Adequate nutrition is fundamental from infancy until adult life. However, the increase in the rates of chronic diseases such as obesity may suggest that children are not using an optimal diet, since studies show that nutritional practice in early childhood has a role in the aetiology of obesity(Reference Robinson and Godfrey1).
There is evidence that many obesity-promoting behaviours, including unhealthy eating habits that are learnt during childhood, track to adulthood(Reference Ells, Campbell and Lidstone2). The social environment is very important for young children to develop eating habits(Reference Patrick and Nicklas3). Not only has the parent's lifestyle been shown to play a significant role in the development of a healthy diet in children(Reference Skinner, Carruth and Bounds4), but also parental education and financial background are associated with children's eating behaviour(Reference North and Emmett5). Similarly, it has been demonstrated that longer television (TV) watching is linked to a higher consumption of salted and sugary snacks in children(Reference Aranceta, Perez-Rodrigo and Ribas6).
Particularly, feeding patterns in the first year of life have been studied to a great extent, but the primary objective of these studies was to assess the socio-demographic determinants of breast- or bottle-feeding(Reference van Rossem, Oenema and Steegers7) along with complementary feeding(Reference Schiess, Grote and Scaglioni8), whereas determinants of a child's diet shortly after the weaning and lactation period have been relatively understudied.
During the last decennium, an alternative approach within nutritional research has been developed by using dietary patterns analysis(Reference Hoffmann, Schulze and Schienkiewitz9, Reference Hu10). This approach takes into account that dietary components can be highly correlated with each other, and hence represents a more comprehensive reflection of food consumption than assessing single nutrients or foods(Reference Hoffmann, Schulze and Schienkiewitz9, Reference Hu10). In addition, several studies have shown in adults that a ‘Western’ dietary pattern is associated with an increased risk of obesity(Reference Kjollesdal, Holmboe-Ottesen and Mosdol11, Reference Paradis, Godin and Perusse12) and metabolic disease(Reference Kant13), whereas ‘Healthy’ dietary patterns are associated with a lower all-cause mortality in adults(Reference Kant13). So far, dietary patterns in toddlers have not had extensive study yet. However, to identify young children at potential risk for unhealthy eating behaviour and in order to develop targeted strategies to improve adequate nutrition during infancy and childhood, knowledge about common dietary patterns in very young children and their determinants will be important. Dietitians and healthcare workers can provide targeted guidance to promote the development of healthy eating when taking into account the combination of different food items that young children eat. Also, to perform further studies on dietary patterns and various health outcomes in toddlers, knowledge about these determinants to elucidate potential confounders or mediators is necessary. For this reason, the aim of the present study was to assess whether certain dietary patterns may already be observed among toddlers and further identify parental and child determinants of adherence to these dietary patterns.
Material and methods
Study population
This study was embedded in a population-based prospective cohort study from fetal life until young adulthood in Rotterdam, the Netherlands and has been described in detail previously(Reference Jaddoe, van Duijn and van der Heijden14).
In total, 9778 mothers with a delivery date between April 2002 and January 2006 were enrolled in the study, of which 7893 provided consent for follow-up. From 2003 onwards, data collection on nutritional intake at the age of 14 months was implemented in the study. In total, 5088 mothers received an FFQ for their child at 14 months of age (Fig. 1). This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Medical Ethical Committee of the Erasmus University Medical Center, Rotterdam, the Netherlands. Written informed consent was obtained from all subjects.

Fig. 1 Flow chart of participants included for analysis.
Dietary assessment of the children
Out of 5088 mothers who received the FFQ to assess the child's nutrition, 3643 (72 %) filled in the FFQ and were eligible for analysis (Fig. 1). The FFQ was developed in cooperation with the division of Human Nutrition of Wageningen University, The Netherlands and based on an existing validated FFQ developed and described in detail previously(Reference Feunekes, Van Staveren and De Vries15). This existing FFQ was modified by only comprising foods frequently consumed during the second year of life according to a National Dutch food consumption survey in 941 Dutch children aged 9–18 months(Reference Hulshof and Breedveld16). In addition, only foods contributing ≥ 0·1 % of the total consumption of energy, protein, fat, carbohydrates and dietary fibre in the latter survey were incorporated in the FFQ. The final FFQ was validated against 3 d 24 h-recalls carried out by trained nutritionists in a representative sample of Dutch children aged 14 months living in Rotterdam, the Netherlands. This validation showed the following intra-class correlation coefficients for macronutrients: total energy, 0·4; total protein, 0·7; total fat, 0·4; carbohydrates, 0·4; dietary fibre, 0·7. The final FFQ consisted of 211 food items and included questions on the frequency of consumption of these food items over the last month, the amount and type of the food item, and preparation methods. Portion sizes in grams per d were estimated using standardised household measures(Reference Donders-Engelen, Heijden van der and Hulshof17). To calculate average daily nutritional values, the Dutch food composition Table 2006 was used(18).
A total energy intake of < 1260 or >12 600 kJ/d ( < 300 or >3000 kcal/d) was considered as implausible values for total energy intake. However, sensitivity analyses showed no different results when excluding these values, and therefore these children were kept in the final dietary pattern analyses.
The interquartile range of the child's age, when the FFQ was filled out, ranged from 12 to 14 months with a minimum age of 11·6 months and a maximum age of 33 months. Since sensitivity analyses showed similar results even when excluding outliers (age above the 99th percentile: 20 months of age), children older than 20 months were kept in the final analyses.
Dietary pattern analysis was restricted to children with a Dutch ethnicity (n 2420), since the definitions of dietary patterns can be race-specific(Reference Tucker19) and because this FFQ was only valid in this population. Ethnicity of the child was defined as follows: if both parents were born in the Netherlands, the ethnicity was defined as Dutch; if one of the parents was born in another country than the Netherlands, that country applied; if parents were born in different countries other than the Netherlands, the country of the mother applied(Reference Swertz, Duimelaar and Thijssen20).
Parental indicators
From obstetric records assessed in mid-wife practices and hospital registries, data on maternal age, BMI and parity at intake were available(Reference Jaddoe, van Duijn and van der Heijden14). Total macronutrient intake during pregnancy was assessed at intake (median 13·5 weeks of gestation, range: 3·4) using a validated semi-quantitative FFQ of Klipstein-Grobusch et al. (Reference Klipstein-Grobusch, den Breeijen and Goldbohm21). Only the energy-providing nutrients – total fat and carbohydrate intake during pregnancy – were used as predictors in this study, since they are associated with both diet and fat mass in the offspring(Reference Brion, Ness and Rogers22). Adjustment for total energy intake was performed by a multivariate nutrient density method(Reference Willett, Howe and Kushi23, Reference Hu, Stampfer and Rimm24). Other prenatal questionnaires completed by the mother included information on mother's educational level, household income, maternal smoking, maternal alcohol consumption, folic acid supplementation during pregnancy, marital status, co-morbidity (i.e. any history of or medical treatment for depression, anxiety, diabetes mellitus, hypertension or hypercholesterolaemia). Folic acid exposure during early pregnancy was assessed by a questionnaire that included the following question: ‘Have you taken folic acid, either as a single supplement or as part of multivitamin supplement during the first trimester?’ Partners of the mothers received one questionnaire in the prenatal phase, which included information on paternal education, age, smoking, and any history or medical treatment for diabetes mellitus, hypertension or hypercholesterolaemia.
Level of maternal and paternal education was defined as follows: (I) low: no education, elementary or middle school or less than 4 years of high school, (II) mid: college, associate degree or Bachelor's degree and (III) high: Master's degree(25).
Child indicators
Sex of the child, birth weight and gestational age were available from obstetric records and hospital registries(Reference Jaddoe, van Duijn and van der Heijden14). Timing of introduction of solids in the first year of life was retrospectively assessed by supplementary questions in addition to the FFQ at the age of 14 months. Parents were asked at what age they had first introduced solids in the child's diet in addition to breast- or bottle-feeding, which we coded in two categories: before the age of 6 months or 6 months and later according to the feeding recommendations of the WHO(26). After the age of 6 months, all children received complementary feeding. Breast-feeding duration was assessed according to five indicators: ever breast-feeding, cessation of breast-feeding and receiving any breast-feeding at the age of 2, 6 and 12 months. Data on ever breast-feeding were collected from delivery reports and data on breast-feeding cessation or continuation were derived from postnatal questionnaires at 2, 6 and 12 months of age. Accordingly, breast-feeding was categorised into the following three groups: (I) never breast-feeding, (II) any partial breast-feeding in the first 4 months of life and (III) any full breast-feeding in the first 4 months of life. A definition of full breast-feeding was established on the basis of whether the child received breast-feeding without any other formula feeding, milk or solids. Partial breast-feeding indicated children receiving both breast-feeding and formula feeding and/or solids in this period.
The presence of cows' milk allergy was obtained by questionnaires at the age of 6 and 12 months, in which parents were retrospectively asked whether their child had a history of doctor-attended cows' milk allergy. At the age of 12 months, questionnaire data were available on additional care-giving of the child by au-pair or daycare. The duration of TV watching during weekdays and weekend days was assessed by questionnaire in the second year of the child's life. According to the American Academy of Paediatrics, the answer categories were divided into < 2 h/d and ≥ 2 h/d(27). Height and weight were measured at the Community Child Health Centres at the age of 14 months. Height was measured in standing position by a Harpenden stadiometer (Holtain Limited). Weight was measured using a mechanical personal scale (SECA). Weight-for-age and height-for-age z-score was calculated from the national reference using the Growth Analyzer program (http://www.growthanalyser.org)(Reference Fredriks, van Buuren and Burgmeijer28).
Statistical methods
All 211 food items from the FFQ data of all Dutch children (n 2420) were classified into twenty-one food groups on the basis of the Dutch food consumption survey among pre-school children(Reference Hulshof and Breedveld16) and nutrient content (Table S1 of the supplementary material, available at http://www.journals.cambridge.org/bjn). Subsequently, we applied principal component analysis on twenty-one food groups (assessed in g/d) of the children to construct overall dietary patterns by explaining the largest proportion of variation in the food group intake(Reference Hu10). To reduce correlation between the factors, the varimax method by maximising the sum of the variance of the loading components was used(Reference Kaiser29). To reduce bias as a result of multiple testing and to better identify common dietary patterns, only the dietary patterns with an eigenvalue of ≥ 1·5 were extracted. This cut-off was on the basis of the scree plots, which indicated a clear break after the second factor (i.e. dietary pattern) with an eigenvalue of 1·7(Reference Hatcher30) (Table 1). These two dietary patterns accounted for 24·5 % of the variability in food consumption within our study population. Accordingly, regression-based factor scores were extracted and used as adherence scores of these dietary patterns.
Table 1 Characteristics of the ‘Health conscious’ and ‘Western-like’ dietary patterns in Dutch children aged 14 months (retaining factor loadings >0·2 or <−0·2)

En%, energy percentage.
* P < 0·05.
† The eigen value was used as indicator of the amount of variation explained by each dietary pattern.
‡ Principal component analysis was used as an extraction method in which the factor score represents the relative contribution of that food group to the identified dietary pattern.
To assess the association between adherence to the dietary pattern and each of the potential parental and child indicators, we included all indicators simultaneously in a multivariate linear regression model. The addition of these potential indicators was predominantly on the basis of previous studies in toddlers and pre-school children(Reference North and Emmett5, Reference Brion, Ness and Rogers22, Reference Gubbels, Kremers and Stafleu31–Reference Hendricks, Briefel and Novak34). Additionally, folic acid supplementation during pregnancy was added as a proxy for other health behaviours of the mother during pregnancy(Reference Dott, Rasmussen and Hogue35). In order to improve the model fit, these analyses were followed-up by a backward stepwise elimination procedure retaining only the strongest predictors, with P = 0·10 as endpoint.
To diminish potential bias associated with attrition, the missing values of subject characteristics (approximately 0·1–28 %) were multiple imputed (n 5 imputed data sets). The multiple imputation was based on the correlation between each variable with missing values with the other subject characteristics as described previously in detail(Reference Sterne, White and Carlin36, Reference Rubin and Schenker37). Briefly, the multiple imputation procedure begins with generating five copies of the original data set, each with missing values replaced by values randomly generated from the predictive distribution on the basis of the correlation between each variable with missing values and the other subject characteristics. The second stage is that the main statistical analyses (i.e. regression analyses) are repeated in each of the five imputed data sets. The final effect sizes (β or regression coefficients) are estimated by taking the average effect size of the five imputed data sets. To calculate the 95 % CI, the combined standard errors from the five imputed data sets were calculated using Rubin's rules(Reference Rubin and Schenker37), taking into account the uncertainty associated with the missing data. Analyses were repeated in the original data and after the multiple imputation procedure. Since we found similar results, the final results in the present paper are presented as the pooled β with its 95 % CI after the multiple imputation procedure. A P value < 0·05 was considered as statistically significant.
Results
The observed correlations of the food groups for the two constructed dietary patterns are presented in Table 1. Component 1 represented a ‘Health conscious’ dietary pattern characterised by high intake of fruit, vegetables, legumes and fish. Component 2 represented a ‘Western-like’ dietary pattern comprising high intakes of savoury and snacks, animal fats, confectionery and sugar-containing beverages (Table 1). Adherence score ranged from − 1·91 to 7·47 for the ‘Health conscious’ dietary pattern and from − 2·09 to 8·43 for the ‘Western-like’ dietary pattern. Characteristics of the study population are shown in Table 2.
Table 2 Parental and child characteristics of the study population (Number of participants and percentages; mean values and standard deviations, n 2420)

En%, energy percentage; TV, television.
Determinants of adherence to a ‘Western-like’ dietary pattern of the child
Multivariate associations between parental and child indicators and adherence to a ‘Western-like’ dietary pattern are presented in Table 3.
Table 3 Predictors of adherence to a ‘Western-like’ dietary pattern of children aged 14 months (Regression coefficients (β) and 95 % confidence intervals, n 2420)
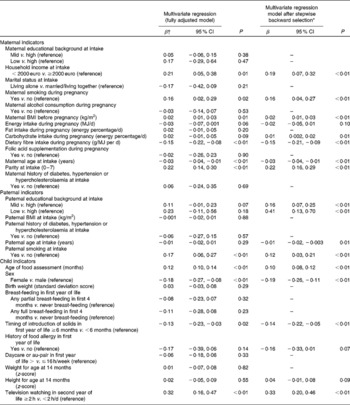
* After using the backward selection procedure with P = 0·10 as endpoint.
† Difference in factor score of a ‘Western-like’ diet compared to reference group or per unit of a continuous variable.
In the multivariate model that included all parental and child indicators kept after the backward selection procedure, low paternal educational background (P <0·01), low household income (P < 0·01), parental smoking (P < 0·01), high maternal BMI (P <0·01), high intake of carbohydrates (P = 0·01, after adjustment for total energy intake) and multiparity (P <0·01) were significantly associated with a higher adherence to a ‘Western-like’ dietary pattern of the child. High dietary fibre intake during pregnancy (P < 0·01, after adjustment for total energy intake) and high parental age (P ≤ 0·01) were inversely associated with adherence to a ‘Western-like’ dietary pattern of the child.
Adherence to a ‘Western-like’ dietary pattern of the child was significantly associated with a higher age of food assessment (P < 0·01) and more TV watching in the second year of the child's life (P < 0·01). Children who where female (P < 0·01) and children who received solids after the recommended age of 6 months (P < 0·01) had a lower adherence score on a ‘Western-like’ dietary pattern (Table 3).
Determinants of adherence to a ‘Health conscious’ dietary pattern of the child
Predictors of adherence to a ‘Health conscious’ dietary pattern of the child are presented in Table 4.
Table 4 Predictors of adherence to a ‘Health conscious’ dietary pattern of children aged 14 months (Regression coefficients (β) and 95 % confidence intervals, n 2420)
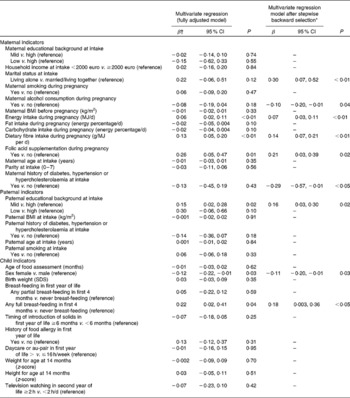
SDS, standard deviation score.
* After using the backward selection procedure with P = 0·10 as endpoint.
† Difference in factor score of a ‘Health conscious’ diet compared to reference group or per unit of a continuous variable.
In multiple regression analyses, children of mothers who consumed alcohol during pregnancy (P = 0·04) and whose mothers had a history of co-morbidity (P < 0·05), and children who were female (P = 0·03) had a lower adherence score on a ‘Health conscious’ dietary pattern. Folic acid supplementation during pregnancy (P = 0·02), high dietary fibre intake of the mother (P < 0·01, after adjustment for total energy intake) and single parenthood (P < 0·01) were positively associated with adherence to a ‘Health conscious’ dietary pattern of the child (Table 4). Children who received any full breast-feeding in the first 4 months of life had a higher score on a ‘Health conscious’ dietary pattern (P < 0·05, Table 4).
Discussion
The results from this prospective cohort study among children aged 14 months have several points with potential health implications that need to be emphasised.
First, a ‘Western-like’ dietary pattern was already identifiable in the second year of the child's life. We found a ‘Western-like’ dietary pattern that was characterised by high consumption of refined grains, savoury snacks, confectionery, animal fats and sugar-containing beverages. An increase in the consumption of sugar-containing beverages is considered to be related to poor diet quality(Reference Libuda, Alexy and Buyken38). Also, consumption of sugar-containing beverages has been shown to be correlated to other unhealthy food choices such as savoury snacks and sweets(Reference Collison, Zaidi and Subhani39). From the Bogalusa Heart Study, it is known that mean intake of sugar-containing beverages, snacks and sweets increases from childhood until adulthood, with an overall decrease in diet quality over the years(Reference Demory-Luce, Morales and Nicklas40). Indeed, we found that the older the child was at the moment of food assessment, the more likely it was to adhere to a ‘Western-like’ dietary pattern. If we assume that adherence to a ‘Western-like’ diet may track from pre-school age to child- and adulthood onwards, then interventions to promote a healthy diet should start early in the child's life(Reference Kleber, Schwarz and Reinehr41).
Second, we demonstrated that paternal education, household income, maternal and paternal smoking, maternal BMI, parental age, parity, early solids introduction and TV watching are important predictors of adherence to a ‘Western-like’ dietary pattern of the child. Maternal BMI has been previously reported to be associated with sweets and sugar intake in 1-year-olds(Reference Brekke, van Odijk and Ludvigsson32). Similarly, maternal smoking during pregnancy and TV viewing more than 2 h a day showed a significant positive association with unhealthy eating behaviour in other studies among children(Reference North and Emmett5, Reference Gubbels, Kremers and Stafleu31, Reference Coon, Goldberg and Rogers42). In infancy, early solid-food introduction has been found to be related to poor dietary quality in the first year of life(Reference Robinson, Marriott and Poole43). The Avon Longitudinal Study of Pregnancy and Childhood (ALSPAC) study showed that the number of older siblings is positively associated with adherence to a ‘Junk’ and ‘Snack’ dietary pattern(Reference North and Emmett5), which is in line with our results since we found a positive correlation between parity and adherence to a ‘Western-like’ dietary pattern of the child. Also, the ALSPAC study found that boys were less likely to adhere to a ‘Healthy’ and ‘Traditional’ dietary pattern(Reference North and Emmett5). In our study, we found that girls had lower scores on the ‘Western-like’ diet along with a ‘Health conscious’ dietary pattern, suggesting differences in food preferences between boys and girls. Low paternal education and low household income were associated with the ‘Western-like’ dietary pattern, which is in accordance with other studies indicating social inequalities in unhealthy eating patterns(Reference Patrick and Nicklas3).
We found that high carbohydrate and low dietary fibre intake of the mother during pregnancy was significantly associated with adherence to a ‘Western-like’ dietary pattern of the child. An earlier study already showed a strong correlation between maternal macronutrient intake during pregnancy and offspring macronutrient intake(Reference Brion, Ness and Rogers22). Another study demonstrated that diet quality of 3-year-old children is highly associated with maternal diet quality(Reference Fisk, Crozier and Inskip44). The latter association appeared to be independent of other maternal characteristics as BMI and educational level(Reference Fisk, Crozier and Inskip44), which is in line with our study results since the inverse association between maternal dietary fibre intake and a ‘Western-like’ dietary pattern of the child was still present after adjusting for maternal BMI and socio-economic background.
Although we did not find a significant association with weight and height z-score at 14 months and the dietary patterns, some of the determinants associated with a ‘Western-like’ dietary pattern have been found to be risk factors of overweight, such as parental smoking, socio-economic background, maternal BMI, maternal diet, early solid-food introduction and TV watching(Reference Robinson and Godfrey1, Reference Beyerlein, Toschke and von Kries45). Taking all these into consideration, our study results imply that children with a ‘Western-like’ dietary pattern might reflect a vulnerable group of children who may be at risk for developing overweight-prone behaviours in later life. Hence, targeting the parents with a low socio-economic background and unfavourable lifestyle factors may be valuable at a very young age of the child to improve the child's eating habits.
Last, we identified a ‘Health conscious’ dietary pattern within our study population, which was characterised by high consumption of pasta and rice, potatoes, legumes, fruit, vegetables, fish and vegetable oils. In schoolchildren, high adherence to a dietary pattern characterised by fish, legumes, fruits and leafy vegetables has been found to be associated with a better diet quality(Reference Lazarou, Panagiotakos and Matalas46). However, not much is known about the determinants and effects of a ‘Health conscious’ dietary pattern in children. Maternal co-morbidity was inversely associated with a ‘Health-conscious dietary pattern’. Similarly, we found that other behaviour associated with health awareness of the mother, such as folic acid supplementation, high fibre consumption, no alcohol consumption during pregnancy and full breast-feeding were predictors of adherence to a ‘Health conscious’ dietary pattern of the child. Strikingly, we found that children of fathers with a mid-educational level had higher scores on the ‘Health conscious’ dietary pattern relative to children of fathers with high educational level, suggesting that a socio-economic gradient in healthy eating habits may be less straightforward than in unhealthy eating patterns early in life.
In contrast, we also found that children of mothers living alone were more likely to adhere to a ‘Health conscious’ dietary pattern. We believe that this might have something to do with other socio-demographic factors such as whether both parents are involved in upbringing or which person is responsible for preparation of the meals. In the ALSPAC study, it was demonstrated that cooking performed by a person other than the mother was negatively correlated with a ‘Healthy’ dietary pattern in 3-year-olds(Reference North and Emmett5). In addition, when the mother lives together with a partner, unhealthy eating habits of the partner might be more incorporated with the child's diet when the partner is involved in raising the child or the preparation of meals. Unfortunately, we did not have data on paternal dietary habits to further clarify these results.
Several study limitations need to be taken into account to appreciate the results. Although we used the varimax rotation to decrease correlation between the two identified patterns, they still might be intercorrelated to some extent(Reference Hu10, Reference Tucker19). In addition, meat consumption was a correlated factor in both the ‘Western-like’ and ‘Health conscious’ dietary patterns in our study group. The identification of dietary patterns in very young children is challenging because dietary patterns may not be strongly formed yet. Also, it cannot be assumed that dietary patterns are perfectly stable throughout early- and mid-childhood as previously demonstrated in ALSPAC(Reference Northstone and Emmett47) and thus, further longitudinal measurements are needed to assess whether unfavourable dietary patterns track during child- and adulthood.
Dietary pattern analysis involves several decisions such as in the division of food items to food groups, the selected method to define these patterns, and the labelling of these components(Reference Hu10), which may have an influence on the final content of the dietary pattern in our study.
The amount of variance (24·5 %) explained by the dietary patterns is small but is similar when compared to previous studies on dietary patterns in young children(Reference North and Emmett5, Reference Robinson, Marriott and Poole43). Nevertheless, this may have consequences on the generalisability of our results in other populations. Therefore, we encourage further study on dietary patterns at this very young age in other populations.
Another limitation of the study is that the FFQ that was used for the collection of maternal nutrition-related data during pregnancy was only validated in an older Dutch population in Rotterdam, the Netherlands(Reference Klipstein-Grobusch, den Breeijen and Goldbohm21). Although we did validate the FFQ of the children against 3 d 24 h recalls, it has been shown that 24 h recalls still underestimate nutrient intake(Reference Kipnis, Subar and Midthune48). Unfortunately, we were not able to validate the FFQ against the doubly labelled water method which is considered to be the ‘gold standard’ to validate total energy intake(Reference Kipnis, Subar and Midthune48).
Missing data can be an important limitation in cohort studies. We aimed to reduce attrition bias as much as possible. For this reason, we used a multiple imputation procedure, which is a very appropriate method to deal with missing data because it requires the least assumptions and exhibits bias when the missing data are not completely at random(Reference Sterne, White and Carlin36). As a result, the 95 % CI in our study reflect the uncertainty associated with the missing values.
We did not have data on the child's preferences, social context of meal consumption and parents' beliefs about nutrition and health. We expect that these factors can be important predictors of a child's adherence to a healthy or unhealthy eating pattern(Reference Patrick and Nicklas3). Therefore, an in-depth study aiming to explore the influence of other social determinants can be worthwhile.
In conclusion, the present study demonstrated that a ‘Western-like’ dietary pattern can already be present in the second year of the child's life and adherence to this diet is associated with other risk factors for obesity. Targeting parents of these children for health promotion might be useful. Future studies should clarify whether this dietary pattern tracks during child- and adulthood, and which other social determinants predict adherence to a ‘Health conscious’ dietary pattern in childhood. Finally, the analyses of this study can form a basis for future studies regarding dietary patterns and various health outcomes in this cohort.
Acknowledgements
The Generation R Study was conducted by the Erasmus Medical Center in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service–Rotterdam Metropolitan Area, the Rotterdam Homecare Foundation, and the Stichting Trombosedienst & Artsenlaboratorium Rijnmond. The authors acknowledge the contributions of children and parents, general practitioners, hospitals and midwives in Rotterdam. The authors also thank Saskia Meyboom, Corine Perenboom and Els Siebelink of the Department of Human Nutrition, Wageningen University, the Netherlands for their contribution in processing the food consumption data. In the funding phase, the Generation R Study was supported by the Erasmus Medical Center, the Erasmus University Rotterdam, the Netherlands Organization for Health Research and Development (Zon Mw) and Europe Container terminals B.V. The funders had no role in the study design, data collection, data analysis, data interpretation, writing of the report or the decision to submit the manuscript. The authors declare that they have no conflicts of interest. The authors' contributions to this study were as follows: J. C. K.-d. J., H. A. M., J. H. d. V., S. E. B., V. W. V. J., A. H. and H. R. designed the research (project conception, development of overall research plan and study overview); J. C. K.-d. J. and H. A. M. conducted the research (data collection); J. H. d. V. provided essential materials (constructs and databases); J. C. K.-d. J. and H. A. M. analysed the nutritional data and performed statistical analysis; J. C. K.-d. J. wrote the paper; and H. A. M. had primary responsibility for the final content.







