Plasminogen activator inhibitor-1 (PAI-1), a primary inhibitor of plasminogen activators, has an anti-fibrinolytic function(Reference Ha, Oh and Lee1). High levels of PAI-1 are associated with an increased risk for developing CHD or stroke(Reference Senno and Pechet2–Reference Thogersen, Jansson and Boman4). This increased risk of CHD may be due to promoting platelet adhesion and acute thrombus formation(Reference Thogersen, Jansson and Boman4).
Epidemiological studies suggest that linoleic acid (LA), a major component of n-6 fatty acids, has beneficial effects on both CHD and its risk factors, whereas some in vitro studies suggest that another n-6 fatty acid, arachidonic acid (AA), may have adverse effects. The Nurses' Health Study showed that a high dietary intake of LA has a strong inverse association with CHD(Reference Oh, Hu and Manson5). Additionally, a recent meta-analysis found that dietary intake of LA had a strong inverse association with non-fatal cardiovascular events(Reference Harris, Poston and Haddock6). The cardioprotective benefits(Reference Laaksonen, Nyyssonen and Niskanen7) of n-6 fatty acids may be due to decreasing blood pressure(Reference Zhao, Zhai and Wang8), reducing thrombosis(Reference Knapp9) and improving insulin sensitivity(Reference Laaksonen, Lakka and Lakka10). In contrast, several eicosanoids derived from AA are pro-inflammatory and pro-thrombotic, promoting vasoconstriction and enhancing platelet aggregation. Thus, AA has been postulated to adversely affect CHD. However, recent studies have identified AA-derived eicosanoids to have several beneficial attributes including vasodilation, platelet aggregation inhibition and anti-inflammatory effects(Reference Calder11). Interestingly, a recent meta-analysis showed that AA was not associated with fatal or non-fatal cardiovascular events(Reference Harris, Poston and Haddock6). These contradictory findings suggest that dietary or serum levels of AA have little association with CHD risk, possibly because AA levels are tightly regulated in the human body(Reference Nelson, Kelley and Emken12–Reference Willett14). Although PAI-1 is known to be involved in developing atherothrombosis(Reference Kohler and Grant15, Reference Levenson, Giral and Razavian16), very few studies have reported their associations with n-6 fatty acids.
The purpose of the present study was to test whether higher levels of serum n-6 fatty acids are associated with lower levels of PAI-1 in men aged 40–49 years. Additionally, we investigated whether higher levels of specific n-6 fatty acids, i.e. LA and AA, are associated with lower levels of PAI-1. To test these hypotheses, we examined data from a population-based cross-sectional study of 926 Caucasian, Japanese and Japanese-American men aged 40–49 years in the Electron Beam Tomography, Risk Factor Assessment among Japanese and US Men in the Post-World War II Birth Cohort (ERA-JUMP) study(Reference Sekikawa, Ueshima and Kadowaki17).
Methods
Study population
The participants were a randomly selected population-based sample of 926 men aged 40–49 years between 2002 and 2006 from three centres: 310 Caucasian men from Allegheny County, Pennsylvania; 313 Japanese men from Kusatsu, Shiga, Japan; 303 Japanese-American men from Honolulu, Hawaii. Those with clinical CVD or other severe diseases were excluded. Detailed descriptions of the study population have been published previously(Reference Sekikawa, Ueshima and Kadowaki17–Reference Abbott, Ueshima and Rodriguez19). Our final sample was 915 men (304 Caucasian, 313 Japanese and 298 Japanese-American men) due to eleven missing data. Informed consent from each participant was obtained. The protocol for the study was approved by the Institutional Review Boards of the University of Pittsburgh (Pittsburgh, PA, USA), Shiga University of Medical Science (Otsu, Japan) and the Kuakini Medical Center (Honolulu, HI, USA).
Venepuncture was performed in all participants in the early morning after a 12 h fast, as described previously(Reference Sekikawa, Ueshima and Kadowaki17). Fasting serum and plasma samples were stored at − 80°C, and shipped on dry ice to the Heinz Laboratory at the University of Pittsburgh, to examine the levels of LDL-cholesterol, HDL-cholesterol, total cholesterol, TAG, insulin and glucose, as published elsewhere(Reference Sekikawa, Ueshima and Kadowaki17). A calorimetric competitive ELISA was utilised to assess C-reactive protein. Hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg or anti-hypertensive medication usage. Diabetes mellitus was defined as fasting glucose level ≥ 7 mmol/l (1260 mg/l) or anti-diabetic medication usage. ‘Alcohol drinker’ was defined as a man who drinks alcohol 2 d or more/week. ‘Current smoking’ was defined as a man who smoked during the previous month.
Measurement of plasminogen activator inhibitor-1
As reported previously(Reference Declerck and Collen20, Reference Macy, Meilahn and Declerck21), plasma PAI-1 was measured at the clinical biochemistry research laboratory of the University of Vermont (Burlington, VT, USA). Briefly, plasma PAI-1 levels were analysed in citrated plasma(Reference Declerck and Collen20) by a two-site ELISA, which was sensitive to the free PAI-1 form but not to complexes between tissue plasminogen activator and PAI-1(Reference Macy, Meilahn and Declerck21), which was developed by Dr Collen and colleagues(Reference Declerck, Alessi and Verstreken22). The inter-assay CV for PAI-1 was 7·7 %.
Measurements of serum fatty acids
To measure serum fatty acids in a percentage of total fatty acid amounts, gas-capillary liquid chromatography (PerkinElmer Clarus 500; PerkinElmer, Waltham, MA, USA) was performed(Reference Sekikawa, Curb and Ueshima23). The intra-assay CV of LA (18 : 2n-6) and AA (20 : 4n-6) in serum n-6 fatty acids were 1·6 and 2·8 %, respectively(Reference Sekikawa, Curb and Ueshima23). The CV for other fatty acids ranged from 2·5 to 9·8 %(Reference Sekikawa, Curb and Ueshima23).
Statistical analyses
Log-transformed PAI-1 was used for the analyses, because the distribution of PAI-1 was skewed. To compare descriptive distributions across centres, an ANOVA for a continuous variable and the Mantel–Haenszel test for a categorical variable were performed. When a significant difference among the three groups existed, we examined multiple comparison tests using Bonferroni's test. To pool the data, we tested interactions according to the centres on associations of PAI-1 with LA as well as with AA. We also assessed the centre-specific associations of n-6 fatty acids on PAI-1 according to the three study centres (Table 3). After confirming no interaction and the same direction of the associations by centres, we pooled the data. To estimate the association between serum n-6 fatty acids and PAI-1 levels, we performed multivariate linear regression analyses, adjusting for covariates as follows: in model I, we adjusted for age and centre; in model II, we further adjusted for BMI, current smoking, alcohol drinking, hypertension and diabetes; in model III, we further adjusted for LDL-cholesterol, HDL-cholesterol, TAG and C-reactive protein; in model IV, we further adjusted for marine n-3 and trans-fatty acids. Because some eicosanoids of n-3 fatty acids were reported to inhibit the production of AA-derived eicosanoids(Reference Calder11), we tested marine n-3 as well as trans-fatty acids as covariates. The level of significance was considered to be P < 0·05. All reported P values were based on two-sided tests. All statistical analyses were performed using SAS 9.2 for Windows (SAS Institute, Inc., Cary, NC, USA).
Results
The general characteristics of the 915 study participants are shown in Table 1. The average age of the study participants was 45 years. In the total study population, there were 24·9 and 7·5 % participants with hypertension and diabetes, respectively. The median level (interquartile range) of PAI-1 was 37·4 (23·3–58·4) ng/ml.
Table 1 General characteristics of the study participants*
(Means or medians with interquartile ranges)
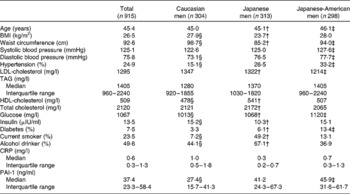
CRP, C-reactive protein; PAI-1, plasminogen activator inhibitor-1.
* Significance test was based on ANOVA, followed by Bonferroni's test if the overall ANOVA was significant.
† Values were significantly different between Japanese and Japanese-American men as determined by Bonferroni's test (P < 0·01).
‡ Values were significantly different between Caucasian and Japanese-American men as determined by Bonferroni's test (P < 0·01).
§ Values were significantly different between Caucasian and Japanese men as determined by Bonferroni's test (P < 0·01).
Serum proportions of fatty acids are listed in Table 2. Total n-6, total n-3, SFA and MUFA made up 39·0, 6·5, 31·2 and 20·4 %, respectively. LA and AA were 28·8 and 8·1 %, respectively. The correlation coefficient between LA and AA was 0·06 (P = 0·059).
Table 2 Distribution of serum fatty acids (%)*
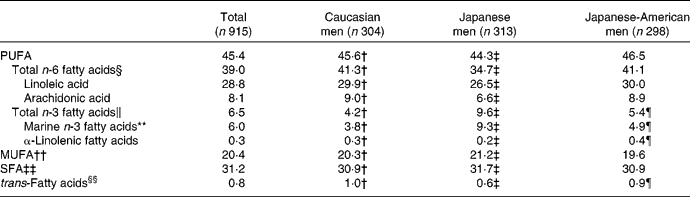
* Significance test was based on ANOVA followed by Bonferroni's test if the overall ANOVA was significant.
† Significant difference between Caucasian and Japanese men as determined by Bonferroni's test (P < 0·01).
‡ Significant difference between Japanese and Japanese-American men as determined by Bonferroni's test (P < 0·01).
§ Total n-6 fatty acids indicate the sum of linoleic acid (18 : 2n-6), γ-linoleic acid (18 : 3n-6), dihomo-γ-linolenic acid (20 : 3n-6) and arachidonic acid (20 : 4n-6).
∥ Total n-3 fatty acids indicate marine-derived n-3 fatty acids, eicosatetraenoic acid (20 : 4n-3) and α-linolenic acid (22 : 18n-3).
¶ Significant difference between Caucasian and Japanese-American men as determined by Bonferroni's test (P < 0·01).
** Marine-derived n-3 fatty acids were defined as EPA (20 : 5n-3), docosapentaenoic acid (22 : 5n-3) and DHA (22 : 6n-3).
†† MUFA indicate the sum of palmitoleic acid (16 : 1n-7), oleic acid (18 : 1n-9) and cis-vaccenic acid (18 : 1n-7).
‡‡ SFA indicate the sum of myristic aicd (14 : 0), palmitic acid (16 : 0) and stearic acid (18 : 0).
§§ trans-Fatty acids indicate the sum of palmitelaidic acid (16n-7 : 1t), trans-9-octadecanoic acid (18n-9 : 1t) and linolelaidic acid (18n-6 : 2tt).
Pooled and centre-specific analyses reveal that serum n-6 fatty acids were inversely associated with PAI-1 in the total population as well as in each of three different populations (Table 3). Pooled analyses showed that serum total n-6 fatty acids, LA and AA, had significant inverse associations with PAI-1 levels, even after adjusting for covariates. In centre-specific analyses, PAI-1 had significant inverse associations with total n-6 fatty acids and LA over three study populations. These significant associations remained after multivariate adjustments. PAI-1 was inversely associated with AA. No significant interaction existed in the associations of serum n-6 fatty acids, LA and AA, with PAI-1 according to the study centres (P = 0·09, 0·08 and 0·24, respectively). Standard parameter estimates indicate a standard deviation unit change in log-transformed PAI-1 per 1 sd unit increase in serum n-6 fatty acids.
Table 3 Centre-specific and pooled associations between n-6 fatty acids and log(PAI-1)

CRP, C-reactive protein.
Marine-derived n-3 fatty acids were defined as EPA (20 : 5n-3), docosapentaenoic acid (22 : 5n-3) and DHA (22 : 6n-3). Total n-3 fatty acids indicate marine-derived n-3 fatty acids, eicosatetraenoic acid (20 : 4n-3) and α-linolenic acid (22 : 18n-3). trans-Fatty acids indicate the sum of palmitelaidic acid (16n-7 : 1t), trans-9-octadecanoic acid (18n-9 : 1t) and linolelaidic acid (18n-6 : 2tt).
Values were significantly different: * P < 0·001; ** P < 0·01; *** P < 0·05.
† Total n-6 fatty acids indicate the sum of linoleic acid (18 : 2n-6), γ-linoleic acid (18 : 3n-6), dihomo-γ-linolenic acid (20 : 3n-6) and arachidonic acid (20 : 4n-6).
‡ Adjusted for age.
§ Additionally adjusted for BMI, current smoking, alcohol drinking, hypertension and diabetes.
∥ Further adjusted for LDL-cholesterol, HDL-cholesterol, TAG and CRP.
¶ Continuously adjusted for marine n-3 fatty acids and trans-fatty acids.
Discussion
The present population-based cross-sectional study found that total serum n-6 fatty acids were inversely and significantly associated with PAI-1 among 915 men, aged 40–49 years. Additionally, both LA and AA showed significant inverse associations with PAI-1 levels (P < 0·0001).
The present study may provide a novel mechanism on the cardioprotective benefits of n-6 fatty acids by improving the fibrinolytic response, such as reducing PAI-1. The present finding of an inverse association between serum n-6 fatty acids and PAI-1 is consistent with the results of several previous studies(Reference Fleischman, Justice and Bierenbaum24, Reference O'Brien, Etherington and Jamieson25), but not all(Reference Byberg, Smedman and Vessby26). These findings of n-6 fatty acids suggest a favourable fibrinolytic response on vascular thrombosis, including a decrease in platelet aggregation. Fleischman et al. (Reference Fleischman, Justice and Bierenbaum24) found an increased platelet aggregation time (P < 0·05) and a decreased disaggregation time (P < 0·01) on a dietary LA in each participant for 2 weeks from about 2·9 % to about 5·0 % of energy among sixty-six subjects. A cross-over study by Thijssen et al. (Reference Thijssen, Hornstra and Mensink27) also demonstrated an increased platelet aggregation time while on a LA diet in comparison with a SFA diet in eighteen men (P = 0·04). O'Brien et al. (Reference O'Brien, Etherington and Jamieson25) conducted a clinical trial in thirty-nine healthy men for 6 weeks with either a PUFA diet (sunflower oil-based foods, 65 % LA) replaced for saturated fat or a normal diet. They found decreased platelet count (P = 0·01) and increased bleed time (P = 0·05)(Reference O'Brien, Etherington and Jamieson25). Furthermore, previous studies, including the Nurses' Health Study(Reference Oh, Hu and Manson5), have suggested that n-6 fatty acids lower the CHD risk, through a decrease in blood pressure(Reference Zhao, Zhai and Wang8), a reduction in thrombosis(Reference Knapp9) and an improvement in insulin sensitivity(Reference Laaksonen, Lakka and Lakka10).
The present results of the inverse association between serum n-6 fatty acids and PAI-1 are partially inconsistent with the results of a previous study. Byberg et al. (Reference Byberg, Smedman and Vessby26) showed that PAI-1 activity has a significant inverse association with serum LA but a significant positive association with serum AA in their sub-analysis with 381 men from a population-based cross-sectional sample of 871 men aged 70 years. The discrepancy in the association of PAI-1 with AA may be attributed to different measurements of PAI-1 and fatty acids or to different ages of participants. In the measurements of PAI-1 and fatty acids, Byberg et al. measured PAI-1 activity (i.e. a free active form) and serum cholesteryl esters for fatty acid measurements in older participants (mean average 70 years), whereas we measured total plasma PAI-1 levels (i.e. free active, free latent and complex with tissue plasminogen activator forms) and fatty acids in serum cholesteryl esters, phospholipids and TAG, in middle-aged men (aged 40–49 years). Although the previous study demonstrated a linear association between PAI-1 activity and PAI-1 antigen (r 0·80 in platelet-poor plasma; r 0·88 in platelet-rich plasma), about 66·7 % of PAI-1 antigen in plasma was active(Reference Declerck, Alessi and Verstreken22). Considering the very short half-life of PAI-1 levels and various processes (e.g. temperature, time or pH) for handling the blood samples as well as significant diurnal change in PAI-1, the PAI-1 antigen measurement as in the present study may have the advantage of detecting comprehensive forms of relatively unstable total plasma PAI-1, rather than measuring only an active form.
Future studies are required in order to elucidate possible reasons of the discrepancy between the in vitro and population studies. Several in vitro studies have shown that LA increases the secretion of PAI-1 in HepG2 cells(Reference Banfi, Rise and Mussoni28, Reference Ye, He and Wang29). In vitro studies have reported that AA-produced eicosanoids promoted neutrophil adhesion(Reference Bates, Ferrante and Smithers30) and IL-1β production by human monocytes(Reference Sinha, Stoll and Weber31). However, more recent studies have demonstrated no effect or a beneficial effect. A double-blind placebo-controlled study with an AA supplementation of 840 mg/d for 4 weeks demonstrated no effect on platelet aggregation in twenty-four healthy Japanese men who had relatively high levels of fish oil consumption(Reference Kusumoto, Ishikura and Kawashima32). In another clinical trial of ten healthy men taking a 200 v. 1500 mg/d AA regimen, Nelson et al. (Reference Nelson, Schmidt and Bartolini33) found borderline significance between higher AA intake and prolonged bleeding time (P = 0·06). Although several AA-derived eicosanoids may indeed have a pro-inflammatory role, recent studies have suggested that several AA-derived eicosanoids may play an anti-inflammatory role(Reference Schmitz and Ecker34).
Mechanisms responsible for the association of n-6 fatty acids with PAI-1 require future studies. However, two possibilities exist. First, n-6 fatty acids may delay platelet aggregation so that PAI-1, acting as an acute-phase reactant, is decreased within the haemodynamic balance and thrombotic response, during vascular injury, in atherosclerosis and CHD. Several previous studies have shown that LA reduces platelet aggregation(Reference Fleischman, Justice and Bierenbaum24, Reference O'Brien, Etherington and Jamieson25). Second, LA may reduce PAI-1 levels through its cholesterol-lowering effect. A previous study from ERA-JUMP found that higher levels of serum LA and AA were associated with lower levels of LDL and VLDL(Reference Choo, Ueshima and Curb35). An in vitro study showed that VLDL led to increased PAI-1(Reference Nilsson, Gafvels and Musakka36). These reduced cholesterol levels may improve or modulate the fibrinolytic response.
We additionally examined the associations of other fatty acids with PAI-1. Serum marine n-3 fatty acids over the three populations showed little overlapped distributions and the lack of consistent associations (see Table S1 of the supplementary material, available online http://www.journals.cambridge.org/bjn). trans-Fatty acids of three populations were positively associated with PAI-1 (see Table S2 of the supplementary material, available online http://www.journals.cambridge.org/bjn).
The strengths of the present study include the following: (1) the association was examined in a randomly selected population-based sample and (2) the sample size was relatively large. However, the present study also has several limitations: (1) the cross-sectional study design could not assess causality; (2) the study population included only men aged 40–49 years, which may limit the generalisability to other populations and (3) as an observational study, the present study may include residual confounding or potentially unmeasured factors, such as total energy intake(Reference Willett, Howe and Kushi37).
In conclusion, serum n-6 fatty acids were inversely and significantly associated with PAI-1 levels in a population-based cross-sectional study. Total n-6 fatty acids, especially LA and AA, were inversely and significantly associated with PAI-1 levels in both univariate and multivariate models. These findings suggest that n-6 fatty acids may have favourable effects on fibrinolysis. A future study to examine the causality between n-6 fatty acid and PAI-1 is warranted.
Acknowledgements
The present study was funded by the National Institutes of Health R01 HL68200 and HL071561 and from the Japanese Ministry of Education, Culture, Sports, Science and Technology, B 16790335 and A 13307016. The authors disclose that there are no conflicts of interest. S. L. wrote the first manuscript. J. D. C., T. K., R. W. E., T. T., A. E.-S., S. K., T. S., D. E., H. U., L. H. K. and A. S. collected the data. S. L., T. O. and A. S. analysed the data. S. L., R. W. E., K. M., C. S., J. C., A. F., T. O., H. U., L. H. K. and A. S. interpreted the data. J. D. C., R. W. E., K. M., J. C., T. S., K. M., D. E., L. H. K. and A. S. gave critical comments. S. L. with A. S. revised the manuscript.





