Lingzhi (Ganoderma lucidum) is a woody mushroom and popular medicinal herb, which is widely used in China and many other Asian countries(Reference Yuen and Gohel1). It has been attributed with a range of beneficial effects including immunomodulation, anti-cancer activity and enhancing longevity(Reference Boh, Berovic and Zhang2). The active constituents include polysaccharides and oxygenated triterpenoids, which have a broad range of biological activities and pharmacological functions(Reference Shiao3). In vitro studies with certain extracts have shown effects which may benefit the cardiovascular system including inhibition of cholesterol synthesis(Reference Komoda, Shimizu and Sonoda4), lowering of blood pressure by decreasing sympathetic outflow from the central nervous system(Reference Lee and Rhee5) and antioxidant effects(Reference Lee, Kwon and Jeong6–Reference Lai, Chan and Tang8).
Hypoglycaemic effects of an aqueous extract of the fruit bodies of Lingzhi were identified many years ago and attributed to two glycans, ganoderans A and B(Reference Hikino, Konno and Mirin9). Subsequent studies have shown that ganoderan B increased the plasma insulin level in normal and glucose-loaded mice(Reference Hikino, Konno and Mirin9), and G. lucidum polysaccharides lowered serum glucose and increased insulin in normal fasted mice by facilitating Ca ion influx into pancreatic β-cells(Reference Zhang and Lin10). A recent study in lean (+db/+m) and obese/diabetic (+db/+db) mice has shown that a water extract of Lingzhi lowered the serum glucose without a change in insulin, with decreased hepatic expression of phosphoenolpyruvate carboxykinase exerting greater effects in diabetic mice than in lean mice(Reference Seto, Lam and Tam11). The diabetic mice also showed decreases in LDL-cholesterol (LDL-C).
Short-term supplementation studies in healthy subjects have shown an acute increase in plasma antioxidant capacity(Reference Wachtel-Galor, Szeto and Tomlinson12) and after 4 weeks' administration of a capsule formulation (1·44 g Lingzhi/d; equivalent to 13·2 g fresh mushroom/d), there was a trend towards lower plasma lipids and increased urine antioxidant capacity(Reference Wachtel-Galor, Tomlinson and Benzie13). The antioxidant effects may also have benefits in diabetic patients. Elevated urine cortisol and catecholamines, markers of stress and sympathetic hyperactivity, are commonly found in hypertensive patients(Reference Stewart14–Reference van Uum, Lenders and Hermus17). With the growing attention on herbal medicine usage, there are insufficient well-designed clinical studies to support the indications that may be suggested by the in vitro and animal data.
The present study was undertaken to examine whether Lingzhi had beneficial effects on a wide range of cardiovascular and metabolic parameters in patients with borderline elevations of blood pressure and/or cholesterol comprehensively, including lipids, glucose and insulin, in addition to markers of antioxidant and oxidative stress status and lymphocyte subsets.
Subjects and methods
Subjects
A total of twenty-six patients with hypertension and/or dyslipidaemia were recruited from the outpatient clinic at the Prince of Wales Hospital, the teaching hospital of The Chinese University of Hong Kong, Shatin, Hong Kong. Pre-hypertensive or stage 1 hypertension (classified by JNC7) subjects with a systolic blood pressure of 130–150 mmHg or a diastolic blood pressure of 85–100 mmHg, or with anti-hypertensive treatment adjusted to the target blood pressure range, were recruited for the present study. Patients with mild to moderate elevation of plasma total cholesterol and/or TAG (total cholesterol ≥ 6·0 mmol/l, or TAG ≥ 1·7 mmol/l and TAG ≥ 5·0 mmol/l) with or without lipid-lowering treatment were also enrolled. Patients with type 2 diabetes were included if reasonably well controlled in terms of fasting glucose and HbA1c ( < 8·5 %). Those with stable CHD could also be included. Patients with recent cardiovascular events (within 6 months) or elevated plasma liver enzymes (>2 × upper limit of normal) were excluded. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Joint CUHK-NTEC Clinical Research Ethics Committee. Written informed consent was obtained at the time of enrolment from all subjects.
Study design and medication
This was a randomised, double-blind, cross-over study with periods of treatment of 12 weeks each on Lingzhi treatment or on matching placebo, two capsules twice daily. There were periods of placebo run-in and cross-over, each of 4 weeks' duration (Fig. 1). Subjects attended the study visits at the end of the placebo run-in and placebo cross-over periods and at the end of each period of 12 weeks' treatment with placebo or Lingzhi. Capsules and matching placebo capsules were prepared and supplied by Vita-Green Health Products Company Limited (Hong Kong, China).
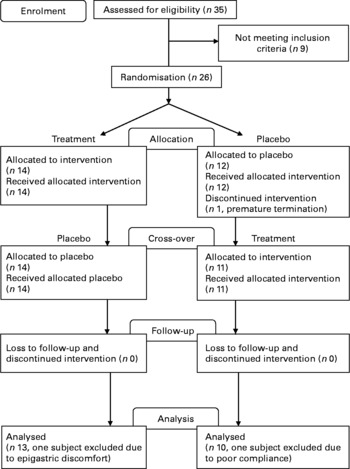
Fig. 1 Consort diagram of patient recruitment in the Lingzhi study.
The Lingzhi extract was prepared as described previously(Reference Lai, Chan and Tang8). The water-soluble polysaccharides from Lingzhi were isolated and extracted with hot water (95–100°C). The concentrated aqueous extract was further extracted with ethanol. The polysaccharide fraction (resulting precipitate) was collected by centrifugation, followed by cleaning and drying. Crude extract of Lingzhi was obtained by a second ethanol extraction and vacuum concentration (60°C, 76 mmHg). The Lingzhi extract was standardised for batch-to-batch consistency with reference to authentic Lingzhi. Moreover, HPLC analysis on two active ingredients, adenosine and ganoderic acid A, was performed. A single stock of extract was prepared for the capsules used in the present study. The dosage was 1·44 g Lingzhi extract/d taken with two capsules (each capsule contains 360 mg extract) twice daily, which is equivalent to 13·2 g of the fresh mushroom. This is within the recommended dose of Lingzhi in the Chinese Materia Medica, with 11·25–18·75 g of fresh material as the usual prescription. The safety of each batch of Lingzhi extract was assured by testing for heavy metals, pesticide residues and microbial levels.
Anthropometry, resting blood pressure measurement, blood and urine sampling
Body weight was recorded, and blood pressure measured by a semi-automatic sphygmomanometer (Critikon Dinamap; GE Medical Systems Information Technologies, Louisville, KY, USA). Fasting venous blood samples were taken at each baseline and at the end of each 12-week treatment. With the collection period starting on the morning before the visit, 24 h urine samples were obtained and the aliquots were stored at − 70°C until analysis.
Plasma haematology and biochemistry measurement
Standard haematology and biochemistry measurements including electrolytes and lipid profile were performed in the Department of Chemical Pathology, Prince of Wales Hospital, Shatin, Hong Kong. Plasma glucose was measured using a standard glucose oxidase method (Diagnostic Chemicals Limited, Prince Edward, Canada) and apo-B by turbidimetric assay, both with a Cobas Mira Analyzer (F Hoffman La Roche Company Limited, Basle, Switzerland). Insulin was measured by Active Insulin ELISA (Diagnostic Systems Laboratories, Inc., Webster, TX, USA).
Plasma antioxidant and oxidative stress status measurements
Assessment of antioxidant status included measurement of plasma total antioxidant power using the ferric-reducing/antioxidant power assay (US patented)(Reference Benzie and Strain18), ascorbic acid using the ferric-reducing/antioxidant power-ascorbic acid assay(Reference Benzie, Chung and Tomlinson19), α-tocopherol by HPLC (Waters Limited, Herts, UK) and calculation of lipid-standardised α-tocopherol(Reference Thurnham, Davies and Crump20). Allantoin was measured by HPLC(Reference Benzie, Chung and Tomlinson19) and uric acid was measured by the enzyme-linked spectrophotometric method (Biosystems, Barcelona, Spain). The antioxidant enzymes, superoxide dismutase and glutathione peroxidase, were measured in erythrocytes using the commercial kit methods (Randox Company, Antrim, UK) and the enzyme activities standardised to Hb content.
Lymphocyte subsets
The MultiTEST IMK Kit with TruCOUNT tubes (Becton Dickinson, San Jose, CA, USA) and the lyse/no-wash method were used for the assessment of absolute counts and the ratio of CD4+ (T-helper lymphocytes), CD8+ (T-suppressor lymphocytes and cytotoxic T-lymphocytes), natural killer cells and B-lymphocytes in whole blood samples using a four-colour FASCalibur flow cytometer (Becton Dickinson).
Urine electrolytes, catecholamines, cortisol and cortisone measurements
Urine catecholamines including adrenaline, noradrenaline and dopamine were measured by an HPLC method. Following collection over a 24 h period, urine cortisol and cortisone were measured by HPLC with tandem MS (liquid chromatography–MS/MS) using an online sample clean-up method, modified from the method of Taylor et al. (Reference Taylor, Machacek and Singh21). A triple-quadrupole API 2000 (Applied Biosystems, Foster City, CA, USA) was connected to a Hewlett Packard Agilent 1100 Series LC system (Waldbronn, Germany) for the analysis. The ratio of cortisol to cortisone in urine reflects the activity of the 11-β hydroxysteroid dehydrogenase type 2 (11-β HSD2) enzyme in the kidney(Reference Stewart14).
Adverse effect assessment
At each visit, patients were asked to report any side effects or change in their activity or well-being, and this information was recorded. Compliance was assessed by counting the number of capsules returned after every treatment period. There was one subject with poor compliance who missed 2 weeks' treatment. The data for this subject were not included in the per-protocol analysis.
Statistical analysis
The data of twenty-three subjects were suitable for the analysis, which was performed using SPSS (version 17; SPSS, Inc., Chicago, IL, USA). We first examined the normality of the data by a Q–Q plot. Insulin, homeostasis model assessment-insulin resistance, TAG and HDL-cholesterol (HDL-C) were log-transformed due to skewness of the data. For a cross-over study, it is important to test for any interaction between treatment and time. Treatment × time interactions between different parameters and Lingzhi treatment effects were tested by a mixed linear model to evaluate the treatment effects and treatment × time interaction in one single analysis. The responses to placebo and Lingzhi from the two treatment periods without significant interaction were combined for the paired t test analysis. Because of significant treatment × time interactions for HDL-C and TAG, only the results in the first treatment period were analysed by an independent t test, with thirteen subjects in the active treatment arm and ten subjects in the placebo arm. The across-treatment changes with placebo and with active treatment for the other variables were evaluated by a paired-sample t test for two purposes: first, testing any significant changes within the placebo or Lingzhi group by comparing the pre- and post-treatment data of each subject; second, testing the difference between the placebo and treatment groups (subtracting the baseline values by the end of treatment values) of various parameters. Data are expressed as mean values and standard deviations, unless otherwise indicated, with statistical significance set at P < 0·05.
Results
Baseline characteristics and medical history
From twenty-six eligible subjects enrolled in the study, one withdrew due to personal reasons, one subject had poor compliance ( < 80 % compliance from capsule counting) with therapy and one subject had intercurrent illness; so their data were not included in the per-protocol analysis (Fig. 1). The demographic details, concomitant illnesses and medication use of the remaining twenty-three subjects according to the randomisation to initial treatment with Lingzhi or placebo are shown in Table 1. There were seventeen subjects receiving treatments for hypertension and five receiving treatments for hyperlipidaemia, which were not changed during the study. Overall, seven patients had impaired fasting glucose or impaired glucose tolerance and were treated with diet alone.
Table 1 Baseline anthropometric and clinical characteristics and plasma lipids of the patients in the per-protocol analysis according to initial treatment
(Mean values, standard deviations, number of patients and percentages)

SBP, systolic blood pressure; DBP, diastolic blood pressure; WHR, waist:hip ratio; TC, total cholesterol; LDL-C, LDL-cholesterol; HDL-C, HDL-cholesterol; FPG, fasting plasma glucose; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; ACEI, angiotensin-converting enzyme inhibitor; CCB, Ca channel blocker.
† No significant difference was found between baseline values of groups receiving placebo and Lingzhi.
‡ No subject in the study was taking any hypoglycaemic agent.
§ Patients having taken more than one anti-hypertensive medication were allowed to enter the study. Anti-hypertensive medications include: ACEI – lisinopril; α-adrenergic blockers – prasosin; β-adrenergic blockers – atenolol and metoprolol; CCB – nifedipine, lercanidipine, diltiazem and amlodipine; diuretics – indapamide, hydrochlorothiazide and triamterene.
∥ Lipid-lowering medications include: fibrate – gemfibrozil and bezafibrate; statin – atovastatin and simvastatin.
Interaction analysis
Responses to the placebo and Lingzhi treatments are shown in Table 2. TAG and HDL-C showed a statistically significant carry-over effect (Fig. 2) whereas the other parameters did not. Data for the two periods were pooled for further analysis except for these two parameters and related ratios (LDL-C:HDL-C and total cholesterol:HDL-C).
Table 2 Changes in lipids in the first 12 weeks' treatment with placebo or Lingzhi for parameters which showed a treatment×time interaction
(Mean values and 95 % confidence intervals)

HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; TC, total cholesterol.
* Absolute change = value of end of treatment − value of baseline.
† No significant difference was found between the responses to the placebo and Lingzhi groups, by unpaired t test comparison.
‡ TAG and HDL-C were analysed with natural log transformation due to skewness of the data.

Fig. 2 Changes in (a) HDL-cholesterol (HDL-C) and (b) TAG during the study treatments. Values are means, with their standard errors represented by vertical bars for subjects randomised to the first group (Lingzhi as the first treatment followed by placebo (–□–, n 13)) and the second group (taking placebo in the first period, then switched to the Lingzhi treatment (–△–, n 10)). There was no significant difference between the baseline and after 12 weeks' treatment of the two groups, while the data showed a significant carry-over effect in these two parameters, HDL-C and TAG.
Anthropometric measurement and glycaemic indices
There were no significant changes in BMI, waist:hip ratio, and systolic and diastolic blood pressure during treatment with Lingzhi or placebo (Table 3). Plasma glucose and insulin tended to increase during both treatment periods, but changes tended to be smaller with Lingzhi treatment than with placebo. The homeostasis model assessment-insulin resistance showed a smaller increase after the Lingzhi treatment, but the difference was not statistically significant compared with placebo (Table 3).
Table 3 Changes in blood pressure, anthropometric measurements, glycaemic control parameters and plasma lipid profile for the twenty-three patients with 12 weeks' treatment with placebo or Lingzhi
(Mean values, standard deviations or 95 % confidence intervals)

SBP, systolic blood pressure; DBP, diastolic blood pressure; WHR, waist:hip ratio; FPG, fasting plasma glucose; HOMA-IR, homeostasis model assessment-insulin resistance; TC, total cholesterol; LDL-C, LDL-cholesterol.
Mean values were significantly different from those of respective baseline: *P < 0·05, **P < 0·02, ***P < 0·005.
† Mean values were significantly different from those of the placebo group (P < 0·05).
‡ Absolute change = value of end of treatment − value of baseline.
§ No significant difference was found between the responses to the placebo and Lingzhi groups by paired t test comparison, unless specified.
∥ Insulin, HOMA-IR and HDL-cholesterol were analysed with natural log transformation due to skewness of the data.¶ 1μIU/ml = 6·945 pmol/l.
Lipid profile
Because of the treatment × time interaction, changes in plasma TAG and HDL-C were only analysed during the first treatment (Table 2). TAG decreased by 8 % and HDL-C increased by 24 % during the treatment with Lingzhi but not with placebo. LDL-C increased to a similar degree with both treatments, whereas apo-B increased significantly (P < 0·02) only during the placebo treatment (Table 3). A trend was observed in absolute changes of apo-B values with placebo and Lingzhi treatment.
Antioxidant and oxidative stress status
Changes in antioxidant capacity and oxidative stress showed no significant differences between the treatments (Table 4). There was a significant decrease in superoxide dismutase during both placebo and Lingzhi treatments. There was a tendency for the ratio of allantoin to uric acid to increase more during the placebo period than during treatment with Lingzhi.
Table 4 Changes in antioxidant and oxidative stress status parameters for the twenty-three patients with 12 weeks' treatment with placebo or Lingzhi
(Mean values, standard deviations or 95 % confidence intervals)

FRAP, ferric-reducing/antioxidant power (plasma total antioxidant power); Vit E LS, vitamin E lipid standardised; TC, total cholesterol; GPx, glutathione peroxidase; SOD, superoxide dismutase.
*** Mean values were significantly different from those of respective baseline (P < 0·005).
† Absolute change = value of end of treatment − value of baseline.
‡ No significant difference was found between the responses to the placebo and Lingzhi groups, by paired t test comparison.
Lymphocyte subsets, urine catecholamines, cortisol and cortisone
B-lymphocytes and T-helper cell counts and their percentages decreased during the Lingzhi treatment, but the changes were not different from those during the placebo treatment (Table 5). The T-lymphocyte count decreased during the placebo treatment, but the change was not different from that during the Lingzhi treatment. There were no significant differences between changes in plasma and urine electrolytes, 24 h urine catecholamines, cortisol, cortisone or the cortisol:cortisone ratio during treatment with Lingzhi or placebo (Table 6).
Table 5 Changes in lymphocyte subsets for the twenty-three patients with 12 weeks' treatment with placebo or Lingzhi
(Mean values, standard deviations or 95 % confidence intervals)

B-lym, B-lymphocytes; T-lym, T-lymphocytes; T-supp cells, T-suppressor cells.
Mean values were significantly different from those of respective baseline: *P < 0·05, **P < 0·02, ***P < 0·005.
† Absolute change = value of end of treatment − value of baseline.
‡ No significant difference was found between the responses to the placebo and Lingzhi groups, by paired t test comparison.
Table 6 Changes in plasma electrolytes and urinary electrolytes, cortisol, cortisone and catecholamines for the twenty-three patients with 12 weeks' treatment with placebo or Lingzhi
(Mean values, standard deviations or 95 % confidence intervals)

Pl K, plasma potassium; Pl Na, plasma sodium; U K, urine potassium; U Na, urine sodium.
Mean values were significantly different from those of respective baseline: **P < 0·02, ***P < 0·005.
† Absolute change = value of end of treatment − value of baseline.
‡ No significant difference was found between the responses to the placebo and Lingzhi groups, by paired t test comparison.
§ Activity of 11-β hydroxysteroid dehydrogenase type 2: the ratio of urinary cortisol to cortisone (value of 24 h cortisol output/value of 24 h cortisone output).
Adverse effect evaluation
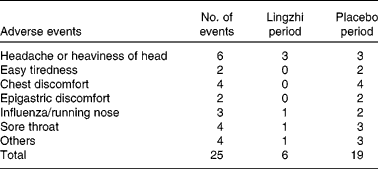
Both active Lingzhi and placebo were well tolerated. No adverse effects in the laboratory safety parameters were detected. A total of twenty-five adverse events were recorded, nineteen during the placebo treatment and six during the Lingzhi treatment (Table 7). None of these was considered clinically significant and no subjects dropped out for reasons which were thought to be related to study medication.
Table 7 Subjective symptoms and adverse events reported by the twenty-three mild dyslipidaemic and hypertensive patients
Discussion
Lingzhi, or G. lucidum, is well known in Chinese traditional medicine and is reputed to promote good health and longevity(Reference Yuen and Gohel1). However, scientific evidence from controlled trials in human subjects is lacking and data from in vitro studies and animal studies cannot always be extrapolated to the clinical setting. Short-term studies in healthy subjects have indicated that some antioxidant material is bioavailable from the same Lingzhi formulation that was used in the present study(Reference Wachtel-Galor, Szeto and Tomlinson12). A double-blind, placebo-controlled, cross-over study in healthy subjects with the same dose (1·44 g Lingzhi/d) of the Lingzhi capsule formulation used in the present study has shown a tendency for reduction in LDL-C after 4 weeks' treatment with Lingzhi compared with a tendency to increase after placebo(Reference Wachtel-Galor, Tomlinson and Benzie13). We considered that repeating the study in patients with higher baseline levels of LDL-C and having a longer period of treatment might produce a more definitive effect. However, we did not find any benefit on the LDL-C as the levels increased during both the placebo and Lingzhi treatment periods.
The effects on HDL-C and TAG were complicated by a treatment × time interaction, and it was felt appropriate to only compare the responses during the first period of treatment so that the numbers in the groups compared in parallel are too small to show significant differences. The treatment × time interaction of TAG and HDL-C might be due to the changes in the diet or lifestyle of the subjects, or other factors related to taking part in a clinical study. The increase in HDL-C and the decrease in TAG seen with the Lingzhi treatment would be compatible with the improvement in dyslipidaemia associated with diabetes when glycaemic control or insulin sensitivity improves(Reference Schwartz22). In the study of Lingzhi extract in obese/diabetic (+db/+db) mice, the decrease in plasma glucose with the higher dose was accompanied by a decrease in LDL-C without an increase in HDL-C(Reference Seto, Lam and Tam11). It has been suggested that db/db mice are a useful model for diabetic dyslipidaemia, but the main lipid abnormality in these mice is an increase in LDL-C in small dense particles rather than elevated TAG and decreased HDL-C as found in human subjects(Reference Kobayashi, Forte and Taniguchi23). As mice do not have cholesteryl ester transfer protein, the inverse relationship between HDL-C and TAG will differ from that in human subjects(Reference de Vries-van der Weij, Zadelaar and Toet24). The expression of human cholesteryl ester transfer protein in db/db mice reduced VLDL-C and LDL-C and prevented the formation of diet-induced aortic atherosclerotic plaques(Reference MacLean, Bower and Vadlamudi25).
We have previously shown that patients with rheumatoid arthritis have insulin resistance and dyslipidaemia and that treatment with the TNF-α inhibitor infliximab improved insulin resistance, reduced C-reactive protein and increased HDL-C(Reference Tam, Tomlinson and Chu26). There was also an increase in LDL-C, but the LDL:apo-B ratio remained unchanged, which may suggest that LDL particle size increased, which would be compatible with an improvement in insulin resistance(Reference Tchernof, Lamarche and Prud'Homme27). The same may be true in the present study where LDL-C appeared to increase more than apo-B with Lingzhi, suggesting that LDL particle size may have increased.
Insulin resistance and inflammation are associated and some anti-inflammatory treatments may improve insulin resistance as in the study with infliximab(Reference Tam, Tomlinson and Chu26). Lingzhi extracts have been shown to suppress inflammation and have free radical-scavenging and anti-cancer effects in various in vitro studies(Reference Lee, Kwon and Jeong6, Reference Lai, Chan and Tang8, Reference Wang, Hsu and Hsu28). In a placebo-controlled study in patients with rheumatoid arthritis, a combination of Lingzhi (4 g) and San Miao San (2·4 g), which contains Rhizoma atractylodis (Cangzhu), Cortex phellodendri (Huangbai) and Radix achyranthes Bidentatae (Niuxi), showed a mild analgesic effect but no obvious anti-inflammatory action(Reference Li, Tam and Wong29). The ex vivo-induced level of inflammatory cytokine IL-18 was lower in the group receiving active treatment, which suggested a beneficial immunomodulatory effect(Reference Xi Bao, Kwok Wong and Kwok Ming Li30). Findings in a rat model of arthritic knees also suggested that the Lingzhi and San Miao San formulation had analgesic and anti-inflammatory effects(Reference Lam, Ko and Ng31). Another study(Reference Wicks, Tong and Wang32) in healthy subjects has shown a tendency for an increase in the count of cells expressing CD56, which identifies natural killer lymphocytes, after 10 d treatment with Lingzhi. We did not find any significant effect on the changes in lymphocyte subsets with Lingzhi compared with placebo in the present study.
Urine cortisol and catecholamines were measured as markers of stress and sympathetic activity. There were no significant differences in changes with Lingzhi compared with those with placebo. The ratio of cortisol to cortisone in urine can be used as a marker of the activity of 11-β HSD2 in the kidney and changes in this have been associated with hypertension and insulin resistance(Reference Stewart14). The 11-β HSD2 enzyme is also inhibited by glycyrrhizic and glycyrrhetinic acids in licorice from Glycyrrhiza glabra, the roots of which are very commonly used in Chinese traditional medicine as gancao(Reference Armanini, Fiore and Mattarello33). Hypertensive patients were found to be more sensitive to the inhibition of 11-β HSD2 enzyme by gancao(Reference Sigurjonsdottir, Manhem and Axelson34). Urine cortisol and cortisone tended to increase with Lingzhi treatment, but this was not significantly different from placebo and there was no change in the cortisol:cortisone ratio.
The present study should be considered exploratory in nature, as the number of patients was small and many different markers were measured, some of which show quite a high biological variation. It was undertaken primarily to look at effects on blood pressure and cholesterol by selecting patients with higher baseline levels of these parameters. The findings of changes in HDL-C, TAG and, potentially, in insulin resistance are of interest and are compatible with some of the animal and in vitro studies. This kind of carefully designed and controlled study is needed to provide evidence to support the clinical use of herbal medicines. Further studies with Lingzhi treatment in patients with more obvious hyperglycaemia with diabetes, or the metabolic syndrome, would be useful to determine whether these abnormalities can be ameliorated.
Acknowledgements
We thank Evelyn Chau and Winnie Yeung for excellent assistance in patient recruitment. B. T. and K. K. C. L. designed the study. T. T. W. C. recruited the subjects, performed the study, data collection and statistical calculations. T. T. W. C., I. F. F. B., B. S. P. F. and C. W. K. L. performed the sample analysis. T. T. W. C. and B. T. wrote the manuscript. T. T. W. C., I. F. F. B., B. S. P. F., C. W. K. L., K. K. C. L. and B. T. contributed to the final discussion of the results. All the authors reviewed the final manuscript. The Lingzhi and placebo capsules were provided free of cost by Vita-Green Health Product Company Limited, Hong Kong. There was no conflict of interest or other funding for this study.











