vacuum tubes (no anticoagulant) were used for collection of venous blood samples (10 ml) from the brachial region of sows at parturition in the first farrowing group (trial) and the subsets of pigs selected in each of the three farrowing groups for tissue sample collections at 0, 3 and 7 weeks for serum Ca, P, parathyroid hormone (PTH) and D analysis. Blood samples were allowed to clot at room temperature and then centrifuged (735 g ) for 20 min. Serum was separated and stored frozen (−20°C) until analysis.
Aliquots of serum were diluted with a lanthanum chloride solution (2000 μg La/ml), and Ca concentrations were determined using atomic absorption spectrophotometry procedures (Perkin-Elmer AAnalyst 400 Spectrometer; Perkin-Elmer Corp.).
A separate aliquot of serum was precipitated with tricarboxylic acid (10 % w/v), and the P concentration in the supernatant was determined using a molybdovanadate, colorimetric procedure. Absorbance was measured at 660 nm using a spectrophotometer (Gilford Spectrophotometer 260; Gilford Instrument Laboratories, Inc.).
Serum intact PTH levels were determined using a porcine specific ELISA kit (#60–3305; Immunotopics International) according to the manufacturer’s instructions. The detection limit was 1·0 pg/ml.
Serum levels of D metabolites (D3; 25-OH-D3; 24,25-OH2-D3) were determined by DSM Nutritional Products Analytical Research Center, Basel, Switzerland. An internal standard was added to the serum followed by protein precipitation. The supernatant was evaporated and the residue reconstituted with an organic-water solution. An aliquot was injected into the liquid chromatography-MS/MS system and detection was performed using a multiple reaction monitoring mode. The lower limit of quantification (LLQ) was 0·500 ng/ml for D3 and 24,25-OH2-D3 and 1·00 ng/ml for 25-OH-D3. The precision (% CV) was 15 % (20 % for LLQ-value) and accuracy was 85–115 % (80–120 % for LLQ-value).
Quantitative real time PCR
Sections of kidney (cortex), distal femur, and the 15th vertebral body tissues were collected immediately after euthanasia of each pig. Samples were frozen in liquid N2, and stored at −80°C until quantitative real time PCR (qPCR) analysis. The RNA isolation and qPCR analysis were completed as described previously( Reference Amundson, Hernandez and Laporta 2 ) with a few modifications. Relative kidney mRNA expression of 24-hydroxylase (CYP24A1) and 1-alpha hydroxylase (CYP27B1) were measured and normalised to the geometrical average of three housekeeping genes (hypoxanthine phosphoribosyltransferase 1, HPRT1; ribosomal protein S18; cyclophilin A). Relative femur and vertebrae mRNA expression of osteocalcin (OCN) were measured and normalised to one reference gene (vitamin D receptor, VDR). Reference genes were verified to be stably expressed across treatments within their respective tissues.
The qPCR data were analysed using the
![]() $$2^{{{\minus}\Delta \Delta C_{t} }} $$
method(
Reference Schmittgen and Livak
18
) with the pigs produced by sows fed –D diets serving as the control for samples at 0 and 3 weeks and pigs produced by sows fed –D diets and fed –DLCaP nursery diets serving as the control for samples at 7 weeks. Primer sequences are listed in the online Supplementary Table S3. Primer sequences were designed with the Primer3 program based on gene sequences obtained from the National Center for Biotechnology Information except for VDR
(
Reference Hittmeier, Grapes and Lensing
19
) and HPRT1
(
Reference Nygard, Jorgensen and Cirera
20
).
$$2^{{{\minus}\Delta \Delta C_{t} }} $$
method(
Reference Schmittgen and Livak
18
) with the pigs produced by sows fed –D diets serving as the control for samples at 0 and 3 weeks and pigs produced by sows fed –D diets and fed –DLCaP nursery diets serving as the control for samples at 7 weeks. Primer sequences are listed in the online Supplementary Table S3. Primer sequences were designed with the Primer3 program based on gene sequences obtained from the National Center for Biotechnology Information except for VDR
(
Reference Hittmeier, Grapes and Lensing
19
) and HPRT1
(
Reference Nygard, Jorgensen and Cirera
20
).
Milk vitamin D analysis
Milk was collected from sows at parturition (colostrum) and approximately 1 week before weaning (day 18 milk) and stored at −20°C until further analysis. Colostrum and day 18 milk concentrations of 25-OH-D3 were determined by DSM Nutritional Products Analytical Research Center, Basel Switzerland. An internal standard was added to the samples, followed by saponification and a liquid-liquid extraction to extract 25-OH-D3. Following evaporation of the extract, the sample was cleaned by solid phase extraction, evaporated, reconstituted in injection solvent, and injected into the LC-MS/MS system. Standards and quality controls were analysed daily to ensure accuracy and precision (±15 %). The LLQ was 0·0169 ng/ml.
Tissue vitamin D analysis
Sections of subcutaneous fat (dorsal cervical region), liver and muscle (psoas major) tissues were collected immediately after euthanasia of each pig at 0, 3 and 7 weeks. Samples were frozen in liquid N2, and stored at −80°C until further analysis. Tissue levels of 25-OH-D3 were determined by DSM Nutritional Products Analytical Research Center, Basel Switzerland. An internal standard was added to each sample followed by extraction with acidic methanol (and SDS solution for fat tissue). The supernatant was cleaned by solid phase extraction and the solvent evaporated by N. The residue was reconstituted in injection solvent and analysed by LC-MS/MS. The method has been validated using poultry tissue according to FDA and EMEA guidelines with an accuracy and precision of ±15 %. The LLQ is 1·00 ng/g for liver, 0·50 ng/g for muscle and 2·00 ng/g for fat tissue.
Statistical analysis
The objective of the current experiment was to assess carryover effects of maternal diets on traits in pigs produced by sows fed the maternal dietary treatments. Variable formulations of nursery diets were used to further assess maternal diet carryover effects on traits in the pigs. Thus, the overall model involved a 3×4 factorial arrangement of treatments analysed as a split-plot design. Fixed effects in the main plot units were maternal diets. Sub-plot unit fixed effects were nursery diets and the two-way interactions with maternal diets and nursery diets. The experiment was conducted in three replicate trials to facilitate tissue collections. Trial was included in the model as a random effect. The complete model used to assess traits (Y) in pigs at completion of the 7-week experiment was: Y hijk =μ+MD h +T i +Sow j (MD T)w j(hi)+ND k +(MD×ND) hk +ε s k(hij); where, MD is the fixed effects of maternal diets; T the random effects of trial; Sow j (MD T)w j(hi) the error A used to test main plot effects; ND the fixed effects of nursery diets; (MD×ND) the interaction effect of maternal diets and nursery diets; and ε s k(hij) the error B. The two-way interactions of trial with maternal diets or with nursery diets were initially analysed and found to be non-significant sources of variation and were included in the error B term in the final analysis. For traits assessed in pigs at birth (0 weeks) and weaning (3 weeks) only the main plot model was used, as the nursery diets had not been fed. The main effect model was also used to assess litter response data where sow and litter effects are synonymous. Models were analysed by regression analysis using mixed models, general linear models, and rank (if data were not normally distributed) procedures using SAS version 9.3 (SAS Institute Inc.). Even though maternal diets were fed to sows, the sow responses to diets were not evaluated in this experiment with exception of the serum and milk samples. The individual pig was considered the experimental unit as only one pig from each litter was sampled at the age periods. Statistical comparisons across the age periods were not made as traits would be expected to change with age. Instead inferences were made about responses to dietary treatments within age periods. Differences due to pig sex were not considered in the analysis. If fixed effects of maternal diets or nursery diets were detected, inferences among treatment groups were based on orthogonal contrasts used to identify the main effects attributed to variation among maternal diets (−D v. +D, ++D and +D v. ++D), effects due to nursery diets (D effect, −D v. +D; CaP effect, LCaP v. HCaP; and the interaction of nursery D×CaP effects); and effects due to the interaction between maternal and nursery diets. Significance was considered at P≤0·05 and trends at P≤0·10.
Results were reported as the average values for each trait within three (0 and 3 weeks) or twelve (7 weeks) dietary treatments groups with a pooled standard error of the mean. The standard error was calculated by dividing the root mean square of the random error term in the regression model by the square root of the average number of observations within treatment groups. For serum and tissue assays, if all observations within one or more treatment groups were below the assay detection limit for the assay, the detection limit value was entered and data were analysed using rank procedures as the data were not normally distributed. Data based on analysis using rank procedures are presented as the analysed mean values and pooled standard errors of detectable values.
Results
Unexplained mortalities (n 9) occurred in the first replicate trial of pigs during the nursery phase of the experiment. The mortalities were distributed across sow treatments. No other unexplained mortalities occurred during other replicate trials of the nursery phase.
Litter traits
Although this experiment was not designed to evaluate the effect of maternal dietary D on reproductive traits due to the limited number of sows, observations were recorded at parturition for the number of live, still and total births (Table 1) to validate the productivity across maternal dietary treatment groups. No differences due to maternal dietary D were detected in live, still or total births. The average total births across all three maternal treatments was 13·1.
Table 1 Effect of maternal diet on litter size, birth weight and weaning weightFootnote * (Mean values with their pooled standard errors)

−D, 0; +D, 8·125; ++D, 43·750 µg cholecalciferol/kg diet.
* Values are for 12, 12 and 13 litters for maternal diets, respectively. Birth (0 weeks) and weaning (3 weeks) weights are averages based on individual values from all pigs. Gain (kg/d) was calculated from weight gained between 0 and 3 weeks divided by the actual days from birth to weaning for each litter. Birth weight is an average of 146–165 pigs and weaning weight is an average of 109–128 pigs/treatment.
† Total births equal the number of live births plus the number of still births.
Pig growth
No differences among maternal treatment groups were detected in pig weights at 0 and 3 weeks or in average daily gain between 0 and 3 weeks (Table 1). Apparently, maternal dietary D levels did not affect pig growth at 0 or 3 weeks.
However, an overall maternal diet effect was unexpectedly detected in pig weights and gain at 7 weeks after pigs were weaned and fed nursery diets (Table 2). Pooled across nursery treatments, pigs produced by +D or ++D sows were approximately 3 kg heavier and gained approximately 100 g/d more during the 4-week nursery phase than their cohorts produced by –D sows. Differences among nursery diet groups were also detected in pig weight and gain during the nursery phase but responses were dependent upon maternal treatment (maternal×nursery diet interaction, P≤0·05). Pigs fed +D nursery diets had increased body weight and gain above that of their –D cohorts if produced by –D or +D sows. If pigs were produced by ++D sows, nursery dietary D did not affect body weight or gain during the nursery phase.
Table 2 Pig body weight and growth at 7 weeks in response to maternal and nursery dietsFootnote * (Mean values with their pooled standard errors)
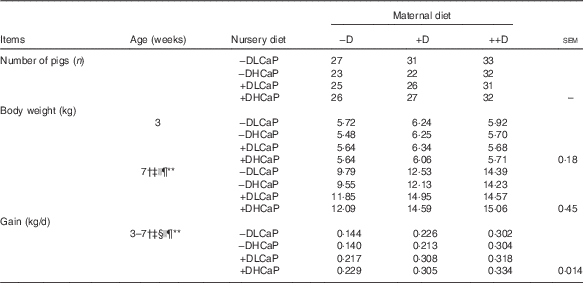
−D, 0; +D, 8·125; or ++D, 43·750 µg cholecalciferol/kg diet; −DLCaP, 0 µg cholecalciferol/kg diet and 75 % of Ca and 95 % of P requirements; −DHCaP, 0 µg cholecalciferol/kg diet and 150 % of Ca and 120 % of P requirements; +DLCaP, 7·0 µg cholecalciferol/kg diet and 75 % of Ca and 95 % of P requirements; +DHCaP, 7·0 µg cholecalciferol/kg diet and 150 % of Ca and 120 % of P requirements for nursery diets.
* Gain was calculated from the weight gained (kg/d) between 3 and 7 weeks and is based on individual pigs.
† Difference due to maternal treatment (P≤0·05).
‡ Maternal treatment −D v. +D and ++D differ (P≤0·05).
§ Maternal +D v. ++D differ (P≤0·05).
|| Difference due to nursery treatment (P≤0·05).
¶ Nursery treatment vitamin D effect (P≤0·05).
** Maternal×nursery treatment interaction (P≤0·05).
Kyphosis prevalence
All kyphosis data were based on subjective, observational scores. The prevalence of kyphosis in pigs at 13 weeks is shown for all 12 treatments in Fig. 2. Pigs produced by –D sows displayed a greater prevalence of kyphosis than cohorts produced by +D and ++D sows (P≤0·05). Pooled across maternal diets, kyphosis was more prevalent in pigs fed –D v. +D nursery diets (P≤0·05). Pigs fed HCaP nursery diets, even with added D, were not protected from developing kyphosis due to the lack of D in maternal diets. Pigs produced by −D sows and fed nursery diets with no supplemental D had the greatest prevalence of kyphosis (P≤0·05).

Fig. 2 Prevalence of kyphosis in pigs at 13 weeks in response to maternal and nursery diets. Maternal diets were formulated to supply either 0 (−D); 8·125 (+D); or 43·750 (++D) µg vitamin D3/kg diet in complete diets fed from breeding through the lactation phase. Nursery diets were formulated to supply either 0 µg vitamin D3/kg (−D) or 7·0 µg vitamin D3/kg (+D) and either 75 and 95 % (L) or 150 and 120 % (H) of the calcium and phosphorus requirements. Prevalence is presented as the number of pigs that scored 3 expressed as a percentage of total pigs in that treatment group. Pigs produced by −D sows displayed the greatest prevalence of kyphosis.
Sow serum analysis
Differences in sow serum Ca and P concentrations were not detected among dietary D treatments during gestation and lactation (Table 3). Consistent with expected responses to dietary additions of D, sows fed –D diets had increased (P≤0·05) PTH concentrations, but sows fed +D and ++D diets had non-detectable concentrations.
Table 3 Sow serum calcium, phosphorus, parathyroid hormone (PTH) and vitamin D metabolites and milk 25-OH-D3 concentrations at parturition and weaning in response to maternal dietFootnote * (Mean values with their pooled standard errors)
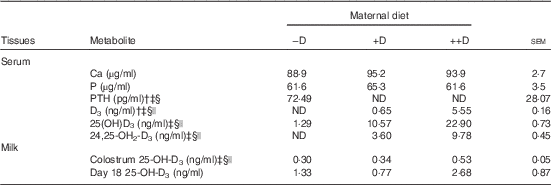
−D, 0; +D, 8·125; ++D, 43·750 µg cholecalciferol/kg diet; ND, not detectable.
* Values are of 5 sows/treatment (serum at farrowing) and 8–11 sows/treatment (colostrum at farrowing and day 18 milk at weaning).
† ND was reported for treatment means when all sows within a treatment had undetectable serum concentrations. Assay detection limit for serum PTH was 1·0 pg/ml. Assay detection limit serum vitamin D3 and serum 24,25-OH2-D3 was 0·5 ng/ml.
‡ Difference due to maternal treatment (P≤0·05).
§ Maternal treatment –D v. +D and ++D differ (P≤0·05).
|| Maternal treatment +D v. ++D differ (P≤0·05).
Sow serum 25-OH-D3 concentrations were altered by maternal dietary D levels (Table 3). At parturition serum 25-OH-D3 was increased (P≤0·05) approximately 2-fold in ++D sows compared with +D cohorts and was increased (P≤0·05) approximately 10-fold in +D sows compared with –D cohorts. Similar to responses in serum 25-OH-D3, sow serum D3 and 24,25-OH2-D3 concentrations at parturition were altered by maternal diets with ++D sows having the highest concentrations (P≤0·05).
Milk analysis
Maternal dietary D altered colostrum but not day 18 milk 25-OH-D3 concentrations (Table 3). Colostrum 25-OH-D3 increased about 1·7-fold (P≤0·05) in ++D sows compared with –D or +D cohorts. Milk samples from sows at 18 d postpartum did not differ in 25-OH-D3 concentrations across maternal dietary treatments.
Pig serum calcium, phosphorus and parathyroid hormone analysis
Differences were not detected in serum Ca, P or PTH concentrations in pigs at birth (0 weeks) across maternal dietary treatments (Table 4). At 3 weeks serum Ca and P were approximately 1·5-fold higher in pigs produced by +D and ++D sows compared with pigs produced by –D sows (P≤0·05). Serum PTH was approximately 6- and 25- fold higher in pigs produced by –D sows compared with pigs produced by +D and ++D sows, respectively (P≤0·05).
Table 4 Pig serum 25-OH-D3, calcium, phosphorus and parathyroid hormone (PTH) concentrations at 0, 3 and 7 weeks in response to maternal and nursery dietsFootnote * (Mean values with their pooled standard errors)

−D, 0; +D, 8·125; ++D, 43·750 µg cholecalciferol/kg diet for maternal diets; −DLCaP, 0 µg cholecalciferol/kg diet and 75 % of Ca and 95 % of P requirements; −DHCaP, 0 µg cholecalciferol/kg diet and 150 % of Ca and 120 % of P requirements; +DLCaP, 7·0 µg cholecalciferol/kg diet and 75 % of Ca and 95 % of P requirements; +DHCaP, 7·0 µg cholecalciferol/kg diet and 150 % of Ca and 120 % of P requirements for nursery diets; ND, not detectable.
* Values are of 9–16 pigs/treatment (0 and 3 weeks) and 5–6 pigs/treatment (7 weeks).
† ND was reported for treatment means when all pigs within a treatment had undetectable serum concentrations. Assay detection limit for serum 25-OH-D3 was 1·00 ng/ml
‡ Difference due to maternal treatment (P≤0·05 and * P≤0·10).
§ Maternal treatment –D v. +D and ++D differ (P≤0·05 and * P≤0·10).
|| Maternal treatment +D v. ++D differ (P≤0·05 and * P≤0·10).
¶ Difference due to nursery treatment (P≤0·05).
** Nursery treatment CaP effect (P≤0·05).
†† Nursery treatment vitamin D effect (P≤0·05).
‡‡ Maternal×nursery treatment interaction (P≤0·10).
§§ Nursery CaP×vitamin D interaction (P≤0·10).
An overall maternal diet effect was detected in pig serum Ca, P and PTH at 7 weeks. Regardless of nursery treatments, pigs produced by sows fed ++D diets had approximately 1·2-fold higher serum Ca compared with pigs produced by –D and +D sows (P≤0·05). Serum P tended to be higher if pigs were produced by +D and ++D sows (P≤0·10). Serum PTH was approximately 2- and 5-fold higher in pigs produced by –D sows compared with pigs produced by +D and ++D sows, respectively (P≤0·05). Nursery dietary treatments also affected pig serum Ca, P and PTH concentrations at 7 weeks. Pooled across maternal dietary treatments, serum Ca was increased approximately 1·5-fold and P approximately 1·2-fold in pigs fed +D v. –D nursery diets (P≤0·05). Serum PTH was 5·2-fold higher in pigs fed –D v. +D nursery diets (P≤0·05). Differences due to nursery dietary Ca and P were only detected in serum P. Pigs fed HCaP diets had increased serum P concentrations (P≤0·05).
Pig serum vitamin D analysis
Serum D3, 25-OH-D3 and 24,25-OH2-D3 concentrations were measured in pigs at 0, 3 and 7 weeks (Table 4 – only 25-OH-D3 data shown). Due to lack of detectable levels for all three metabolites in pigs within entire treatment groups, data were ranked for statistical analyses. Serum D3 concentrations were not detectable in any pigs at 0 and 7 weeks and only slightly above detection limits in pigs produced by ++D sows at 3 weeks (average=0·97 (sem 0·21) ng/ml). Serum 24,25-OH2-D3 concentrations were not detectable in pigs at 7 weeks and only slightly above detection limits in pigs produced by ++D sows at 3 weeks (average=1·14 (sem 0·15) ng/ml). At 0 weeks there was an increase (P≤0·05) in serum 24,25-OH2-D3 concentrations across all maternal treatments. (−D average=not detectable; +D average=0·93 (sem 0·07) ng/ml; ++D average=2·53 (sem 0·15) ng/ml).
Treatment averages for serum 25-OH-D3 at 0, 3 and 7 weeks are in Table 4. Not detectable was recorded for means if all values in that treatment group were not detectable. At 0 and 3 weeks there was an increase (P≤0·05) in serum 25-OH-D3 concentrations across maternal dietary treatments with pigs produced by ++D sows having the highest levels. At 7 weeks only pigs fed +D nursery diets had increased serum 25-OH-D3 concentrations (P≤0·05). However, the highest average concentration only reached 4·91 ng/ml. Regardless of nursery diet pigs produced by +D and ++D sows had higher serum 25-OH-D3 concentrations at 7 weeks compared with cohorts produced by –D sows (P≤0·05).
Pig tissue store analysis
Tissue 25-OH-D3 concentrations in fat samples from pigs at 0, 3 and 7 weeks were not detectable. Tissue 25-OH-D3 concentrations in liver samples from pigs at 0 and 3 weeks were also not detectable. Only one pig at 7 weeks had a detectable liver 25-OH-D3 concentration (1·69 ng/g). Muscle 25-OH-D3 concentrations were ranked before statistical analysis due to lack of detectable levels in all pigs within entire treatment groups. Muscle 25-OH-D3 concentrations were just above detection limits in all treatments at 0, 3 and 7 weeks with the highest amount (0·84 ng/g) detected in a pig at 7 weeks produced by a ++D sow (data not shown). Differences in muscle 25-OH-D3 concentrations were only detected in pigs at 7 weeks. Pigs fed +D nursery diets had higher muscle 25-OH-D3 concentrations compared with cohorts fed –D nursery diets (P≤0·05, data not shown).
Kidney mRNA expression analysis
No differences in kidney mRNA expression of CYP24A1 or CYP27B1 were detected at 0 weeks (Table 5). At 3 weeks kidney mRNA expression of CYP24A1 was increased (P≤0·05) 9-fold in pigs produced by ++D sows, whereas expression of CYP27B1 was decreased (P≤0·05) to 0·4-fold in pigs produced by +D or ++D sows. Pooled across maternal dietary treatments, pigs at 7 weeks that were fed +D nursery diets had a 2·75-fold increase (P≤0·05) in kidney mRNA expression of CYP24A1 and expression of CYP27B1 was reduced (P≤0·05) to 0·4-fold.
Table 5 Pig kidney, femur and vertebrae relative mRNA expression at 0, 3 and 7 weeks in response to maternal and nursery dietsFootnote * (Mean values with their pooled standard errors)

−D, 0; +D, 8·125; ++D, 43·750 µg cholecalciferol/kg diet for maternal diets; −DLCaP, 0 µg cholecalciferol/kg diet and 75 % of Ca and 95 % of P requirements; −DHCaP, 0 µg cholecalciferol/kg diet and 150 % of Ca and 120 % of P requirements; +DLCaP, 7·0 µg cholecalciferol/kg diet and 75 % of Ca and 95 % of P requirements; +DHCaP, 7·0 µg cholecalciferol/kg diet and 150 % of Ca and 120 % of P requirements for nursery diets; OCN, osteocalcin.
* Values reflect averages of the relative fold change compared with the control. The control treatment was pigs produced by sows fed –D at 0 and 3 weeks and pigs produced by sows fed –D and -DLCaP during the nursery at 7 weeks. The geometrical average of three housekeeping genes (hypoxanthine phosphoribosyltransferase 1; ribosomal protein S18; cyclophilin A) were used as the housekeeping gene for kidney tissues. The femur and vertebrae OCN mRNA expression were normalised to one reference gene (vitamin D receptor).The data were analysed using the
![]() $$2^{{{\minus}\Delta \Delta C_{t} }} $$
method(
Reference Schmittgen and Livak
18
). Values are of 6–9 tissues/treatment (0 and 3 weeks) and 3–6 pigs/treatment (7 weeks).
$$2^{{{\minus}\Delta \Delta C_{t} }} $$
method(
Reference Schmittgen and Livak
18
). Values are of 6–9 tissues/treatment (0 and 3 weeks) and 3–6 pigs/treatment (7 weeks).
† Difference due to maternal treatment (P≤0·05 and * P≤0·10).
‡ Maternal treatment –D v. +D and ++D differ (P≤0·05).
§ Maternal treatment +D v. ++D differ (P≤0·05 and * P≤0·10).
|| Difference due to nursery treatment (P≤0·05).
¶ Nursery treatment CaP effect (P≤0·05).
** Nursery treatment vitamin D effect (P≤0·05).
†† Nursery CaP×vitamin D interaction (P≤0·05 and * P≤0·10).
More intriguing were the maternal dietary D effects evident at 7 weeks regardless of nursery diet. At 7 weeks kidney mRNA expression of CYP24A1 was increased (P≤0·05) more than 3-fold in pigs produced by +D and ++D sows compared with –D sows. Concomitantly kidney mRNA expression of CYP27B1 was reduced (P≤0·05) to 0·7-fold in pigs produced by +D and ++D sows.
Bone mRNA expression analysis
At 0 weeks differences in expression of OCN were detected in vertebrae but not femur (Table 5).
Pigs produced by +D or ++D sows had approximately 3-fold higher vertebrae mRNA expression of OCN (P≤0·05). At 3 weeks differences in expression of OCN were detected in femur but not vertebrae. Pigs produced by +D or ++D sows tended to have increased femur mRNA expression of OCN (P≤0·10).
Differences due to nursery dietary D were detected in femur and vertebrae mRNA expression of OCN at 7 weeks. Pig femur and vertebrae mRNA expression of OCN was approximately 2-fold higher in pigs fed +D v. –D nursery diets (P≤0·05).
Discussion
Skeletal abnormalities and kyphosis have been replicated as in earlier experiments( Reference Rortvedt and Crenshaw 1 , Reference Amundson, Hernandez and Laporta 2 ). Under controlled experimental conditions manipulation of maternal dietary D and nursery dietary D, Ca, and P has been used to develop the hypovitaminosis D kyphotic pig model. Due to priorities given to tissue sample collections at early ages in previous studies, we were not able to evaluate the ability of excess maternal dietary D to prevent pigs from developing kyphosis. One objective of the current study was to evaluate the prevalence of kyphosis and evaluate its relationship to parameters commonly associated with D status. The results from the current study provided evidence for persistent maternal dietary D effects on pig traits and skeletal development at both gross and molecular levels.
Although the prevalence of kyphosis reported herein was presented across all twelve nursery treatments, the maternal diet was obviously of greater influence than nursery diet. Pooled across nursery treatments 34 % of pigs produced by –D sows developed kyphosis compared with only 4 and 1 % of pigs produced by +D and ++D sows, respectively. The identification of one pig produced by a ++D sow is most likely due to experimental error associated with subjective observational traits. Therefore, maternal dietary D was the major dietary risk factor in the development of kyphosis.
The highest prevalence of kyphosis was in pigs produced by –D sows and fed –DHCaP nursery diets. The same pattern was evident in pigs fed –DHCaP v. −DLCaP nursery diets if pigs were produced by +D sows but the overall prevalence was reduced. These results infer that a lack of Ca and P available for bone mineralisation was not the limiting factor in the development of kyphosis. The presence of nursery dietary D did not completely prevent the development of kyphosis if pigs were produced by –D sows, but did if pigs were produced by +D or ++D sows. One conclusion from these results may be that pigs produced by –D sows have such low tissue D stores that supplements of nursery dietary D was not sufficient to compensate and prevent kyphosis. However, tissue analyses from this experiment refute that hypothesis.
Fat, liver, and muscle 25-OH-D3 concentrations were measured in pigs at 0, 3 and 7 weeks. The undetectable and barely detectable concentrations in these tissues from pigs at all time points infer that D storage is not predictive of developing kyphosis, otherwise one would expect all pigs to have developed bone abnormalities. Alternatively, the tissue concentrations of D, even at values below detection limits may still differentially supply sufficient amounts of D to meet the needs for synthesis of the active hormone, even if the assay is not sensitive enough to detect these changes.
Studies to investigate the effects of maternal D status on offspring D status, as measured by serum 25-OH-D3 levels, reveal a close relationship( Reference Goff, Horst and Littledike 21 , Reference Halloran, Barthell and DeLuca 22 ). The inferences from these studies were based on injections or mega doses of D rather than physiologically relevant dietary D supplements in the maternal diet. Although serum 25-OH-D3 is one of the main parameters available to evaluate D status, results reported herein infer that serum concentrations do not provide a sensitive measure to assess D sufficiency in the hypovitaminosis D kyphotic pig model. Increases in pig serum 25-OH-D3 concentrations at birth and weaning were not proportional to increases in sow serum 25-OH-D3 across maternal treatments. At 7 weeks, even pigs produced by ++D sows had concentrations below detection limits. Therefore, the modest increase evident at birth and weaning due to excess maternal dietary D was not maintained throughout the nursery phase. In addition, serum 25-OH-D3 concentrations were not sensitive enough to indicate risk of kyphosis and changes in D homoeostatic genes at a molecular level.
Both sow and pig 25-OH-D3 concentrations at all ages evaluated in this experiment would be classified as deficient as defined by veterinary guidelines for adequacy( Reference Arnold, Madson and Ensley 23 ). The guidelines for pigs are consistent with concentrations suggested for humans which can vary depending on the response criteria evaluated. The committee for the 2011 Institute of Medicine recommendations for D and Ca concluded that there is uncertainty in the use of 25-OH-D3 as a biomarker for D sufficiency and that the prevalence of D deficiency based on serum 25-OH-D3 concentration ranges are grossly overestimated based on the available data( 16 ). However, a more sensitive and accurate biomarker has yet to be identified.
Ca is presumed to drive the classical homoeostatic D, Ca and PTH pathway. A decrease in serum Ca or D stimulates PTH release which activates D and subsequently bone mobilisation of Ca and P to restore serum Ca levels. The presence of D is also necessary for active absorption of Ca to maintain physiological ranges of Ca which are tightly regulated. Therefore, serum Ca is a potential indicator of D sufficiency.
All pigs fed +D nursery diets maintained serum Ca concentrations within the physiological range (8–120 µg/ml), regardless of maternal dietary treatment. Pigs produced by ++D sows and fed –D nursery diets were still able to maintain physiological serum Ca despite having undetectable serum 25-OH-D3 concentrations. However, that was not true for their cohorts with undetectable serum 25-OH-D3 produced by –D or +D sows, as their serum Ca dropped below 80 µg/ml. Therefore, the magnitude of difference in serum Ca between pigs fed –D and +D nursery diets was greater if pigs were produced by –D or +D sows v. ++D sows. This pattern of responses was also reflected in serum PTH, verifying the classical pathway for Ca homoeostasis. Unexplainably, serum Ca appeared to decrease in pigs fed –DHCaP compared with –DLCaP cohorts across maternal dietary treatments. However, if D was present in the nursery diet, dietary Ca and P did not appear to alter serum Ca. This response was not evident in pig serum P. Although there was a trend for maternal dietary effects and a nursery dietary D effect on serum P, all pigs maintained serum P levels within the physiological range (60–90 µg/ml).
Sow milk levels have been altered by a pharmacological dose of D( Reference Goff, Horst and Littledike 21 ). Injection of a single dose (5×106 µg) of D3 to sows before parturition was positively correlated with an increase in milk D3 concentrations( Reference Goff, Horst and Littledike 21 ). In the current study we fed physiologically relevant concentrations of D and measured 25-OH-D3 concentrations. However, differences which were evident in colostrum at parturition across maternal treatments were not maintained through the lactation phase. Although the difference in colostrum 25-OH-D3 concentrations were statistically significant, biological relevance of such a small difference is not fully understood. Although this study was not designed to discern between gestation and lactation effects, colostrum and milk results reported herein infer pig responses were not likely attributed to differences in milk 25-OH-D3 concentrations, especially at weaning given the high variability and lack of a detectable difference in the D18 milk.
The enzymes responsible for activation (CYP27B1) and degradation (CYP24A1) of D are implicated in its homoeostatic regulation. Understanding the changes in expression of these genes may help determine D status and molecular characterisation of the bone abnormalities in the hypovitaminosis D kyphotic pig model. Kidney mRNA expression of CYP24A1 and CYP27B1 reflected differences due to maternal dietary treatment at weaning despite only modest increases in serum 25-OH-D3 and no difference in milk 25-OH-D3 concentrations at that time. Therefore, adherence to guidelines based on attempts to increase serum 25-OH-D3 concentrations may proportionately increase anabolic and catabolic activities without a net increase in D benefits.
Surprisingly, an influence of maternal dietary treatments was maintained on pig kidney mRNA expression of CYP24A1 and CYP27B1 at 7 weeks. Nursery dietary D did not appear to alter kidney CYP24A1 expression if pigs were produced by –D sows. The magnitude of difference between pigs fed –D v. +D nursery diets was larger if pigs were produced by +D or ++D sows with the overall highest expression in pigs produced by ++D sows. Kidney mRNA expression of CYP27B1 revealed a slightly different pattern in response to nursery dietary D. The difference between expression in pigs fed –D v. +D was evident across all maternal treatments, with +D pigs having an overall lower expression, as expected. Again, as with other parameters, these differences would not be expected if sole observations were made on serum 25-OH-D3 concentrations. For example, all pigs fed –D diets had similar, albeit near detection limit, 25-OH-D3 concentrations but if produced by ++D sows, the pigs had approximately a 1·5- and 2-fold higher kidney mRNA expression of CYP24A1 compared with those produced by –D and +D sows, respectively.
As previously mentioned, kyphosis is not the only bone abnormality evident in the hypovitaminosis D kyphotic pig model. In previous studies whole body bone mineralisation and femur strength properties were examined( Reference Amundson, Hernandez and Laporta 2 ). Gross bone traits were not evaluated in this experiment as the focus was on tissue collections for molecular analysis and an effort to retain a large number of pigs for detection of kyphosis prevalence. However, mRNA expression of OCN, a marker of bone formation( Reference Lian, Stewart and Puchacz 24 ), was evaluated in vertebral and femoral tissues. Femur mRNA expression of OCN tended to be up-regulated in pigs produced by +D and ++D sows at weaning. However, at birth, vertebrae mRNA expression of OCN was up-regulated in pigs produced by +D and ++D sows. The discrepancy in the age at which differences were evident between these bone tissues is not completely understood, however the different responses may be important in the development of the types of lesions formed in these bones.
Maternal dietary influence on pig femur and vertebrae mRNA expression of OCN was not detected in pigs at 7 weeks. However, in previous experiments with similar maternal and nursery diets an overall maternal diet effect was detected in whole body bone mineral content and bone mineral density (BMD) traits. Pig BMD was reduced by 25 % in pigs produced by –D sows( Reference Amundson, Hernandez and Laporta 2 ). Cautious extrapolation of these observations across trials infers that mRNA expression of OCN may not be a sensitive measure for predicting whole body bone mineralisation responses to maternal dietary D.
Nursery diets did influence pig femur and vertebrae mRNA expression of OCN. Unexpectedly, if D was omitted from the nursery diet, HCaP decreased OCN mRNA expression. This response was similar in femur and vertebrae tissues and was consistent with the response of kyphosis prevalence. The increased concentrations of dietary Ca and P did not reduce the prevalence of kyphosis.
None of the classical parameters to evaluate D status measured herein were sufficiently sensitive to detect changes due to maternal dietary D. Thus, identification of a trait to predict the maternal diet effects in this model remains unknown. As described previously( Reference Amundson, Hernandez and Laporta 2 ), gross observation of excised vertebrae and femurs from pigs that were fed diets that led to the development of kyphosis revealed aberrant growth plates characterised by irregular physis widths. The abnormalities in the growth plates imply that an uncoupling of matrix formation and degradation within this region may be responsible for the bone abnormalities present in this model. Extracellular matrix remodelling is the rate limiting factor in endochondral ossification( Reference Ortega, Behonick and Werb 25 ). Critical enzymes implicated in this process are matrix metalloproteinases and vascular endothelial growth factor( Reference Ortega, Behonick and Werb 25 ). Therefore, these proteins are potentially dysregulated and lead to the bone abnormalities evident in the hypovitaminosis D kyphotic pig model. Future efforts should focus on assessments of these proteins.
Conclusions
In this experiment, whole animal traits and molecular characteristics of the hypovitaminosis D kyphotic pig model were further characterised. Maternal dietary D influenced pig growth, development of kyphosis, serum parameters and tissue mRNA expression regardless of nursery diet. Pigs produced by –D sows were more likely to develop kyphosis. However, the classical parameters used to measure D sufficiency (serum 25-OH-D3) failed to accurately predict the prevalence of kyphosis and other bone abnormalities described in previous studies. Therefore, more work is warranted to understand the mode of action by which maternal dietary D influences physiological changes resulting in the development of skeletal abnormalities. Further work can then be conducted to develop a sensitive biomarker to predict such endpoints. Inferences gathered from the hypovitaminosis D kyphotic pig model will advance both animal and human nutritional understanding of the effects and successful evaluation of maternal D status.
Acknowledgements
The authors acknowledge the University of Wisconsin Swine Research and Teaching Center staff for their meticulous animal care and DSM Nutritional Products for laboratory analysis of vitamin D.
Funds to support this project were provided by the University of Wisconsin Hatch fund (PRJ42HD) and an unrestricted research fund (233-Q893) to support swine nutrition research. DSM Nutritional Products North America (45 Waterview Blvd., Parsippany, NJ 07054) donated the supplemental vitamin D3 used in diet supplements, but had no role in the experimental design, analysis or writing of the manuscript.
L. A. A. and T. D. C. designed and conducted research and wrote the paper; L. A. A., T. D. C. and L. L. H. analysed data; L. A. A. had primary responsibility for final content. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary Material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517001751










