Mg is the fourth most abundant cation in the human body. Clinical observational studies have found that Mg deficiency is involved in various pathological conditions, especially CVD. A meta-analysis of community-based cohorts concluded that both low Mg intake and low serum Mg levels are significant risk factors for CVD events( Reference Xu, Sun and Xu 1 ). Low serum Mg has also been associated with vascular calcification in patients with end-stage renal disease (ESRD)( Reference Molnar, Biyani and Hammond 2 ); a significant association has been found between low serum Mg and increased cardiovascular risk in both pre-dialysis chronic kidney disease patients and those undergoing dialysis( Reference Kanbay, Yilmaz and Apetrii 3 – Reference Markaki, Kyriazis and Stylianou 7 ).
Peritoneal dialysis (PD) is a well-accepted method of home-based renal replacement therapy for ESRD patients. PD removes dissolved body wastes and extra fluid from the patient’s blood via the peritoneal cavity by using dialysate exchanges. The reported prevalence of hypomagnesaemia is high in the PD population, mainly because the routinely used commercial peritoneal dialysate contains a low Mg level of 0·25 mol/l( Reference Ye, Zhang and Guo 8 , Reference Ejaz, McShane and Gandhi 9 ). Although several retrospective studies have shown that low serum Mg was associated with an increased risk of hospitalisation and all-cause mortality( Reference Yang, Soohoo and Streja 10 , Reference Cai, Luo and Dai 11 ), the relationship between serum Mg and cardiovascular mortality in PD patients has not been rigorously studied. In addition, female patients in the general population are more vulnerable to CVD( Reference Wenger 12 , Reference Xuereb, Magri and Xuereb 13 ). A prospective cohort study reported that higher plasma Mg concentrations and dietary Mg intake were associated with a lower risk of sudden cardiac death in women( Reference Chiuve, Korngold and Januzzi 14 ). However, sex differences in the relationship between serum Mg and cardiovascular mortality in patients receiving PD have rarely been described. Therefore, the aim of the present study was to explore the association between serum Mg and cardiovascular mortality, as well as sex differences in this association, in a prospective cohort of the PD population.
Methods
Patient information
This prospective cohort study included prevalent patients from our previous cross-sectional study( Reference Ye, Zhang and Guo 8 ), which was primarily designed to explore the prevalence and associated factors of hypomagnesaemia at a single centre. Enrolled patients had received PD treatment for more than 1 month and were regularly followed up from February to June 2011. Tenckhoff catheters were placed with sterile surgical technique in all patients. Patients and their caregivers received standardised training after catheterisation. A low-Mg dextrose peritoneal dialysate (containing Mg2+ 0·25 mmol/l, Ca2+ 1·25 mmol/l or 1·77 mmol/l) was used for dialysis. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving patients were approved by the Ethics Committee of The First Affiliated Hospital, Sun Yat-sen University. Written informed consent was obtained from all participants at enrolment.
Demographic and biochemical data
Demographic and clinical characteristics, including age, sex, primary renal disease, comorbidity conditions, PD duration, daily PD exchange volume, ultrafiltration volume and urine output, as well as history of CVD, which included cerebrovascular disease, IHD, congestive heart failure and peripheral vascular disease at enrolment, were reviewed. Biochemical data, including serum albumin, serum Mg, high-sensitivity C-reactive protein, serum lipid profile, serum Ca, serum P, serum K, intact parathyroid hormone, serum creatinine and blood urea N, were collected at enrolment. All biochemical data were determined with an automatic chemistry analyser (Hitachi 7180 (Boehringer Mannheim) or Abbott Aeroset (Abbott Laboratories)). The serum Mg level was determined with the xylidyl blue method; the normal reference range in this study was 0·70–1·10 mmol/l. Hypomagnesaemia was defined as a serum Mg level <0·7 mmol/l. Parameters for adequacy of PD, including the total Kt/V, weekly creatinine clearance and residual renal function, were also assessed at enrolment according to standard methods( Reference Nolph, Moore and Twardowski 15 , Reference van Olden, Krediet and Struijk 16 ). The normalised protein equivalent of N appearance was calculated with the modified Bergstrom formula( Reference Bergstrom, Furst and Alvestrand 17 ), and normalised according to ideal body weight.
Follow-up and study endpoints
All patients were followed up to the study endpoint, administrative censoring (including renal transplantation, switch to haemodialysis, transfer to another PD centre, loss to follow-up or withdrawal from treatment) or the end of the study follow-up period (31 December 2016). The primary study endpoint was cardiovascular mortality. The secondary endpoint was all-cause mortality. cardiovascular death was defined as death due to IHD, arrhythmia, cardiac sudden death, congestive heart failure, other heart disease or cerebrovascular events( Reference Cheung, Sarnak and Yan 18 , Reference Delmez, Yan and Bailey 19 ). The PD team consisted of three nephrologists at our centre who reviewed the details of individual medical records and identified the causes of death. If a death had two or more potential causes, we generally ascribed the death to the primary cause for hospitalisation or the initial presenting condition.
Statistical analysis
Results were expressed as frequencies and percentages for categorical variables, means and standard deviations for continuous variables and medians and interquartile ranges (IQR) for skewed distributions. Baseline serum Mg was evaluated as a continuous variable; values were categorised as hypomagnesaemia or control group, and also into quartiles: quartile 1 (≤0·66 mmol/l), quartile 2 (0·67–0·73 mmol/l), quartile 3 (0·74–0·79 mmol/l) and quartile 4 (≥0·80 mmol/l). To compare sex differences in hypomagnesaemia, patients were divided into four groups (Group 1: females with hypomagnesaemia; Group 2: females without hypomagnesaemia; Group 3: males with hypomagnesaemia; Group 4: males without hypomagnesaemia). For comparisons among these groups, χ 2 tests and one-way ANOVA tests or Kruskal–Wallis tests were used to evaluate categorical variables and continuous variables, respectively.
The cumulative risks for all-cause and cardiovascular mortality were evaluated with the Kaplan–Meier method. The associations between serum Mg and outcomes were assessed with Cox proportional hazards regression models. Multivariate models were adjusted for conventional confounding factors and for confounders that have been identified as risk factors related to cardiovascular and all-cause mortality in dialysis patients. The interactions between sex and serum Mg in the associations with mortality were checked with the Cox regression model. Competing-risks regression models were used to examine the associations between serum Mg and cardiovascular mortality. In these models, cardiovascular deaths were considered principal, whereas other causes of death were considered competing events.
Calculations were performed with statistical software SPSS (version 19.0; SPSS Inc. and IBM Inc.); the competing risk models were performed with the cmprsk package for R statistical software. P values of <0·05 were considered significant.
Results
Baseline clinical data
A total of 402 continuous ambulatory PD patients were enrolled in this study. The mean age of participants was 49·3 (sd 14·9) years, 57 % were male, and the median PD duration at enrolment was 23·3 months (IQR: 11·9–38·1). The leading cause of ESRD was glomerulonephritis (59·7 %), followed by diabetic nephropathy (19·4 %). At enrolment, median daily urine volume was 500 ml/d (IQR: 100–900). The baseline serum Mg level was 0·73 (SD 0·11) mmol/l (IQR: 0·66–0·79). According to the reference range, 163 (40·5 %) patients had hypomagnesaemia. Normality testing of the distribution of serum Mg in the overall study population indicated non-normal distribution (P<0·001; Fig. 1). Stratification of the distribution according to sex is shown in Fig. 2.
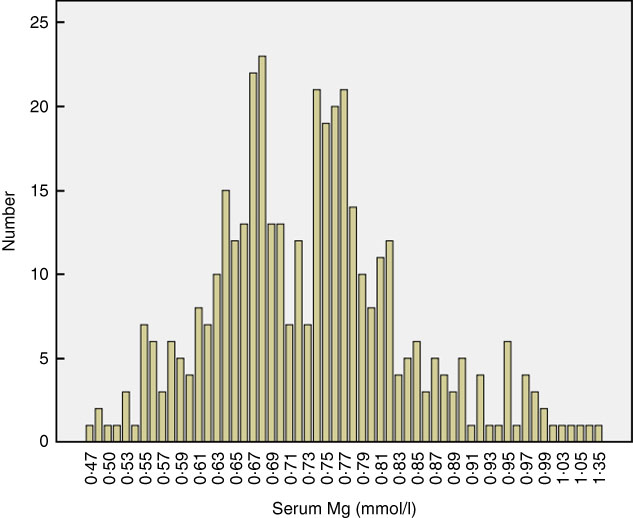
Fig. 1 Distribution of baseline serum magnesium in the study population (P for normality test<0·001). Hypomagnesaemia is defined as a serum magnesium level <0·7 mmol/l.
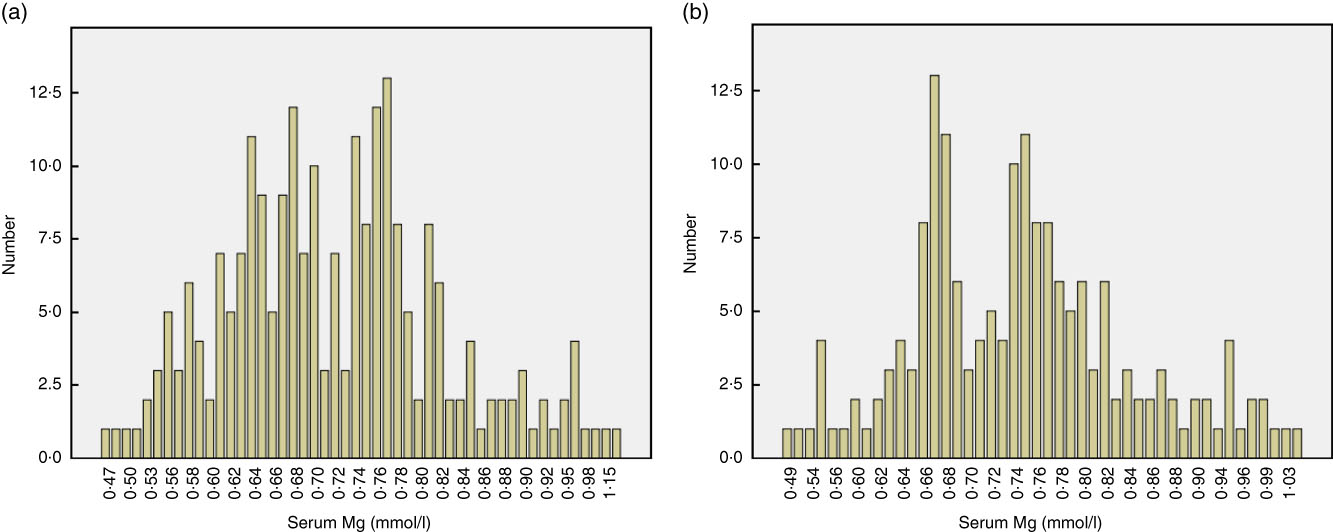
Fig. 2 Stratification of the distribution of baseline serum magnesium according to sex: (a) male and (b) female.
Comparisons of clinical characteristics stratified according to hypomagnesaemia and sex subgroups
Patients were divided into four groups according to sex and the presence of hypomagnesaemia (Group 1: females with hypomagnesaemia; Group 2: females without hypomagnesaemia; Group 3: males with hypomagnesaemia; Group 4: males without hypomagnesaemia). Compared with the other three groups, female patients with hypomagnesaemia (Group 1) had a shorter time of PD treatment at enrolment (P<0·001); lower daily exchange volume (P=0·001); lower serum Ca (P=0·005), albumin (P<0·001), K (P<0·001), phosphate (P<0·001), urea N (P<0·001) and creatinine (P=0·003); and higher total cholesterol (P<0·001) and total Kt/V (P<0·001; Table 1).
Table 1 Comparisons of clinical characteristics stratified according to hypomagnesaemia and sex subgroupsFootnote * (Mean values and standard deviations; medians and interquartile ranges (IQR); percentages)
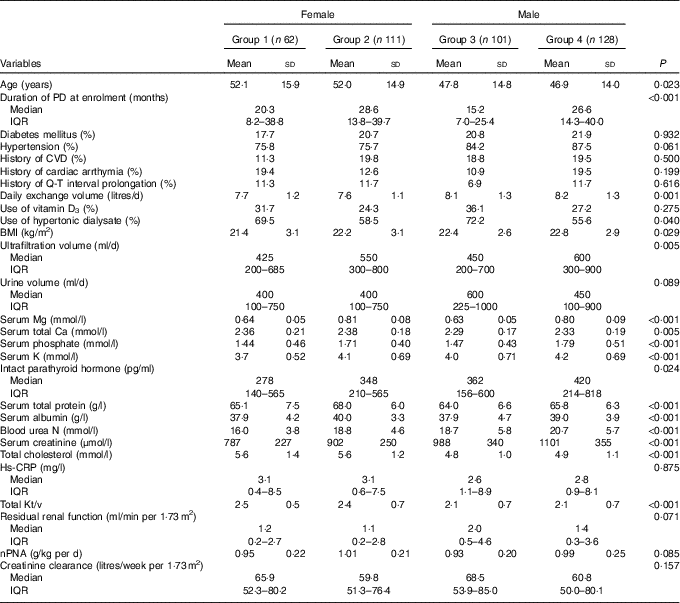
PD, peritoneal dialysis; Hs-CRP, high-sensitivity C-reactive protein; nPNA, normalised protein equivalent of N appearance.
* Group 1: female with hypomagnesaemia; Group 2: female without hypomagnesaemia; Group 3: male with hypomagnesaemia; Group 4: male without hypomagnesaemia.
Serum magnesium and cardiovascular and all-cause mortality
During a median of 49·9 months (IQR: 25·9–68·3) of follow-up, 104 (25·8 %) of the 402 patients died, 72 (17·9 %) were transferred to haemodialysis, 66 (16·4 %) received renal transplantation and 150 (37·3 %) remained on PD. Among the deaths, 62 (59·6 %) resulted from CVD, 23 (22·1 %) from infection, five (4·8 %) from malignancy, five (4·8 %) from cachexia and nine (8·7 %) from other causes.
Kaplan–Meier survival analysis revealed a trend that patients with hypomagnesaemia or in the lowest quartile of serum Mg level had a higher risk for cardiovascular mortality than other patients. However, this trend did not reach statistical significance (Fig. 3). The univariate Cox regression model showed no significant association between serum Mg and cardiovascular or all-cause mortality (Table 2). After adjustment for the conventional confounders, the lower quartile of serum Mg level was independently associated with an increased risk of cardiovascular mortality, with hazards ratios (HR) of 2·28 (95 % CI1·04, 5·01), 1·41 (95 % CI 0·63, 3·16) and 1·62 (95 % CI 0·75, 3·51) for the lowest, second and third quartiles, respectively. A similar trend was observed when all-cause mortality was considered as the study endpoint (Table 2).
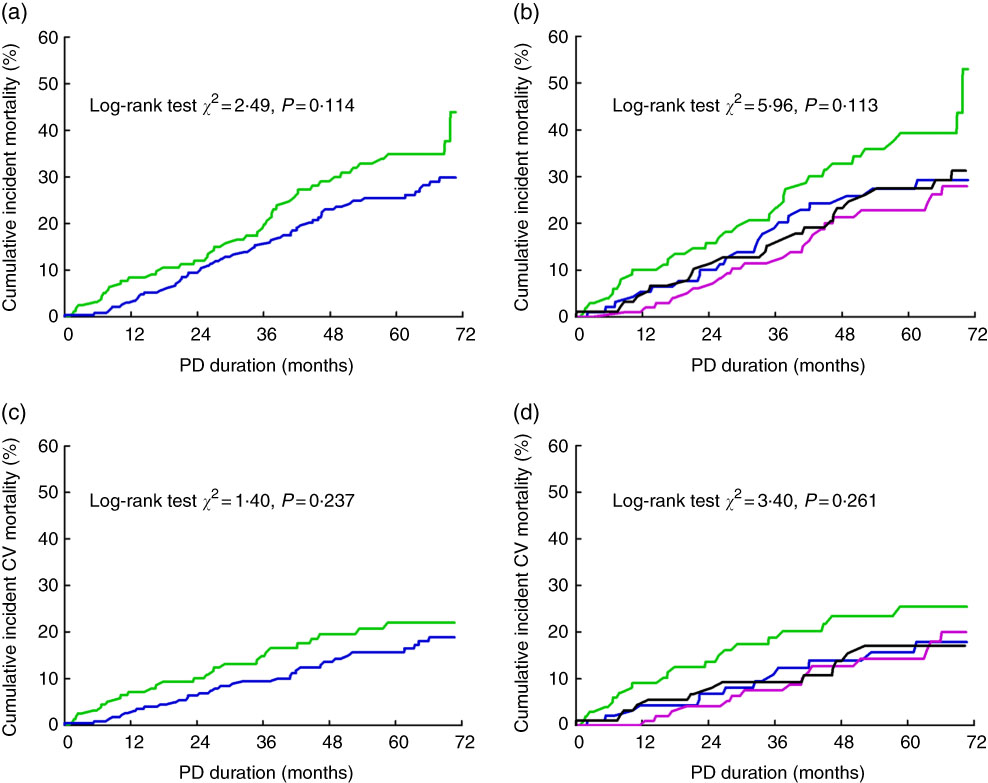
Fig. 3 Serum magnesium and mortality in peritoneal dialysis (PD) patients. Cumulative risk for all-cause mortality in the entire patients stratified by hypomagnesaemia (a) and magnesium quartiles (b); cumulative risk for cardiovascular (CV) mortality in the entire patients stratified by hypomagnesaemia (c) and magnesium quartiles (d). (a,c): ![]() , Hypomagnesaemia group;
, Hypomagnesaemia group; ![]() , control group. (b,d):
, control group. (b,d): ![]() , Quartile 1;
, Quartile 1; ![]() , quartile 2;
, quartile 2; ![]() , quartile 3;
, quartile 3; ![]() , quartile 4.
, quartile 4.
Table 2 Associations between serum magnesium and mortality in the Cox regression modelsFootnote * (Hazard ratios (HR) and 95 % confidence intervals)
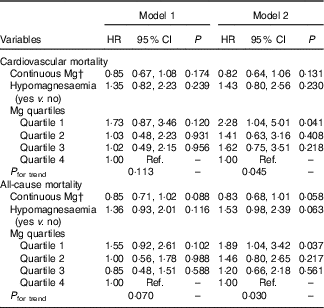
Ref., referent values.
* Model 1: univariate analysis; model 2: adjusted for age, sex, hypertension, diabetes, history of CVD, peritoneal dialysis duration at enrolment, serum phosphate and urine volume. Mg concentrations for quartiles: quartile 1 (≤0·66 mmol/l), quartile 2 (0·67–0·73 mmol/l), quartile 3 (0·74–0·79 mmol/l) and quartile 4 (≥0·80 mmol/l).
† Per 0·1 mmol/l increase of Mg.
Sex differences in associations between serum magnesium and mortality
The test for interactions indicated that the associations of hypomagnesaemia with cardiovascular and all-cause mortality differed according to sex (P=0·008 and P=0·011, respectively; Table 3). We further analysed the relationships between serum Mg and mortality according to sex. According to Kaplan–Meier survival analysis, hypomagnesaemia was associated with a significantly increased risk for all-cause and cardiovascular mortality in female patients, but not in male patients (Fig. 4). After adjustment for confounders in the multivariate Cox models, lower serum Mg (HR: 0·58; 95 % CI 0·43, 0·78) and hypomagnesaemia (HR: 2·89; 95 % CI 1·58, 5·28) were independently associated with a higher risk of cardiovascular mortality only in the female subgroup. The risk of cardiovascular mortality was more than three times higher in quartile 1 for serum Mg than in quartile 4 (HR: 4·16; 95 % CI 1·72, 10·07). Similar results were also observed when all-cause mortality was considered as the study endpoint (Table 3).
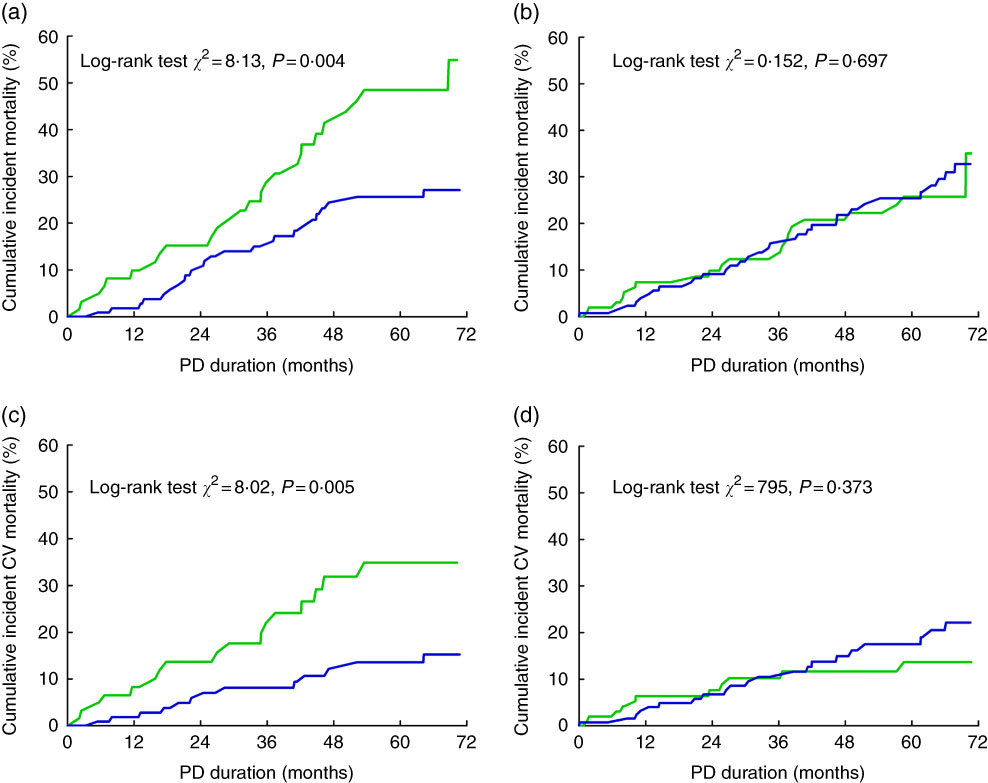
Fig. 4 Hypomagnesaemia and mortality stratified by sex in peritoneal dialysis (PD) patients. Cumulative risk for all-cause mortality in female patients (a) and male patients (b); cumulative risk for cardiovascular (CV) mortality in female patients (c) and male patients (d). ![]() , Hypomagnesaemia group;
, Hypomagnesaemia group; ![]() , control group.
, control group.
Table 3 Interaction tests of hypomagnesaemia and sex and mortality, and the sexual difference in the associations between magnesium and mortalityFootnote * (Hazard ratios (HR) and 95 % confidence intervals)
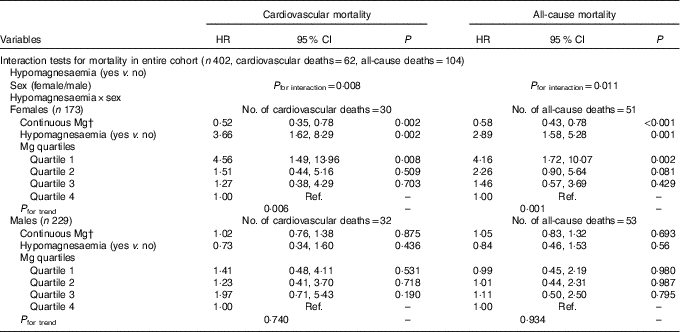
Ref., referent values.
* All the models were adjusted for age, diabetes, hypertension, history of CVD, PD duration at enrolment, serum phosphate and urine volume in the COX regression models; Mg concentrations for quartiles: quartile 1 (≤0·66 mmol/l), quartile 2 (0·67–0·73 mmol/l), quartile 3 (0·74–0·79 mmol/l) and quartile 4 (≥0·80 mmol/l).
† Per 0·1 mmol/l increase of Mg.
Considering the potential competing effects of other causes of death on cardiovascular mortality, competing-risks regression models were used to examine the associations between serum Mg and cardiovascular mortality. The results from both models give the same message (online Supplementary Tables S1–S3).
Discussion
Large prospective cohort studies on the relationships between serum Mg and cardiovascular and all-cause mortality are lacking in the PD population. In this prospective cohort study that included 402 PD patients followed for up to 5 years, we found that a lower serum Mg was independently associated with a higher risk of cardiovascular and all-cause mortality. Further analysis revealed that the negative effects of lower Mg on mortality were only observed in female patients.
CVD is the leading cause of death in ESRD patients( Reference de Jager, Grootendorst and Jager 20 ). In addition to the traditional factors, there are numerous other CVD risk factors in ESRD patients, such as inflammation, oxidative stress, malnutrition and vascular calcification( Reference Stenvinkel, Carrero and Axelsson 21 ). Close associations between low serum Mg and an increased risk of CVD or cardiovascular mortality have been observed both among pre-dialysis chronic kidney disease patients and those undergoing haemodialysis( Reference Kanbay, Yilmaz and Apetrii 3 , Reference Joao Matias, Azevedo and Laranjinha 5 , Reference Lacson, Wang and Ma 6 ). However, the relationship between serum Mg and CVD has not been fully investigated in the PD population. Yang et al. 10 reported that lower serum Mg levels were associated with an increased risk of hospitalisation and all-cause mortality in a large cohort of incident PD patients in the USA; however, cardiovascular mortality was not discussed in that study. Another retrospective cohort study of PD patients reported that serum Mg was an independent negative predictor of all-cause and cardiovascular mortality( Reference Cai, Luo and Dai 11 ); however, that study included only thirty-six patients with hypomagnesaemia and the multivariate models did not adjust for a history of CVD, both of which may have affected the results. In the current prospective cohort study, we included a larger sample size of PD patients and demonstrated that lower serum Mg may increase the risk of cardiovascular and all-cause mortality, providing further evidence for the close relationship.
The underlying mechanisms for the association between serum Mg and CVD remain unclear; several potential mechanisms may be involved. First, hypomagnesaemia has been linked with increased comorbidity and cardiovascular risk factors( Reference Joao Matias, Azevedo and Laranjinha 5 ), including C-reactive protein, atherosclerosis, hypertension and dyslipidaemia, which are important for the development of CVD. Second, low serum Mg in PD patients may be associated with vascular calcification( Reference Molnar, Biyani and Hammond 2 ), which is a significant pathophysiological cause of CVD. In a mouse model of pseudoxanthoma elasticum, Mg supplementation reduced carotid intima–media thickness, probably by inhibiting hydroxyapatite formation and increasing natural inhibitors of calcification( Reference Kupetsky-Rincon, Li and Uitto 22 , Reference Kupetsky, Rincon and Uitto 23 ). In clinical observation, close associations have also been described between low serum Mg and vascular calcification in haemodialysis and PD patients( Reference Molnar, Biyani and Hammond 2 , Reference Ishimura, Okuno and Kitatani 24 ). Third, Mg is recognised as a regulatory factor in endothelial dysfunction. In an animal model, deficiency of dietary Mg was found to induce endothelial dysfunction( Reference Maier 25 ), whereas oral Mg supplementation improved endothelial dysfunction in patients with coronary artery disease( Reference Shechter, Sharir and Labrador 26 ). In addition, higher Mg exposure was shown to halt smooth muscle cell proliferation and stimulate endothelial cell proliferation in vitro ( Reference Sternberg, Gratz and Koeck 27 ).
We found that serum Mg was not significantly associated with cardiovascular mortality in univariate analysis, indicating that the effects of serum Mg on mortality might be influenced by confounders. In addition, interaction analysis indicated that sex was the most important effect modifier in the current study. In subgroup analysis, hypomagnesaemia (HR: 2·89; 95 % CI 1·58, 5·28) was independently associated with a higher risk of cardiovascular mortality only in the female subgroup, further demonstrating the sex difference. Such sex differences in the association between lower serum Mg and cardiovascular mortality in the dialysis population have rarely been reported. Chiuve et al. (14) reported that higher plasma Mg concentrations and dietary Mg intake were associated with a lower risk of sudden cardiac death in a prospective cohort (Nurses’ Health Study) of women without chronic kidney disease. Interestingly, a meta-analysis that included several prospective studies also showed a protective effect of dietary Mg intake on CVD mortality risk in women but not in men( Reference Xu, Sun and Xu 1 ). This evidence suggests that cardiac responses to Mg deficiency may be different in women than in men. However, none of these studies has described a clear underlying mechanism of this phenomenon.
The different pathophysiological conditions of heart disease in women and the adverse cardiac outcomes caused by serum Mg deficiency may account for the sex-related differences found in this study. Women are more vulnerable than men to CVD( Reference Wenger 12 , Reference Xuereb, Magri and Xuereb 13 ); women also more often have a longer QTc interval than men( Reference Hreiche, Morissette and Turgeon 28 , Reference Whang, Julien and Higginbotham 29 ), which is associated with an increased risk of life-threatening cardiac arrhythmias (such as torsade de pointes and polymorphic ventricular tachycardia) and sudden cardiac death( Reference Velat and Culic 30 , Reference Straus, Kors and De Bruin 31 ). Severe serum Mg deficiency has been correlated with torsade de pointes and certain ventricular tachycardia events; intravenous Mg is regarded as the treatment of choice in these cases, even if hypomagnesaemia is not present( Reference Martin, Gonzalez and Slatopolsky 32 , Reference Agus 33 ). Moreover, the phenomenon that oral Mg shortens the prolonged QTc interval caused by medications also suggests that Mg may be an important regulator in the electrocardio physiological process( Reference Bachman 34 ). As described above, the incidence of hypomagnesaemia is high among PD patients in whom a low-Mg dialysate is routinely used( Reference Ejaz, McShane and Gandhi 9 , Reference Shah, Winer and Cutler 35 , Reference Katopodis, Koliousi and Andrikos 36 ). Taking these factors together, including women’s susceptibility to CVD, the close association between serum Mg and life-threatening cardiac arrhythmias, and the high prevalence of hypomagnesaemia among PD patients, it is not surprising that female PD patients with lower serum Mg had a higher cardiovascular mortality than other patients.
Another explanation for the relationship between hypomagnesaemia and cardiovascular mortality may be the close association between hypomagnesaemia and poor nutritional status( Reference Markaki, Kyriazis and Stylianou 7 , Reference Ye, Zhang and Guo 8 , Reference Wada, Hirayama and Hibino 37 , Reference Alhosaini and Leehey 38 ). Malnutrition is a strong risk factor for CVD and atherosclerosis in patients with advanced chronic renal failure( Reference Stenvinkel, Heimburger and Paultre 39 , Reference Suliman, Qureshi and Barany 40 ). Although malnutrition is rarely the direct cause of cardiac death, it may predispose to reduced cardiac contractility and atherosclerotic vascular disease and may contribute to cardiovascular death by aggravating pre-existing cardiovascular disease( Reference Bergstrom and Lindholm 41 ). In the current study, female patients with hypomagnesaemia also had significantly lower levels of serum albumin, phosphate, urea nitrogen and creatinine than other patients, all of which are regarded as nutritional markers of chronic renal failure( 42 ). Therefore, it is possible that the female PD patients with low serum Mg had poor nutritional status, which may have contributed to their higher risk of cardiovascular mortality.
The strengths of this study include its large and prospective cohort of patients receiving PD with a follow-up time of up to 5 years and complete data on clinical outcomes. However, our study has several limitations that should be considered. First, the patients enrolled were from a single PD centre and our previous cross-sectional study did not include the entire population of PD patients, inevitably introducing selection bias. Second, we did not collect data on dietary Mg intake or Mg loss in dialysate and urine; therefore, we cannot perform an in-depth analysis of Mg metabolism and mortality in this population. In addition, because the current prospective study did not involve any intervention for low serum Mg, we cannot say whether Mg supplementation is beneficial for PD patients. Finally, electrocardiogram data were not collected for most deaths that occurred outside the hospital. Therefore, a direct relationship between hypomagnesaemia and fatal cardiac arrhythmia is difficult to confirm.
Conclusion
Lower serum Mg was associated with a higher risk of cardiovascular and all-cause mortality in PD patients; however, the impact of lower serum Mg on mortality was mainly found in female patients. The current results suggest that we should pay close attention to serum Mg in PD patients, especially in female patients. Regular monitoring and supplementation may be helpful for patients with hypomagnesaemia.
Acknowledgements
The authors thank all nephrologists and nurses in our PD centre for their excellent management of PD patients. Thanks for the kind suggestions for the statistical methodology by Li Fan, MD and Hui Zhang, PhD. The authors thank Rebecca Tollefson, DVM, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
This work was supported by the Natural Science Foundation of China (grant nos. 81570614, 81774069), National Key Research and Development program (grant no. 2016YFC0906101), the Guangdong Science Foundation of China (grant nos. 2014A030313139, 2014B020212020, 2017A050503003, 2017B020227006,), Foundation of Guangdong Key Laboratory of Nephrology (grant no. 2014B030301023) and the Guangzhou Committee of Science and Technology, China (grant nos. 2014Y2-00543, 201704020167).
The authors’ contributions are as follows: H. Y., X. Yu and X. Yang contributed to the study design, data analyses, interpretation of the findings and wrote the manuscript; P. C., X. Z., J. L., Q. G. and H. M. contributed to the study design, subject briefings and data collection. All authors read and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518001599










