Consumption of soya proteins and isoflavones (ISF) has been associated with many health benefits(Reference Hermansen, Sondergaard and Hoie1, Reference Xiao2). However, soyabeans have been listed as one of the most significant sources of food allergens(3), and contain diabetogenic agents and autoimmunogens(Reference Hoorfar, Scott and Cloutier4), inducing autoimmune diseases such as type 1 diabetes in BioBreeding rats and non-obese diabetic mice(Reference Scott5).
Sex hormones such as oestradiol play an important role in mediating immune responses(Reference Cook6, Reference Kovacs, Messingham and Gregory7). Soya ISF mainly consist of genistein, daidzein and glycitein, and have oestrogenic and anti-oestrogenic effects because of their structural similarity to that of mammalian oestradiol(Reference Whitten and Patisaul8, Reference Lephart, Setchell and Handa9). Many post-menopausal women choose to consume soya products or ISF supplements as an alternative to hormone replacement therapy(Reference Hutchins, McIver and Johnston10). Meanwhile, a large number of infants take high amounts of ISF from soya-based formulas. However, existing knowledge on the effects of soya proteins and ISF ingested from food sources on immune functions of different age groups such as infants, youths and adults as well as the impact of maternal intake during pregnancy and lactation on their offspring is quite limited. The purpose of the present study was to examine the effects of sex and age of exposure to dietary soya components on the content of serum total and soya protein-specific antibodies using rats as a model.
Materials and methods
Chemicals and reagents
Alcohol-washed soya protein isolate (SPI, Pro Fam 930) and Novasoya ISF concentrate were purchased from Archer Daniels Midland Company (Decatur, IL, USA). Casein protein (90 % protein) was from ICN (Cleveland, OH, USA). Rat IgA, IgE, IgG and IgM ELISA quantification kits were purchased from Bethyl Laboratories, Inc. (Montgomery, TX, USA).
Animals and diets
Animal experimental protocols were approved by the Health Canada Animal Care Committee, and all animal handling and care followed the guidelines of the Canadian Council for Animal Care. Rats were kept in an environmentally controlled room with a 12 h light–12 h dark cycle. At the end of the study, rats were killed by exsanguination through cardiac puncture under general anaesthesia with isoflurane. Sera were collected, immediately frozen in liquid N2 and stored at − 80°C until analysis.
In Expt 1, pubertal Sprague–Dawley rats (Charles River, St Constant, PQ, Canada) at 50 d of age were randomly allocated into three groups (twenty-three males and twenty-three females per group) as the parental generation. Rats were given free access to tap water and one of the three diets. The specifications of the AIN93G diet(Reference Reeves, Nielsen and Fahey11) were followed in the preparation of all diets, except that in diets 2 and 3, 20 % casein was replaced by 20 % alcohol-washed SPI. Additionally, diet 3 was supplemented with ISF (250 mg/kg diet). After being fed for 70, 190 or 310 d, six males and six females from each diet group were randomly selected and killed, and sera were collected and stored as described earlier. At 120 d of age, five males and five females from the same diet group were randomly selected and housed together for mating to produce offspring generation (F1). The F1 pups were weaned at 21 d of age, and twenty-four pups of each sex from each diet group were randomly selected and fed the same diet as their parents throughout life. At 28, 70, 120 and 240 d of age, six males and six females from each diet group were randomly selected and killed, and sera were collected as stated earlier.
In Expt 2, juvenile Sprague–Dawley rats at 30 d of age (six males and six females per group) were fed diets containing 20 % casein in the absence or presence of 50 mg/kg diet of supplemental ISF or increasing amounts of alcohol-washed SPI (5, 10, or 20 %) with decreased amounts of casein (15, 10 or 0 %) for 90 d. At the end of the feeding period, rats were killed and sera were collected. The actual total ISF including genistein, daidzein and glycitein were measured as aglycones by Waters HPLC linear gradient with UV detection monitored at 254 nm(Reference Wang and Murphy12). Casein was used in the control diets in consideration of the popular consumption of dairy products in Western diets and being one of the major sources of proteins in infant formulas.
Measurement of serum total and soya protein-specific antibodies
Serum IgA, IgE, IgG and IgM concentrations were measured using commercial rat ELISA quantification kits according to the manufacturer's instructions. The concentrations of Ig were calculated against the standard.
For the measurement of soya protein-specific antibodies, diluted serum samples (1:10 for IgA, IgE, and IgG and 1:50 for IgM) were added into alcohol-washed SPI (10 mg/l)-coated plates. After incubation with either horseradish peroxidase-conjugated goat anti-rat IgA (1:80 000), sheep anti-rat IgE (1:1000), rabbit anti-rat IgG (1:50 000) or goat anti-rat IgM (1:30 000) antibodies, the plates were added with horseradish peroxidase substrate. The absorbance was read at 650 nm. The relative content of serum soya protein-specific Ig was expressed as the optical density at 650 nm after subtraction of the respective background. The average backgrounds for IgA, IgG, IgM and IgE were 0·03, 0·05, 0·05 and 0·04, respectively. The inter-assay CV for IgA, IgG, IgM and IgE were 4·2, 6·5, 5·3 and 4·8 %, respectively.
Statistical analyses
Results are expressed as means with their standard errors. Serum SPI-specific IgA, IgG, IgM and IgE contents were transformed to logarithms to eliminate heterogeneity of variance. Age or sex and dietary effects on serum total or SPI-specific IgA, IgG, IgM and IgE were analysed using one-way or two-way ANOVA. Differences between individual means were determined by Fisher's least significant difference test. A probability of P < 0·05 was considered to be significant. All data were analysed using STATISTICA version 6.1 (StatSoft, Inc., Tulsa, OK, USA). The sample size was n 6.
Results
Total isoflavones in diets
In Expt 1, the actual total ISF in 20 % casein, 20 % SPI or 20 % SPI+ISF were 0, 31·7 and 235·6 mg/kg diet, respectively. In Expt 2, the actual total ISF in 20 % casein, 20 % casein+ISF, 5 % SPI+15 % casein, 10 % SPI+10 % casein or 20 % SPI were 0, 42·8, 7·9, 15·9 or 31·7 mg/kg diet, respectively.
Total IgA, IgE, IgG and IgM content
Serum total IgA was significantly increased in parental generation female rats at the ages of 240 d (90·4 (sem 10·6) v. 51·3 (sem 2·9) mg/l, P < 0·01) and 360 d (94·5 (sem 7·9) v. 72·5 (sem 4·1) mg/l, P < 0·05), and decreased in male rats at the age of 360 d (91·2 (sem 10·6) v. 129·0 (sem 13·8) mg/l, P < 0·05) by dietary SPI compared with a casein-based diet. Addition of ISF to the SPI diet elevated IgA concentration in female rats at 120 d of age (61·7 (sem 5·8) v. 46·0 (sem 6·3) mg/l, P < 0·05) and further decreased IgA concentration in male rats at 360 d of age (62·5 (sem 3·3) v. 91·2 (sem 10·6) mg/l, P < 0·01) compared with rats consuming SPI diets.
In F1, female rats fed the diet containing SPI alone had significantly higher IgA and IgM content at 28 d of age (Fig. 1(A) and (C)) and lower IgG level at 240 d of age (Fig. 1(B)) than those fed the casein-based diet (P < 0·05). Addition of ISF to the SPI-based diet further increased the serum IgA and IgM in female rats at 28 d of age (Fig. 1(A) and (C), P < 0·01), and markedly increased IgG content in rats at 28, 70 and 120 d of age (Fig. 1(B)) compared with casein and SPI alone. Male rats fed SPI-based diets had higher IgA at 28 d of age (Fig. 1(D)) and lower IgM at 240 d of age (Fig. 1(F)). Supplemental ISF increased IgG at 28 d of age. Male rats fed the soya-based diet had much lower total IgG at 240 d of age than those fed the casein-based diet (Fig. 1(E)).
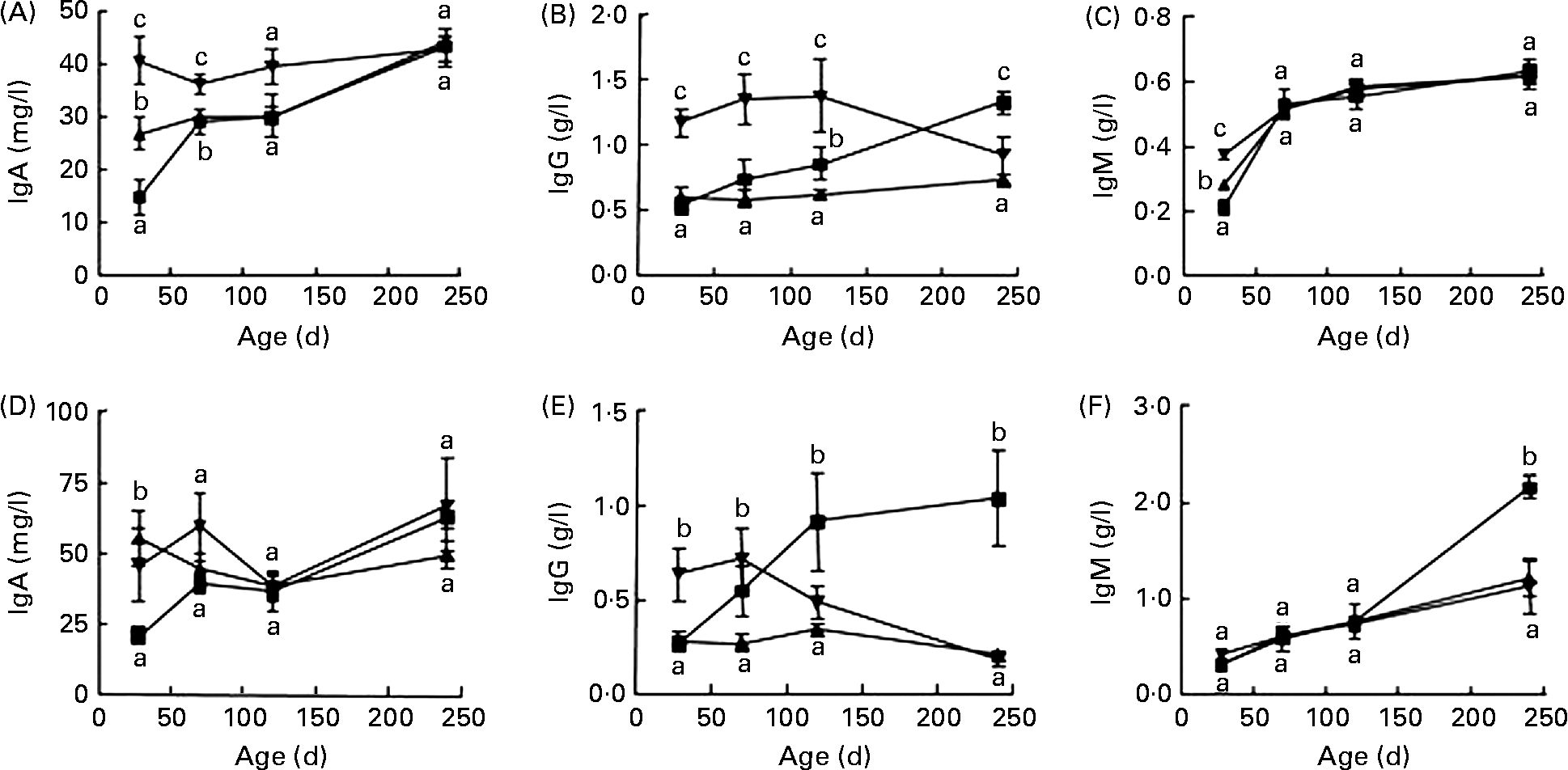
Fig. 1 Serum total IgA, IgG and IgM contents in F1 female (A–C) and male (D–F) rats fed diets containing either casein (![]() ) or alcohol-washed soya protein isolate (SPI,
) or alcohol-washed soya protein isolate (SPI, ![]() ) in the absence or presence of supplemental isoflavones (ISF, 250 mg/kg diet) and necropsied at 28, 70, 120 or 240 d of age. Values are means, with their standard errors represented by vertical bars (n 6). a,b,c Mean values with unlike letters were significantly different at the same time point (P < 0·05).
) in the absence or presence of supplemental isoflavones (ISF, 250 mg/kg diet) and necropsied at 28, 70, 120 or 240 d of age. Values are means, with their standard errors represented by vertical bars (n 6). a,b,c Mean values with unlike letters were significantly different at the same time point (P < 0·05). ![]() , SPI+ISF.
, SPI+ISF.
Soya protein isolate-specific antibodies in rats exposed to dietary soya before 28 d of age or after 30 d of age
In both Expt 1 and 2, the SPI diet markedly elevated SPI-specific IgM in female rats and IgG in male rats compared with the casein-based diet at 120 d of age (P < 0·05; Fig. 2). In F1 female rats fed 20 % SPI diets at day 28, all SPI-specific antibodies including IgA, IgG, IgE and IgM increased; addition of ISF to the SPI diet had no significant effect on IgA, IgM and IgE, and inhibited the stimulatory effect of SPI on IgG (data not shown).

Fig. 2 Differential effects of soya protein isolate (SPI) and isoflavones (ISF) on SPI-specific IgM and IgG in male and female rats fed diets containing either casein or alcohol-washed SPI with or without supplemental ISF (250 mg/kg diet) in Expt 1 (A and B) or diets containing casein in the absence or presence of 50 mg/kg diet of supplemental ISF or increasing amounts of alcohol-washed SPI (5, 10, or 20 %) in Expt 2 (C and D). Values are means, with their standard errors represented by vertical bars (n 6). a,b Mean values with unlike letters were significantly different (P < 0·05). OD, optical density.
Discussion
The present study showed that exposure to soya-derived ISF at early life significantly increased serum total IgA, IgM and IgG levels in the weanling female rats compared with other diets but had no significant effect on IgA or IgM levels in male rats. This suggests that ISF may have sex-associated immunomodulatory functions. Similar effects have also been observed in an in vitro study, showing that 17β-oestradiol or daidzein increased IgM and IgE production, while genistein reduced IgM and increased IgE concentrations in cultured mouse splenocytes(Reference Han, Denison and Tachibana13). Although the mechanism(s) by which soya ISF affects the serum total Ig content remains unclear, existing evidence suggests a possible role of soya ISF in modulating the number of antibody-producing cells. For example, ingestion of daidzein increased spleen IgM-producing cells in mice(Reference Zhang, Li and Wang14). Feeding the female rats with genistein markedly increased splenic B-cells in their offspring of both sexes and elevated IgM antibody-forming cells only in female but not in male offspring(Reference Guo, White and Brown15). The present study used soya-derived ISF rather than pure isolated genistein or daidzein, which may better represent the situation of human intake of soya foods, soya-based infant formulas or ISF supplements.
Circulating IgG and IgM play important roles in anti-infection through engaging the phagocytic system and activating the complement system, whereas IgA may compete with IgG and IgM for the same antigen and block complement activation. Additionally, serum IgA inhibits phagocytosis, chemotaxis and antibody-dependent cellular cytotoxicity(Reference Wolf, Fischer and Puhringer16). Therefore, changes in serum Ig isotypes by dietary soya ISF and proteins may affect the immune function of rats.
The oestrogenic effects and selective binding of soya ISF to oestrogen receptors may play a key role in the sex-dependent modulation of antibody production. The binding affinity of soya ISF to oestrogen receptor β is twenty times greater than that to oestrogen receptor α(Reference Kostelac, Rechkemmer and Briviba17), which is very different from the endogenous oestradiol. The type of oestrogen receptors (i.e. α or β) bound is believed to have a profound impact on humoral immunity(Reference Guo, White and Brown15). Therefore, the intake of soya ISF supplements may alter the humoral immunity in post-menopausal women.
The present study also demonstrated that SPI-specific antibodies (IgA, IgG, IgM and IgE) were present in sera of F1 female rats fed soya diets at 28 d of age for 1 week before necropsy. These antibodies, especially IgA, IgG and IgE, might have been produced in response to the dietary source of soya proteins rather than transferred from the maternal source, because only SPI-specific IgM was detected in their dams. This suggests that gastrointestinal tracts in the newly weaned rats may have not been well developed, and soya protein fragments or peptides can be absorbed into the circulation and thereby stimulating the specific immune response.
In summary, the present study has demonstrated that dietary soya ISF significantly affect the content of serum total antibodies in a sex- and age-dependent manner. Furthermore, exposure to dietary soya proteins at early life ( < 28 d of age) induces production of SPI-specific antibodies, suggesting that those rats may be vulnerable to the development of soya allergy or autoimmune diseases. Whether the consumption of soya foods has similar effects in humans warrants further investigation.
Acknowledgements
The present study was funded by Health Canada. S. M. C. was a recipient of Health Canada Postdoctoral Fellowship. None of the authors had any conflicts of interest. C. W. X. contributed to the experimental design, animal studies and data analysis. S. M. C. and C. M. W. conducted the sample analyses. G. S. G. analysed the dietary isoflavone content. M. R. L. A., G. M. C. and I. H. C. contributed to the design and animal study of Expt 1.




