Riboflavin (vitamin B2) is a water-soluble vitamin belonging to the B complex group, which participates in a wide range of biological redox reactions through its biologically active forms FAD and FMN. These flavins act as oxidising agents through their ability to accept a pair of hydrogen atoms and participate in energy production as cofactors of various enzymes including some involved in the Kreb’s cycle( Reference Abbas and Sibirny 1 ).
Riboflavin is essential in the diet, because unlike plants and some micro-organisms the human body is unable to synthesise this vitamin( Reference Powers 2 ). Even though riboflavin is present in a wide range of foods, there is a large percentage of the world population that does not meet the daily nutritional requirement of this vitamin, estimated at 1·3 mg for an average adult according to the Food and Nutrition Board, 2000. There are many causes for riboflavin deficiencies including inadequate ingestion of food (caused by economic or psychological problems), different eating disorders (especially malnutrition) and malabsorption of the vitamin as a consequence of certain diseases or treatment protocols( Reference O’Brien, Kiely and Harrington 3 ). Furthermore, it has been shown that riboflavin can be produced by certain micro-organisms present in the human microbiota; however, the concentrations produced are insufficient to prevent vitamin deficiencies( Reference LeBlanc, Milani and de Giori 4 , Reference Magnúsdóttir, Ravcheev and de Crécy-Lagard 5 ).
In order to understand the importance of adequate ingestion of riboflavin, numerous animals studies have been performed to study the consequences of its absence in diets( Reference Powers, Weaver and Austin 6 – Reference Powers, Hill and Mushtaq 9 ). Extreme riboflavin deficiency, called ariboflavinosis, can cause many disorders such as reduced growth rates, oral lesions including cracked and red lips, glossitis, angular cheilitis, atrophy of taste buds, ulcers and sore throat( Reference Wilson 10 , Reference Basu and Dickerson 11 ), and hyperhomocysteinaemia( Reference Moat, Ashfield-Watt and Powers 12 ), among others. Subclinical riboflavin deficiencies have also been associated with lower antioxidant potential, because FAD is the cofactor of glutathione reductase (GR) that plays an essential role in resisting oxidative stress at the cellular level( Reference Deponte 13 ).
In order to prevent riboflavin deficiencies, many countries have included this vitamin in mandatory fortification programmes of foods of mass consumption. However, because these programmes have not been successful in eliminating deficiencies and that modern populations are reluctant to consume chemical-based ingredients, more natural methods of increasing vitamin concentrations have been the focus of many research groups. In this sense, it has been proposed that beneficial micro-organisms such as lactic acid bacteria (LAB) have the potential to increase B group vitamin concentrations in foods based on the de novo biosynthetic capacity of certain strains( Reference LeBlanc, Laino and Juarez del Valle 14 , Reference LeBlanc, Laiño and Juarez del Valle 15 ). Riboflavin-producing LAB have been obtained from a wide range of ecological niches( Reference Thakur, Tomar and De 16 , Reference Thakur and Tomar 17 ) such as dairy products( Reference Juarez del Valle, Laiño and Savoy de Giori 18 , Reference Jayashree, Jayaraman and Kalaichelvan 19 ), Andean grains (personal data), the human gastrointestinal tract (GIT)( Reference LeBlanc, Milani and de Giori 4 , Reference Magnúsdóttir, Ravcheev and de Crécy-Lagard 5 ), wheat and other cereals( Reference Capozzi, Menga and Digesu 20 – Reference Russo, Capozzi and Arena 22 ). In addition, it has been shown that the exposition to roseoflavin, a riboflavin toxic analogue, can be used to isolate over-producing strains( Reference Burgess, O’Connell-Motherway and Sybesma 23 – Reference Capozzi, Russo and Dueñas 26 ).
Previously, our group was able to bio-enrich soyamilk with riboflavin using a riboflavin over-producing LAB strain( Reference Juarez del Valle, Laiño and Savoy de Giori 18 ). Thus, the aim of the present study was to evaluate the efficiency of soyamilk bio-enriched with riboflavin as a result of fermentation with Lactobacillus plantarum CRL 2130 to revert and/or prevent the nutritional deficiency of riboflavin using different animal models.
Methods
Strains and culture conditions
L. plantarum CRL 2130 (previously identified as CRL 725 G) and L. plantarum CRL 691, a riboflavin-producing strain and a riboflavin consumer, respectively( Reference Juarez del Valle, Laiño and Savoy de Giori 18 ), were obtained from the culture collection (CRL) of the Centro de Referencia para Lactobacilos (CERELA-CONICET, Tucuman, Argentina). Before experimental use, cultures were propagated (2 %, v/v) twice in MRS broth (Difco) and incubated at 30°C for 16 h.
Fermented soyamilk preparation
Fermented soyamilk was prepared according to previously described techniques( Reference Juarez del Valle, Laiño and Savoy de Giori 18 ). In brief, whole commercial soyabeans (100 g) were washed and hydrated with distilled water for 16 h and manually peeled. A slurry was obtained by grinding soyabeans with 1 volume of distilled water (300 ml) using a kitchen blender (TS-696; Home Electric). The slurry was cooked after adding 3 volumes of water at 80°C for 15 min, and then 2 volumes of water were added before filtering using a double-layered cheese cloth. The water-soluble extract (soyamilk) was then autoclaved for 15 min at 121°C.
After activation in MRS broth, L. plantarum strains were harvested by centrifugation at 5000 g for 5 min and washed twice with 1 volume of sterile saline solution to eliminate extracellular riboflavin. This cell suspension was used to inoculate soyamilk at an initial optical density at 600 nm (OD600) of 0·2 (calculated), which was then incubated statically at 30°C 12 h.
In these conditions, L. plantarum CRL 2130, a roseoflavin-resistant strain, was able to produce 1551 (sd 20) ng of riboflavin/ml of soyamilk, whereas L. plantarum CRL 691 consumed this vitamin. Considering that the initial concentration of riboflavin in unfermented soyamilk was 309 (sd 9) ng/ml, the product fermented with L. plantarum CRL 2130 contained 1860 (sd 20) ng/ml, whereas in soyamilk fermented with L. plantarum CRL 691 the concentration decreased slightly (but not significantly) to 302 (sd 10) ng/ml.
Depletion–repletion animal model
In total, thirty-five 21-d-old female BALB/c mice were obtained from the closed colony maintained at CERELA. They were maintained under controlled environmental conditions (temperature 20±2°C, humidity 55±2 %) with cycles of 12 h light–12 h dark in the same facilities. The mice had free access to water and solid food unless otherwise indicated.
During the depletion period (21 d), the animals were divided into two groups (see Fig. 1): (i) the depleted group (D), fed a riboflavin-deficient diet (RDD, Dyets # 316755; Dyets Inc.) and (ii) the non-depleted group (C), fed a control diet (CD) consisting of RDD with 7 mg of B2/kg, which is the amount of riboflavin present in normal diets (Dyets # 517933; Dyets Inc.).
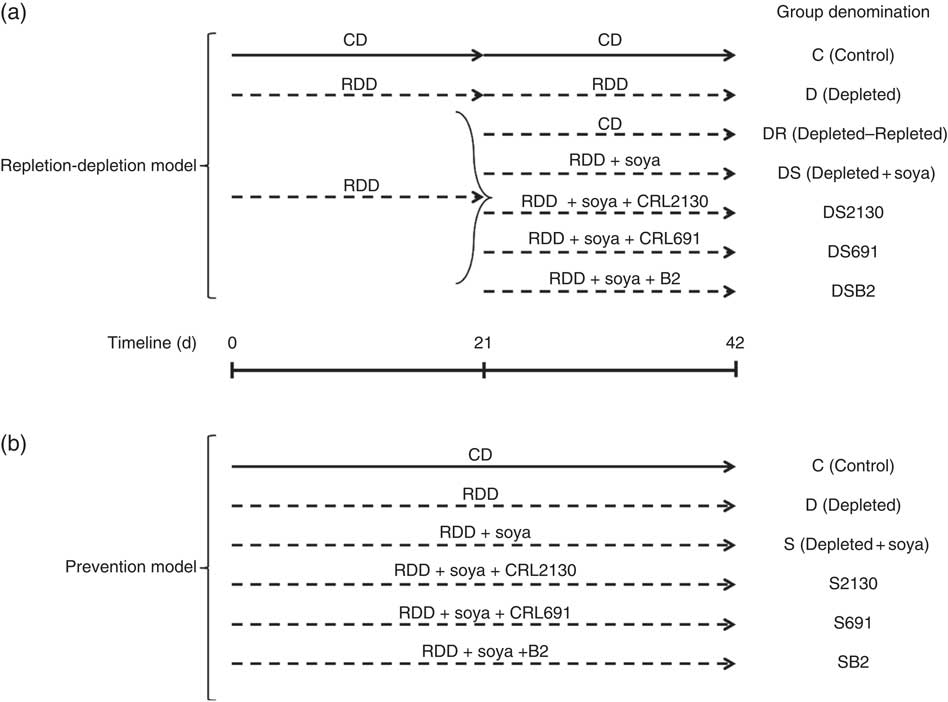
Fig. 1 In the riboflavin depletion–repletion model (a), mice received either the control diet (CD, ![]() ) or the riboflavin-deficient diet (RDD,
) or the riboflavin-deficient diet (RDD, ![]() ) during the depletion period (first 21 d) or the repletion period (from day 21 to 42). During the repletion period, some animals receiving the RDD (n 5 per group) were supplemented with unfermented soya (DS), soyamilk fermented with the riboflavin-producing strain L. plantarum CRL 2130 (DS2130), soyamilk fermented with the riboflavin-consuming strain L. plantarum CRL 691 (DS691) or soyamilk with added riboflavin (DSB2) or they only received the RDD (D) or the control diet (DR). In the preventative model (b), animals received these same control (C) or RDD diets (D) during 42 d of the experimental protocol and some animals (n 5 per group) received RDD supplemented with soya (S), soya+CRL 2130 (S2130), soya+CRL 691 (S691) or soya+commercial B2 (SB2).
) during the depletion period (first 21 d) or the repletion period (from day 21 to 42). During the repletion period, some animals receiving the RDD (n 5 per group) were supplemented with unfermented soya (DS), soyamilk fermented with the riboflavin-producing strain L. plantarum CRL 2130 (DS2130), soyamilk fermented with the riboflavin-consuming strain L. plantarum CRL 691 (DS691) or soyamilk with added riboflavin (DSB2) or they only received the RDD (D) or the control diet (DR). In the preventative model (b), animals received these same control (C) or RDD diets (D) during 42 d of the experimental protocol and some animals (n 5 per group) received RDD supplemented with soya (S), soya+CRL 2130 (S2130), soya+CRL 691 (S691) or soya+commercial B2 (SB2).
The depletion period was followed by a repletion period (21 d), where the depleted group was sub-divided into six groups: (i) the D group, where animals continued to receive the same RDD; (ii) the depleted–repleted (DR) group, where animals were fed the CD; (iii) the DR group, where animals were fed the RDD supplemented with soyamilk fermented with the riboflavin-producing strain L. plantarum CRL 2130 (DS2130); (iv): the DR group, where animals were fed the RDD supplemented with soyamilk fermented with the riboflavin-consuming strain L. plantarum CRL 691 (DS691); (v) the DR group, where animals were fed the RDD supplemented with unfermented soyamilk acidified to pH 4·5 with lactic acid, which is the same pH obtained after fermenting soyamilk (DS); and (iv) the DR group, where animals were fed the RDD supplemented with the same unfermented soyamilk containing commercial riboflavin (1·7 µg/ml; Fluka, Biochemika, which is the equivalent produced by L. plantarum CRL 2130) (DSB2). Each subgroup contained a minimum of five animals. Animal live weight and food intake were determined three times a week.
Preventative riboflavin deficiency animal model
For this model, mice were divided into six groups (see Fig. 1), fed for 42 consecutive d with the following diets: (i) the RDD supplemented with riboflavin (control, C); (ii) the RDD (depleted, D); (iii) the RDD supplemented with soyamilk fermented with L. plantarum CRL 2130 (S2130); (iv) the RDD supplemented with soyamilk fermented with L. plantarum CRL 691 (S691); (v) the RDD supplemented with unfermented soyamilk acidified to pH 4·5 with lactic acid (S); and (vi) the RDD supplemented with soyamilk containing commercial riboflavin (SB2). Each subgroup contained a minimum of five animals. Animal live weight and food intake (given ad libitum) were determined three times a week.
All animal protocols were pre-approved by the Animal Protection Committee of CERELA (CRL-BIOT-LT-2014-1A) and followed the latest recommendations of the Federation of European Laboratory Animal Science Associations and the Asociación Argentina para la Ciencia y Tecnología de Animales de Laboratorio. All the experiments complied with the current laws of Argentina.
Sample collection and procedure
At the end of the trial, animals were anaesthetised with an i.p. injection of ketamine (Holliday, Scott S.A.) and xylazine (Rompun, Bayer S.A.) to obtain a final concentration of 100 and 5 µg/kg live body weight, respectively, and bled by cardiac puncture. Blood samples were transferred into a tube containing EDTA as anticoagulant (1·5 mg/ml of blood) – 100 µl was used for haematological studies and the remaining samples were centrifuged (2000 g for 15 min). The sedimented cells were washed three times with cold 0·15 m-NaCl.
Erythrocytes (0·5 ml) were haemolysed by adding distilled water (9·5 ml) and stored at −70°C. Freshly excised organs (spleen and kidneys) were removed, cleaned and weighed.
For the study of microbial translocation, the liver was aseptically removed, weighed and homogenised in 5·0-ml sterile 0·1 % (w/v) peptone solution. The homogenate was plated in triplicate in the following media: De Man-Rogosa-Sharpe (MRS), brain heart infusion (BHI) and MacConkey (Britania Laboratories). Bacterial growth was evaluated after incubation at 37°C for 48–72 h.
The small intestine was gently washed with ice-cold PBS and divided into segments, which were fixed in formaldehyde solution (10 % (v/v)) in PBS for 24 h at room temperature. Subsequently, the tissues were completely dehydrated using a graded series of alcohol and embedded in paraffin at 56°C. From the paraffin block, 4-µm-thick sections of the processed tissue were cut using a microtome. The paraffin sections were mounted on slides followed by staining with haematoxylin–eosin. The histological sections were observed using a trinocular microscope (Carl Zeiss Primo Star model; Zeiss) with a digital camera (Canon Powershot G9 – 12 megapixels). The captured images were processed and analysed using the programme AxioVision Rel. 4.8.
Riboflavin status
Riboflavin status was determined by measuring the erythrocyte glutathione reductase activation coefficient (EGRAC) using a modification of the technique described previously( Reference LeBlanc, Burgess and Sesma 27 – Reference LeBlanc, Rutten and Bruinenberg 29 ). The haemolysed blood samples were thawed at room temperature (21°C) in the dark. Haemolysed blood samples (31·3 µl) were added to 1 ml of phosphate buffer (0·1 m-PBS, pH 7·4) containing 2·3 mm-EDTA and 0·89 mm-GSSG with or without FAD (1 mm). Aliquots of the mixture (200 µl) were placed in each well of a sterile ninety-six-well microplate (Deltalab) and preincubated at 37°C for 30 min. Next, 2 mm-NADPH (8 µl) was added to start the reaction, which was followed by OD measurement at 340 nm every 5 min for 1 h at 37°C using a microplate reader (VERSAmax; Molecular Devices). The riboflavin status was calculated as the ratio of the variation rate of absorbance per unit time in the presence and absence of FAD. EGRAC determination was performed in triplicate for each sample according to the following equation: EGRAC=((∆A34010 min with FAD)/(∆A34010 min without FAD))×100.
Statistical analysis
All values are expressed as means and standard deviations. Statistical analyses were performed using the software package SigmaPlot for Windows, version 12.0 (Systat Software Inc.) using ANOVA generalised linear model (GLM) followed by a Tukey’s post hoc test. Differences were considered statistically significant at P≤0·05.
Results and discussion
Because of the continuous presence of people with riboflavin deficiencies in all parts of the world, many research groups have focused their attention on obtaining more natural methods to increase riboflavin intakes in foods, because current fortification programmes have not been completely effective. In this sense, certain LAB strains have been shown to be able to produce different concentrations of riboflavin, making them organisms of interest to produce novel bio-enriched foods. Although many riboflavin-producing LAB have been identified, there are a very few studies where the vitamin produced by these strains were evaluated in an in vivo model( Reference LeBlanc, Burgess and Sesma 27 – Reference LeBlanc, Rutten and Bruinenberg 29 ). In the current study, soyamilk bio-enriched with riboflavin produced by L. plantarum CRL 2130 was evaluated using two different animal models.
It is well documented that when experimental animals are deprived of riboflavin, there is a decrease in growth( Reference LeBlanc, Burgess and Sesma 27 – Reference LeBlanc, Rutten and Bruinenberg 29 ). Our results confirmed these previous observations, as at the end of the depletion period (21 d) and the experimental period (42 d), the live body weight of the animals that only received a RDD (depleted group) was significantly lower compared with the non-depleted control group (Fig. 2). This was an important first step, as previous studies evaluating the biological effect of riboflavin, produced by genetically engineered LAB, were performed using rats, and in this study mice were used, showing similar results( Reference LeBlanc, Burgess and Sesma 27 , Reference LeBlanc, Burgess and Sesma 28 ). Using this depletion–repletion model, it was shown that mice supplemented with (i) soyamilk fermented with the riboflavin over-producing strain (L. plantarum CRL 2130) possessed similar growth rates as those receiving (ii) the CD or (iii) those supplemented soyamilk containing commercial riboflavin (1·7 µg/ml), the same concentration produced by the LAB, reaching live weights of 26 (sd 1), 28 (sd 1) and 29 (sd 2), respectively (Fig. 2(A)). These results demonstrate that the riboflavin produced by L. plantarum CRL 2130 was just as effective as the commercial vitamin in reverting ariboflavinosis in our mouse model. Animals of the depleted group and those supplemented with unfermented soyamilk or soyamilk fermented with the non-riboflavin-producing strain (L. plantarum CRL 691) recorded significant lower (between 33 and 36 % decrease) live weights at the end of the experiment (42 d) compared with the control group or the group supplemented with the riboflavin-producing LAB-fermented product (Fig. 2(A)).
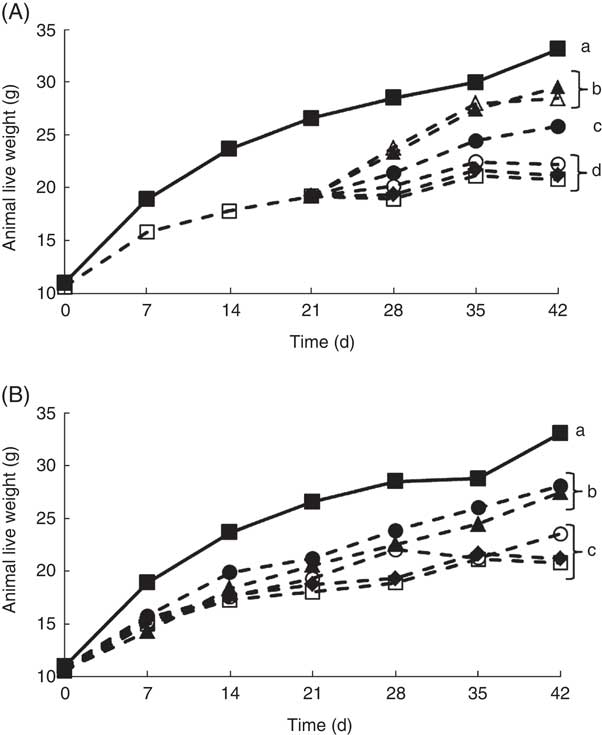
Fig. 2 Live weight of animals of the experimental groups described in the methods section in the depletion–repletion (A) or the preventative model of riboflavin deficiency (B). a,b,c,d Mean values with unlike letters at different levels were significantly different (P≤0·05) within each individual graph.
In the preventive model, where animals were not previously depleted, similar results were obtained. Mice fed the RDD supplemented with soyamilk containing 1·7 µg B2/ml or soyamilk fermented with L. plantarum CRL 2130 showed mean body weights (27 (sd 1) and 28 (sd 1) g, respectively) that were significantly higher than that of animals belonging to the other groups (depleted, unfermented soyamilk and soyamilk fermented with L. plantarum CRL 691) (Fig. 3(B)). Here again, the riboflavin produced by L. plantarum CRL 2130 was just as bioavailable as the commercial vitamin as both were able to prevent stunted growth as a consequence of vitamin deficiency.
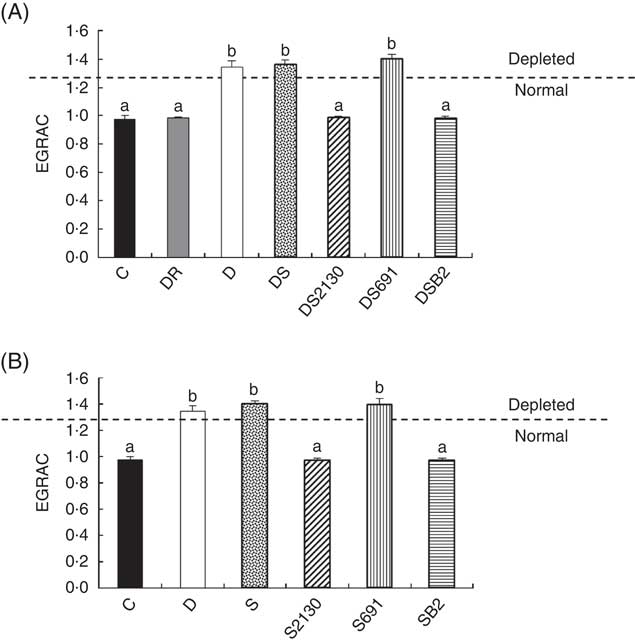
Fig. 3 Erythrocyte glutathione reductase activation coefficient (EGRAC) values of animals of the experimental groups described in the methods section in the depletion–repletion (A) or the preventative model of riboflavin deficiency (B). ![]() , Animals are considered depleted or normal in function of EGRAC values. a,b Mean values with unlike letters at different levels were significantly different (P≤0·05) within each individual graph.
, Animals are considered depleted or normal in function of EGRAC values. a,b Mean values with unlike letters at different levels were significantly different (P≤0·05) within each individual graph.
It must be clarified that the differences in live body weight between the different groups were not related to the food consumed by animals as they all consumed the same amount of food (average of 3·4 (sd 0·2) g/mouse daily, data not shown) nor were there any significant differences in soyamilk consumption between groups.
In addition to growth, the EGRAC is a biological marker to determine the riboflavin status of animals. As GR is a FAD-dependent antioxidant enzyme, and riboflavin is immediately converted into FAD or FMN after its absorption in the intestines, maximal enzymatic activity is only observed when adequate absorption of riboflavin occurs. The EGRAC measures this enzyme before and after the addition of exogenous FAD in haemolysed erythrocytes, and if the EGRAC values (consisting the relation of GR with FAD on the same activity without the addition of FAD) are ≥1·3 this indicates that the blood concentration of FAD (riboflavin) is insufficient and these animals are defined as being deficient in riboflavin.
After the depletion period (21 d), animals showed an elevated EGRAC value (>1·3), indicating that FAD was insufficient for maximal GR activity. When these mice were supplemented with soyamilk fermented with the riboflavin-producing strain L. plantarum CRL 2130 for 21 d, the EGRAC values decreased to normal levels (Fig. 3(A)). The same effect was observed in animals supplemented with soyamilk containing commercial riboflavin or when a CD containing an excess of B2 was used, inferring that the riboflavin produced by L. plantarum CRL 2130 was just as bioavailable as the synthetic form of the vitamin. On the other hand, consumption of unfermented soyamilk or soyamilk fermented with L. plantarum CRL 691 (the non-riboflavin-producing strain) together with the riboflavin-free diet did not improve the B2 status (EGRAC) of the animals compared with those of the depleted group whose EGRAC values were >1·3 (Fig. 3(A)).
In the preventive model, supplementation of the RDD with soyamilk fermented with L. plantarum CRL 2130 or a commercial B2 was able to avoid development of riboflavin deficiency in mice, where EGRAC values were similar to those of the animals that received the CD. Moreover, the EGRAC values of animals supplemented with unfermented soyamilk or soyamilk fermented with the riboflavin-consuming strain (CRL 691) increased significantly (>1·3), indicating that these animals have insufficient riboflavin concentrations in the blood (Fig. 3(B)).
It was previously reported that riboflavin deficiency could lead to anaemia( Reference Lane and Alfrey 30 , Reference Sirivech, Driskell and Frieden 31 ); however, in the present study, no changes were observed in the haematological parameters or in the relative weight of the organs in all experimental groups (data not shown). This could be due to an insufficient depletion period. Nevertheless, these results are important as they show the safety of the fermented products and that they do not alter these important physiological parameters of animals that consume them.
Furthermore, it is known that the maturation of the function of the GIT in rats is regulated in part by the composition of the diet immediately after weaning( Reference Henning and Guerin 32 ). In this sense, it was previously shown that riboflavin deficiency can directly affect the development of the duodenum in rats during this period( Reference Yates, Evans and Powers 8 ). In the present study, a significant increase in the length of intestinal villus was observed in the depleted group of animals or in those that received unfermented soyamilk or soyamilk fermented with L. plantarum CRL 691 compared with those in the control group (Fig. 4(A)). These results were similar to those obtained by Williams et al.( Reference Williams, Powers and Rumsey 33 ), who observed that when a RDD was administered to rats there were alterations in the morphology of the intestinal villus displaying a larger size than the controls. Animals previously depleted with RDD supplemented with soyamilk fermented with the riboflavin-producing strain (CRL 2130) or with soyamilk supplemented with commercial B2 had normal-sized villi (Fig. 4(A)). In the case of the preventive model, supplementation with soyamilk fermented with L. plantarum CRL 2130 or with soyamilk with commercial B2 to the RDD was able to preserve the morphology of the villus similar to that found in the control group (Fig. 4(B)). This prevention of hypertrophy observed in intestinal villus was not possible with the supplementation of unfermented soyamilk or soyamilk fermented with L. plantarum CRL 691.

Fig. 4 Mean small intestinal villus height of animals of the experimental groups described in the methods section in the depletion–repletion (A) or the preventative model of riboflavin deficiency (B). a,b Mean values with unlike letters at different levels were significantly different (P≤0·05) within each individual graph.
Apart from the size of the villus observed in the deficient group compared with the control group, no other changes were observed in the muscle layer or in the mucous layer of the small intestine. Moreover, no microbial translocation was observed in all the animal groups, including those that received soyamilk fermented with L. plantarum CRL 2130 (data not shown). These results indicate that the fermented soyabean product did not cause alterations in the intestinal mucosa, and may be considered as an indicator of the biological safety of the product and the LAB used in its preparation( Reference LeBlanc, Van Sinderen and Hugenholtz 34 ).
Conclusions
The results of this study clearly show that soyamilk fermented with the riboflavin-producing strain L. plantarum CRL 2130 was able to avoid ariboflavinosis as shown by preventing stunted animal growth, normalised EGRAC values and reversion of histological changes in a mouse depletion–repletion model. Furthermore, this fermented soyamilk was also able to prevent development of vitamin B2 deficiency in a different animal feeding protocol. Thus, this bio-fortified product could be considered a suitable source of riboflavin in two different populations – those already with clinical or subclinical riboflavin deficiencies or in a large part of the population that does not consume adequate amounts of this vitamin. A 200-ml portion of this fermented soyamilk would provide 28 % of the recommended daily intake for an average person. This novel fermented food with proven functionality constitutes a viable and economically attractive alternative to fortification with commercial riboflavin, as its effect on the prevention and reversion of vitamin deficiency was similar to that obtained using synthetic riboflavin.
Acknowledgements
This work study supported by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) (grant no. PIP 2015-0697 and PIP 2011-0006) and the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) (grant no. PICT 2012-3045 and PICT 2012-2859). CONICET and ANPCyT had no role in the design, analysis or writing of this article.
M. J. d. V., J. E. L., A. d. M. d. L. and J. G. L. carried out the study and analysed the data. M. J. d. V., J. E. L., G. S. d. G. and J. G. L. formulated the research question and designed the study. A. d. M. d. L., G. S. d. G. and J. G. L. wrote the article.
The authors declare that there are no conflicts of interest.







