Fe deficiency is the most prevalent micronutrient deficiency, affecting over three billion people worldwide1. Infants and children are especially at risk of Fe deficiency as growth increases nutrient requirements, including FeReference Allen2. Fe deficiency not only leads to anaemia, but may, even before the onset of anaemia, cause impairment of psycho-motor development, which is in part irreversibleReference Pollitt3–Reference Grantham-McGregor and Ani5. For example, infants with Hb concentrations < 95 g/l at 8 months of age scored significantly lower for locomotor scores at 18 months of ageReference Sherriff, Emond, Bell and Golding6. In many developing countries, over 50 % of infants are anaemic by 1 year of ageReference Dijkhuizen, Wieringa, West, Muherdiyantiningsih and Muhilal7, and blanket Fe supplementation for children is being considered as one option to reduce anaemia prevalence in childhood. However, Fe supplementation may negatively influence Zn uptake and Zn statusReference Whittaker8. Moreover, a recent study comparing the effects of Fe supplementation on infants in Sweden and Honduras suggests that Fe supplementation in Fe-replete infants can cause growth falteringReference Dewey, Domellof, Cohen, Landa Rivera, Hernell and Lonnerdal9, and Fe supplementation in malarious areas could increase morbidity and mortalityReference Oppenheimer10, Reference Sazawal, Black and Ramsan11. Hence, Fe supplementation in infants is not as straightforward as once thought. Furthermore, although sex differences in Fe status and Fe requirements are generally thought to arise only after the menarche, some studies have pointed to substantial sex differences in Fe status in infants12 and pre-pubertal children13.
On the initiative of UNICEF, four parallel studies on Fe and Zn supplementation in infants were conducted in South-East Asia (Thailand, Vietnam and Indonesia (two sites)) between 1996 and 2000 to investigate effects of Fe and Zn supplementation on Fe and Zn status and growth. All trials were randomized, placebo-controlled, double-blind trials. A shared core-protocol was used, specifying basic characteristics for the trials, and allowing pooling of data after completion of the trials. Main outcomes for each site were Fe status, Zn status and growth and these will be reported elsewhere. The aim of the present analysis of the pooled database was to investigate sex differences in infants with respect to anaemia and Fe deficiency prevalence and efficacy of Fe supplementation in view of earlier reports on differences in Fe status between boys and girls.
Materials and methods
Core protocol and design
The core protocol of the South-East Asia Multi-country Trial on Iron and Zinc supplementation in Infants (SEAMTIZI) was developed in a meeting with all principal investigators, prior to the start of the studies. Agreements were made on the dosage and chemical form of the Fe and Zn supplement (10 mg Fe and/or 10 mg Zn /d, both as sulphate salts), the age of recruitment (between 4 and 6 months of age), the duration of supplementation (6 months), the design of the studies (2 × 2 factorial), and core measurements to be included (concentrations of Hb and Zn, weight and height). At the time of the studies, national policies recommended exclusive breastfeeding for the first 4 months, so it was decided to start supplementation after 4 months of age.
Description of study sites
The research in Thailand was conducted by the Institute of Nutrition, Mahidol University (INMU) in Khon-kaen province in the north-east of ThailandReference Wasantwisut, Winichagoon, Chitchumroonchokchai, Yamborisut, Boonpraderm, Pongcharoen, Sranacharoenpong and Russameesopaphorn14. Infants were recruited from 106 rural villages. A survey prior to the study showed the prevalence of anaemia in infants aged 4–6 months to be about 50 % in this area. Supplements were given daily by the mother under supervision of health volunteers participating in the study.
The research in Vietnam was conducted by the National Institute of Nutrition (NIN), Hanoi and the Institute of Research for Development (IRD), Montpellier, France, in the rural Que Vo district, in Bac Ninh province in the north-west of VietnamReference Berger, Ninh, Khan, Nhien, Lien, Trung and Khoi15. Infants were recruited from 120 villages. Similar to the other study areas, most people depend on farming as the main source of income. A recent study showed that approximately 60 % of infants are anaemic in this areaReference Nguyen, Berger, Dao, Nguyen, Traissac and Ha16. Supplementation was given daily by trained field workers especially recruited for the study.
The research in Indramayu, Indonesia was conducted by the University of Trisakti and University of Indonesia (UT/UI), in a rural area in the province of Indramayu in West Java. Supplements were given 6 d/week by the village health workers participating in the study.
The research in Bogor District, Indonesia was conducted by the Nutrition Research and Development Centre (NRDC) in Bogor district, West JavaReference Dijkhuizen, Wieringa, West, Martuti and Muhilal17. Infants were recruited from six rural villages. In an earlier study in the same area, about 50 % of the infants were anaemic, and 20 % had low plasma Zn concentrationsReference Dijkhuizen, Wieringa, West, Muherdiyantiningsih and Muhilal7. Supplements were given 5 d/week by the health volunteers recruited for the study.
Subjects and procedures
Mothers of eligible infants were invited to participate in the study, informed of the procedures and purpose of the study, and informed consent was obtained. Infants were assigned to one of the four supplementation groups on the basis of individual randomization, following a computer-generated allocation list using blocks of twelve. Three of the four sites gave a high-dose vitamin A capsule to all infants prior to the study, with one site giving 50·000 IU (INMU) and two sites giving 100·000 IU vitamin A (NIN and UT/UI). These sites also took baseline blood samples in all (NIN), or a subsample of the infants (INMU, UT/UI). Exclusion before recruitment was on grounds of chronic or severe illness, severe clinical malnutrition, or congenital anomalies. In addition, infants with Hb concentrations < 70 g/l were not included, but referred to the health centre for Fe supplementation therapy. After 6 months of supplementation, a blood sample of the infant was taken for biochemical assessment of nutritional status. All infants with a Hb concentration of < 110 g/l were given Fe supplementation treatment at the end of the study. All study sites received ethical approval from their respective ethical boards.
Supplements
Both Fe and Zn were supplemented as sulphate salts in a sugar-based syrup, with 15 mg vitamin C/ml added. Infants received either Fe (10 mg/d), Zn (10 mg/d), Fe+Zn (10 mg each/d) or placebo as 2 ml syrup, 5–7 d/week according to site. Supplements for all sites were made by the same pharmaceutical company (PT. Kenrose, Jakarta, Indonesia) in cooperation with UNICEF-Jakarta. Supplementation was double-blind, and the supplements were coded with a letter at the production site. The code was made known only after all subjects had completed the trials and biochemical analyses were completed.
Biochemical analyses
Blood samples were obtained either by venepuncture (INMU, NIN, NRDC) or heel prick (UT/UI). Hb concentrations were measured by standard cyanmethaemoglobin method (INMU, NIN, NRDC) or Hemocue (UT/UI). Serum (INMU, NIN) or plasma (NRDC) ferritin concentrations were measured with ELISA. Anaemia was defined as a Hb concentration < 110 g/l, and Fe deficiency anaemia was defined as anaemia combined with a ferritin concentration < 12 μg/l18.
Statistical analysis
The effect of supplementation on biochemical indicators was investigated using a general linear model controlling for site and sex, using a full factorial model. Ferritin concentrations were transformed to natural logarithms to achieve normality. Differences between boys and girls were analysed with ANCOVA, controlling for site and age, and effect sizes were calculated from estimated means, using the full factorial model. Differences in prevalence of anaemia and Fe deficiency among groups were analysed using χReference Allen2 statistics.
Baseline biochemistry was not available for all subjects. One site (NRDC) did not take baseline blood samples, and two sites (INMU, UT/UI) did baseline biochemistry in subgroups only. However, the subgroup of subjects with baseline blood samples in the pooled analysis did not differ from the other subjects in end-point indicators of micronutrient status or anthropometry.
Results
Of the 2604 recruited infants, 2452 completed the study (86 %; Fig. 1). Although there were significant differences at recruitment among the different sites in several parameters, including Hb concentrations, there were no significant differences among the four supplementation groups at recruitment in the pooled database. However, there were significant differences in Fe status between boy and girl infants at recruitment, when the infants had a mean age of 5·2 months (sd 0·9) (all infants). Boy infants had significantly lower Hb concentrations than girls (108·7 and 111·4 g/l respectively, all groups combined; P = 0·04, ANCOVA controlling for age and site), and boy infants also had a significantly higher prevalence of anaemia (54·3 % and 47·4 % respectively, P < 0·01, Pearson χ2) at recruitment.

Fig. 1 Trial profile of the study and the number of blood samples available for haemoglobin (Hb) and ferritin (Fer) determination.
As reported earlier, Fe supplementation significantly improved Hb concentrations (P < 0·001), with a mean estimated effect size of 9·4 g/l (95 % CI 8·1, 10·7) in infants receiving Fe compared with infants not receiving FeReference Wieringa, Berger, Dijkhuizen, Hidayat, Ninh, Utomo, Wasantwisut and Winichagoon19. Hb concentrations were higher in both the Fe and Fe+Zn groups compared with the placebo and Zn groups (P < 0·001). Sex however significantly affected end-point Hb concentrations (P < 0·001; Fig. 2). End-point Hb concentrations were the same in boys and girls receiving Fe (118·2 and 118·4 g/l respectively; Table 1), but significantly lower in boys not receiving Fe compared with girls not receiving Fe (106·2 and 111·0 g/l respectively, P < 0·001, ANOVA controlling for age, site and Zn supplementation, Table 1). Hence, the estimated effect size of Fe supplementation on end-point Hb concentrations in boys was 12·0 g/l (95 % CI 10·2, 13·8) whereas in girls it was only 6·8 g/l (95 % CI 4·9, 8·7).

Fig. 2 Cumulative frequency of final Hb concentrations (g/l) in boy and girl infants receiving Fe (boy, – –; girl, ······) (P = 0·7) or not (boy, –––; girl,–––) (P < 0·001) for 6 months. The vertical line indicates the current cut-off for anaemia in infants at 110 g/l.
Table 1 Hb and ferritin concentrations in boy and girl infants at recruitment and after 6 months of supplementation

Anaemia was defined as haemoglobin < 110 g/l, and iron deficiency anaemia as anaemia combined with a ferritin concentration < 12 μg/l
aFerritin concentrations were transformed to natural logarithms prior to statistical analysis.
Significantly different from girl infants at recruitment: ANOVA, controlling for age and site bP = 0·041; cP < 0·01 and dChi-square, P < 0·01
Significantly different from girl infants not receiving iron: ANOVA, controlling for age, zinc supplementation and site eP < 0·01; fChi-square, P < 0·01
Significantly different from infants receiving iron: ANOVA, controlling for age, zinc supplementation and site gP < 0·01; hChi-square, P < 0·01
The same sex-specific effect was observed for plasma ferritin concentrations. At recruitment, ferritin concentrations were significantly higher in girl infants than in boy infants (geometric means 46·9 and 32·4 respectively, P < 0·001, ANOVA controlling for age and site). At the end of the supplementation period, ferritin concentration had declined in the infants not receiving Fe, with boy infants still having significantly lower ferritin concentrations than girl infants (geometric means 14·4 μg/l (95% CI 13·1, 15·7 μg/l) and 21·4 μg/l (95% CI 19·4, 23·5 μg/l) respectively, P < 0·001). In contrast, there was no statistically significant difference in end-point ferritin concentration between boys and girls receiving Fe (geometric means 44·2 (95% CI 40·2, 48·5) and 50·2 μg/l (95% CI 46·0, 55·8) respectively, P = 0·28, Table 1).
The lower Hb and ferritin concentrations in boy infants not receiving Fe resulted in a much higher prevalence of anaemia and Fe deficiency anaemia compared with girl infants not receiving Fe (Table 1) at the end of the supplementation period, when the infants were on average 11 months old. Overall, in the infants not receiving Fe, boys had a relative risk for anaemia of 1·6 (95 % CI 1·3, 2·1), and a relative risk for having Fe deficiency anaemia of 3·3 (95 % CI 2·1, 5·0) compared with girls at the end of the supplementation period (Table 2). Similarly, Fe deficiency, as indicated by a ferritin concentration < 12 μg/l regardless of Hb concentrations, was also significantly more prevalent in boy infants not receiving Fe compared with girl infants not receiving Fe (42·5 % v. 21·4 %, P < 0·001) with boys having a relative risk of 2·7 (95 % CI 1·9, 3·8) of being Fe deficient compared with girls.
Table 2 Prevalence of anaemia and Fe deficiency anaemia in boy and girl infants receiving Fe or not, and relative risks for boy infants as compared to girl infants
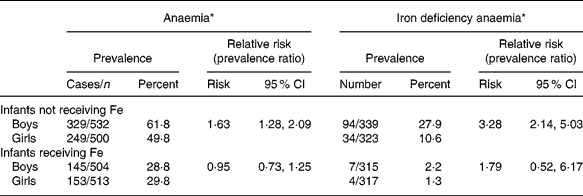
* Anaemia was defined as haemoglobin < 110 g/l, and Fe deficiency anaemia as anaemia combined with a ferritin concentration < 12 μg/l
Interestingly, in the subgroup of infants in which blood samples were taken at recruitment (mean age of these infants 5·6 months), differences in Hb concentrations between boy and girl infants were already statistically significant, but less distinct than at 11 months of age. Furthermore, boy infants not receiving Fe had a decrease in Hb concentrations over the study period from 108·4 g/l to 106·2 g/l (placebo and Zn groups combined), whereas Hb concentrations of girl infants not receiving Fe decreased only slightly over the study period (111·8 g/l to 111·0 g/l, Table 1). Infants not receiving Fe, and who were anaemic at 5 months of age had a significantly higher risk of also being anaemic at 11 months compared with those who were not anaemic at 5 months (odds ratio 2·1, 95 % CI 1·5, 3·0; χ2, placebo and Zn groups combined). Boy infants had a significantly higher risk to remain anaemic than girls (32 % of the boys remained anaemic v. 19 % of the girls). Vice versa, girls had a significantly higher chance of remaining non-anaemic (28 % of the boys and 41 % of the girls remained non-anaemic).
Discussion
In this pooled analysis of data from four large trials on the effects of Fe and Zn supplementation on nutritional status in infants, we found significant differences in Fe status between boy and girl infants. Prevalences of anaemia and Fe deficiency at the end of the study were significantly higher in boy infants not receiving Fe than in girl infants not receiving Fe. After Fe supplementation however, boys and girls achieved similar Hb concentrations.
At recruitment, when the infants where approximately 5 months of age, the sex difference in Hb concentrations and Fe status was already present, but not as distinct as at the end of the study. Hence the largest part of the difference in Hb concentrations between boy and girl infants not receiving Fe developed during the second half of infancy. It is important to note in this context that it is unlikely that there is a cultural bias towards girls in the three countries that could cause such a sex difference in nutritional status20. Moreover, sex differences in anaemia prevalence and Fe status in infancy have been reported from other regions as well, for example the UK and SwedenReference Domellof, Lonnerdal, Dewey, Cohen, Rivera and Hernell12, Reference Sherriff, Emond, Hawkins and Golding13. As both girls and boys achieved similar Hb concentrations after Fe supplementation, there is no reason to surmise physiological sex differences, e.g. in the physiological ranges of Fe status indicators or in Hb set-points. Furthermore, as the infants received a relatively low dose of Fe, close to the RDA, the ranges in Hb and ferritin concentrations after Fe supplementation probably reflect the physiological ranges, and are not due to Fe overdosing.
The most likely explanation for the findings of the present study is that these boy infants experienced a stronger decline in Fe status during the second half of infancy compared with girl infants, due to higher Fe requirements. The low ferritin concentrations at the end of the study in the boy infants not receiving Fe further strengthens this interpretation. One reason for higher Fe requirements in boy infants could be the higher growth rate of boy infants. The role of Fe in growth is complex however. Fe supplementation has been shown to improve length and ponderal growth in some studiesReference Allen2, but may also negatively affect growth, especially in Fe-replete childrenReference Dewey, Domellof, Cohen, Landa Rivera, Hernell and Lonnerdal9. Regardless of the underlying mechanism, important implications are that boy infants are more at risk for anaemia and Fe deficiency than girl infants, and that the recommendations for RDA of Fe for infants should either reflect these sex differences, or be high enough to ensure adequate intake of Fe for boy infants. Currently, RDA for boy and girl infants under the age of 1 year are the same however, ranging from 6 to 10 mg/d21-23.
Although transferrin receptor concentrations were not measured in this study, hampering accurate estimation of total body Fe, a very rough estimation of the extra Fe requirement for boy infants can be made, using the differences in ferritin concentrations at the end of study between boy and girl infants not receiving Fe. Ferritin concentrations in the range 15 to 300 μg/l reflect Fe stores, with 1 μg/l serum ferritin reflecting 140 μg/kg body weight stored FeReference Sharieff, Zlotkin, Tondeur, Feldman and Tomlinson24. Models using this relationship have been shown to predict effects of Fe supplementation on Fe status indices accuratelyReference Sharieff, Zlotkin, Tondeur, Feldman and Tomlinson24. The difference in ferritin concentrations between boy and girl infants not receiving Fe at the end of the study (7 μg/l) would correspond to a difference of stored Fe of 8·3 mg (mean body weight 8·5 kg). To account for this difference over a 6-month period, assuming that only 5 % of the ingested Fe is absorbed, boys would need to consume 166 mg more Fe than girls or approximately 0·9 mg/d, or >10 % higher than most current recommendations. Isotope studies on absorption of intrinsically labelled microencapsulated Fe fumerate (sprinkles) showed absorption percentages from 4 to 9 %Reference Tondeur, Schauer, Christofides, Asante, Newton, Serfass and Zlotkin25. The estimate of 0·9 mg/d extra Fe for boy infants is a conservative estimate, as Fe body stores are considered depleted at serum ferritin concentrations < 15 μg/l, and we do not know at what time point the boy infants became depleted. Clearly, more research specifically investigating Fe requirements in infants taking these sex differences into account is urgently needed, as the current study was not designed to investigate this. The current study clearly shows however that, with the assumption that infants of both sexes have access to the same diets, Fe needs are higher in boy than in girl infants, and that under ‘normal’ conditions in three countries in South-East Asia standard infant feeding practices do not meet the Fe requirements, especially of boys but also of girls. These findings are likely to be valid for most developing countries.
Furthermore, this study shows that although Fe supplementation was very effective in eliminating Fe deficiency anaemia, with less than 3 % of the infants receiving Fe still having Fe deficiency anaemia at the end of the 6-month supplementation period. The addition of vitamin C to the supplement syrups may have improved absorption of the ferrous sulphate, but could not prevent the decrease in Fe status in the placebo group, perhaps because the syrup was given in one daily dose, minimizing the effect of vitamin C on dietary Fe absorption. Despite Fe supplementation, anaemia prevalence remained high at 29 %. Various other causes may well underlie the non-Fe deficient anaemia still present after 6 months of Fe supplementation, including haemoglobinopathies such as Hb E and thalassaemia, and other micronutrient deficiencies such as vitamin B12 or vitamin A deficiencyReference Thurlow, Winichagoon, Green, Wasantwisut, Pongcharoen, Bailey and Gibson26. The prevalence of haemoglobinopathies is estimated to be between 5 and 60 % in the region, with very large regional differencesReference Weatherall and Clegg27. Sub-optimal vitamin A nutrition is also prevalent in the region, and may contribute to non-Fe deficient anaemia, as vitamin A has been shown to play a role in the utilization of FeReference Thurlow, Winichagoon, Green, Wasantwisut, Pongcharoen, Bailey and Gibson26, Reference Suharno, West, Muhilal, Karyadi and Hautvast28. Furthermore, chronic low-grade inflammation such as caused by intestinal parasites might also contribute to lower Hb concentrations independently of Fe statusReference Wieringa, Dijkhuizen, West, Northrop-Clewes and Muhilal29. As apparent from this study, the problem of non-Fe deficient anaemia in infants is an important issue in this region, and warrants further research.
To conclude, this study shows that, regardless of sex, in all infants not receiving Fe, Fe status was poor at the age of 11 months, with over 50 % of these infants having ferritin concentrations < 20 μg/l. Daily supplementation with 10 mg Fe was sufficient to virtually eliminate Fe deficiency anaemia. Therefore, in view of the high risk of Fe deficiency, not only in boy infants but also in girl infants, and the serious consequences of Fe deficiency for psycho-motor development, action is urgently needed. Strategies to improve Fe status in infants during the first years of life, including fortification of infant foods and perhaps even supplementation, are urgently needed in South-East Asia.
Acknowledgements
We thank all mothers and infants involved in this study, and UNICEF for funding the study. UNICEF did not participate in the analysis of the data or in the writing of the manuscript. All authors were principal investigators at their study site, contributing to the core-protocol and overseeing collection and entering of data. F.T.W and M.A.D were responsible for pooling of the data and data analysis, and drafting of the first draft. All authors contributed to the final manuscript.
Other contributors were involved in the SEAMTIZI Study Group as follows: UI Study site: Utomo B, Krause V, Sunawang and Dibley M; INMU Study site: Yamborisut U, Chitchumroonchokchai C, Thamrongvaranggoon T, Thasanasuwan W, Rojroongwasinkul, Russameesopaphorn W, Boonpraderm A, Sranacharoenpong K and Pongcharoen T; NIN study site: Trung NQ, Nhien NV, Lien DK, Quyen DT, Hien VT, Thu NN, Khan NC, Khoi HH and Tolvanen M; NRDC study site: West CE†, Muhilal, Martuti S, Muhardiyantisih and Permeasih D; UNICEF: Shrimpton R, Gross R†, Yip R, Schultink W, Darnton-Hill I and Esrey S†.
(† indicates deceased.)






