It was recently proven that cumulative exposure to hyperlipidaemia during early adulthood increases the risk of CVD in a dose-dependent manner( Reference Navar-Boggan, Peterson and D’Agostino 1 ). In fact, prolonged exposure to decreased LDL-cholesterol beginning in early life significantly reduces the risk of CVD( Reference Ference, Yoo and Alesh 2 ).
Recently, some strains of probiotics, such as multi-strain probiotic capsules (Streptococcus thermophilus, Lactobacillus plantarum, Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium lactis, Bifidobacterium longum and Bifidobacterium breve), were reported to significantly reduce serum cholesterol, waist circumference and body weight in adults with a BMI>25kg/m2 during an 8-week treatment( Reference McNulty, Yatsunenko and Hsiao 3 , Reference Lee, Bose and Seo 4 ). In addition, Lactobacillus reuteri NCIMB 30242, a probiotic associated with cardiovascular health, claims to clinically lower LDL-cholesterol levels by 11·6 % in hyperlipidaemic adults, and it has been approved by Health Canada( Reference Joyce, MacSharry and Casey 5 , Reference Jones, Martoni and Parent 6 ).
Supplementary L. reuteri may reduce serum cholesterol because of its high bile salt hydrolase (BSH) activity( Reference Elkins, Moser and Savage 7 , Reference Kumar, Nagpal and Kumar 8 ). Probiotics with high BSH activity promote secondary amino acid conjugates and the deconjugation of bile acids. Finally, probiotics process bile salts and block cholesterol absorption in the gut( Reference Tsai, Lin and Hsieh 9 , Reference Joyce, MacSharry and Casey 10 ). Previously, L. reuteri GMNL-263 (Lr263), a probiotic strain, was reported to have activity that may improve insulin resistance and ameliorate hepatic steatosis in hamsters fed a high-fructose diet( Reference Hsieh, Lee and Chai 11 ).
However, there are still some doubts about whether probiotics arrive alive in the gut after gastric acid exposure. In 2013, Shinkai et al.( Reference Shinkai, Toba and Saito 12 ) performed a randomised, double-blind, placebo-controlled trial that proved that oral intake of the heat-killed Lactobacillus pentosus strain b240 had similar immunoprotective effects as probiotics. In the current study, heart-protective and anti-hyperlipidaemic effects were investigated using a high-fat diet to induce hyperlipidaemia in hamsters. The hamsters were treated with different doses of heat-killed L. reuteri GMNL-263 (Lr263) via oral gavage for 8 weeks.
Methods
Preparation of probiotic suspensions
Heat-killed L. reuteri GMNL-263 was provided by GenMont Biotech Inc. Two concentrations of L. reuteri GMNL-263 (5×108 and 2·5×109 cells/ml) were prepared in PBS for oral gavage treatments.
Animal model
In all, twenty-four male golden Syrian hamsters without spontaneous cardiomyopathy (Mesocricetus auratus, 6 weeks old) were purchased from the National Laboratory Animal Center and divided into four groups (n 6 each). The experimental protocol used in this study was approved by the Institutional Animal Care and Use Committee of China Medical University (No.100-4-B). The normal control group hamsters were fed a normal diet with water. An HF in the results represents the group of hamsters fed a high-fat diet only with normal water. The standard diet is Laboratory Rodent Diet 5001 purchased from LabDiet. The high-fat diet formula had 15 % (w/w) lard added to the standard diet. HKL represents the group of hamsters fed the high-fat diet with normal water and heat-killed L. reuteri GMNL-263 5×108 cells/kg per d via oral gavage. HKH represents the group of hamsters fed the high-fat diet with normal water and heat-killed L. reuteri GMNL-263 2·5×109 cells/kg per d via oral gavage. This protocol lasted 8 weeks.
Serum lipid profile analysis
All plasma samples were collected and analysed by the National Laboratory Animal Center. The following parameters were analysed: TAG, total cholesterol (TC), LDL-cholesterol, HDL-cholesterol and plasma malondialdehyde (P-MDA).
Echocardiography
Echocardiography was performed using a Vivid i ultrasound system (GE Healthcare) with a 10-MHz transducer (GE 10S-RS; GE Healthcare). Left ventricular (LV) M-mode measurements at the papillary muscle level included left ventricular internal end-diastolic dimensions (LVIDd) and left ventricular internal end-systolic dimensions (LVIDs). Fractional shortening (%FS) was calculated according to the following equation: [(LVIDd–LVIDs)/LVIDd]×100. Ejection fraction (EF) was defined as the ratio between the volume of blood pumped out of the LV and the total volume of blood in the LV.
Haematoxylin–eosin staining
Hearts of the hamsters in each group were soaked in formalin, dehydrated using graded concentrations of alcohol and embedded in paraffin wax. Paraffin sections of 2 μm thickness were sliced from the paraffin-embedded tissue blocks. The tissue slices were deparaffinised by immersion in xylene and then rehydrated. All the slices were dyed with haematoxylin–eosin (H&E), and then rinsed with water. Each slide was dehydrated using graded concentrations of alcohol. Finally, they were soaked in xylene twice. Photomicrographs were obtained using Zeiss Axiophot microscopes (Carl Zeiss NTS, LLC).
Tissue protein extraction
Heart tissue extracts from six hamsters in each group were obtained by homogenising heart tissue in a lysis buffer (0·05 m Tris-HCl, pH 7·4, 0·15 m NaCl, 0·25 % deoxycholic acid, 1 % nonyl phenoxypolyethoxylethanol and 1 mm EDTA) at a ratio of 100 mg tissue per 1 ml of buffer. Homogenates were placed on ice and centrifuged at 13 000 rpm for 40 min. Supernatants were collected and stored at –80°C for subsequent experiments.
Western blot assay
Heart tissue protein concentrations were determined using a Lowry protein assay. Protein samples were separated using a 12 % SDS-PAGE with a constant voltage of 75 V for 150 min. Proteins were then transferred to Hybond-C membranes (GE Healthcare UK Ltd) using 50 V for 3 h. Polyvinylidene difluoride membranes were incubated in 5 % bovine serum albumin in tricine buffer saline. Primary antibodies, including β-actin (sc-47778; Santa Cruz Biotechnology), Fas (sc-956; Santa Cruz Biotechnology), Fas-associated protein with death domain (FADD) (sc-6035; Santa Cruz Biotechnology), caspase-8 (sc-6134; Santa Cruz Biotechnology), FAS (sc-1023; Santa Cruz Biotechnology), heat-shock protein 27 (HSP27) (sc-1048; Santa Cruz Biotechnology) and AIF (sc-9416; Santa Cruz Biotechnology) were diluted 1000 times in tris-buffered saline according to manuals and added to the membranes. Finally, horseradish peroxidase-labelled antibodies were used. Pictures were then taken with a Fujifilm LAS-4000 (GE Healthcare UK Ltd).
Statistical analysis
All results were obtained from six hamsters in each experimental group and are presented as the group mean values and standard deviations. A one-way ANOVA was used to indicate an overall statistical significance from the means of the four experimental groups. A P value<0·05 was considered significant. Statistical analyses were performed using the SigmaPlot v.10.0 software.
Results
Serum lipid profile
After 8 weeks, the TAG, TC, LDL-cholesterol, HDL-cholesterol and P-MDA serum levels were significantly increased in the hamsters fed a high-fat diet. These results are shown in Table 1. In the hamsters treated with GMNL-263 5×108 cells/kg per d and fed the high-fat diet for 8 weeks, LDL-C and P-MDA were reduced. Further, after 8 weeks, the TC and P-MDA serum levels were further reduced in the hamsters treated with GMNL-263 2·5×109 cells/kg per d and fed the high-fat diet. There was no difference in the ratio of LDL-cholesterol/HDL-cholesterol between the HF and HKL groups (GMNL-263 5×108 cells/kg per d treatment group). However, the LDL-cholesterol/HDL-cholesterol ratio was reduced in the HKH group (GMNL-263 2·5×109 cells/kg per d treatment group) compared with the HF group.
Table 1 Serum lipid profile (Mean values and standard deviations)
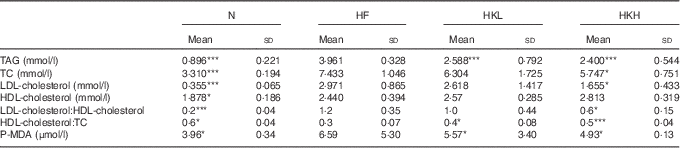
N, normal control; HF, group of hamsters fed a high-fat diet only with normal water; HKL, group of hamsters fed the high-fat diet with normal water and heat-killed Lactobacillus reuteri GMNL-263 5×108 cells/kg per d via oral gavage; HKH, group of hamsters fed the high-fat diet with normal water and heat-killed L. reuteri GMNL-263 2·5×109 cells/kg per d via oral gavage; TC, total cholesterol; P-MDA, plasma malondialdehyde.
* P<0·05; *** P<0·001 compared with HF group.
Echocardiography
After 8 weeks, the EF of hamster hearts was reduced from 89·79 (sd 0·72) % in the control group to 85·71 (sd 2·36) % in the HF group. These results are shown in Table 2. The %FS of hamster hearts was reduced from 53·80 (sd 2·17) % in the control group to 46·93 (sd 5·04) % in the HF group. In the hamsters treated with GMNL-263 5×108 cells/kg per d and fed the high-fat diet, the EF was slightly increased to 87·18 (sd 2·41) %, and the %FS increased to 51·31 (sd 3·15) %. Furthermore, in the group treated with GMNL-263 2·5×109 cells/kg per d and fed the HKH diet, the EF improved up to 91·81 (sd 0·71) % and the %FS also improved up to 57·92 (sd 1·30) %.
Table 2 Echocardioghraphy (Mean values and standard deviations)

N, normal control; HF, group of hamsters fed a high-fat diet only with normal water; HKL, group of hamsters fed the high-fat diet with normal water and heat-killed Lactobacillus reuteri GMNL-263 5×108 cells/kg per d via oral gavage; HKH, group of hamsters fed the high-fat diet with normal water and heat-killed L. reuteri GMNL-263 2·5×109 cells/kg per d via oral gavage; IVSd, interventricular septum diastolic; LVIDd, left ventricular dimension diastolic; IVSs, interventricular septum systolic; LVIDs, left ventricular dimension systolic; LVPWs, left ventricular posterior wall systolic; EDV, end-diastolic velocity; ESV, end-systolic velocity; EF, ejection fraction; %FS, fractional shortening; LV mass, left ventricular mass.
* P<0·05 compared with the normal group, ** P<0·01 compared with the normal group, *** P<0·001 compared with the normal group, **** P<0·05 compared with the HF group, ***** P<0·01 compared with the HF group, ****** P<0·001 compared with the HF group.
Myocardial biopsy
H&E staining was used for the hearts from each group. After 8 weeks, the cardiomyocytes from the HF group were in disarray and had more space between the cells (Fig. 1). In the group treated with GMNL-263 5×108 cells/kg per d and fed the high-fat diet, the myocardial disarray and space between the cells were slightly improved. In the group treated with GMNL-263 2·5×109 cells/kg per d and fed the high-fat diet, the myocardial disarray and space between the cardiomyocytes were significantly reduced.

Fig. 1. Haematoxylin–eosin staining of heart slides. The nuclei of the cells are stained blue, and the other cells are stained pink. The arrows indicate the spaces between the cardiomyocytes. N, normal control; HKL, group of hamsters fed the high-fat diet with normal water and heat-killed Lactobacillus reuteri GMNL-263 5×108 cells/kg per d via oral gavage; HF, group of hamsters fed a high-fat diet only with normal water; HKH, group of hamsters fed the high-fat diet with normal water and heat-killed L. reuteri GMNL-263 2·5×109 cells/kg per d via oral gavage. (Bar length=100 μm).
Myocardial apoptosis signalling analysis
In the protein analysis, the Fas ligand protein level was higher in the HF hamster hearts compared with those of the control group (Fig. 2). The proteins downstream of Fas ligand, especially caspase-8, were more highly expressed and had higher levels of cleaved active form in the HF hamster hearts compared with the controls. In the GMNL-263 5×108 cells/kg per d treatment plus HKL and the GMNL-263 2·5×109 cells/kg per d treatment plus HKH hamster hearts, Fas ligand expression was reduced. The protein levels of active caspase-8 and AIF were also reduced. Further, HSP27 was increased in both the GMNL-263 5×108 cells/kg per d treatment plus HKL and the GMNL-263 2·5×109 cells/kg per d treatment plus HKH hamster hearts.

Fig. 2. Fas-induced apoptosis signalling analysis. (a) The western blot analysis of the protein expression in the FAS signalling pathway. (b) The normalised protein expression of FAS-L/β-actin. (c) The normalised protein expression of FAS/β-actin. (d) The normalised protein expression of Fas-associated protein with death domain (FADD)/β-actin. (e) The normalised protein expression of caspase-8/β-actin. (f) The normalised protein expression of heat-shock protein 27 (HSP27)/β-actin. (g) The normalised protein expression of AIF/β-actin. N, normal control; HKL, group of hamsters fed the high-fat diet with normal water and heat-killed Lactobacillus reuteri GMNL-263 5×108 cells/kg per d via oral gavage; HF, group of hamsters fed a high-fat diet only with normal water; HKH, group of hamsters fed the high-fat diet with normal water and heat-killed L. reuteri GMNL-263 2·5×109 cells/kg per d via oral gavage. ** P<0·01 compared with the normal group, *** P<0·001 compared with the normal group, **** P<0·001 compared with the HF group.
A DAPI and TUNEL dual staining assay was used to evaluate myocardial apoptosis in the hamster heart sections from each group. Some apoptotic cardiomyocytes were labelled by green TUNEL stain in the high-fat diet-only hamster hearts (Fig. 3). After 8 weeks, the number of apoptotic cardiomyocytes in the GMNL-263 5×108 cells/kg per d treatment plus HKL hamster hearts was reduced. Moreover, apoptosis of the cardiomyocytes in the GMNL-263 2·5×109 cells/kg per d treatment plus the HKH hamster heart group was inhibited.

Fig. 3. DAPI and TUNEL staining on hamster heart sections. The nuclei of the cells are stained with DAPI in blue, and nuclei of apoptotic cells are indicated by the TUNEL stain in green. The apoptotic cardiomyocytes are indicated by arrows. N, normal control; HKL, group of hamsters fed the high-fat diet with normal water and heat-killed Lactobacillus reuteri GMNL-263 5×108 cells/kg per d via oral gavage; HF, group of hamsters fed a high-fat diet only with normal water; HKH, group of hamsters fed the high-fat diet with normal water and heat-killed L. reuteri GMNL-263 2·5×109 cells/kg per d via oral gavage. (Bar length=50 μm).
Myocardial survival signalling analysis
There were no significant differences in the p-IGF1R and downstream p-PI3K, p-Akt, Bcl-2 and p-Bad protein levels between the control and the HF hamster hearts (Fig. 4). After 8 weeks, the expression levels of p-IGF1R downstream proteins p-PI3K, p-Akt and p-Bad were significantly increased in the GMNL-263 5×108 cells/kg per d-treated HKL group and GMNL-263 2·5×109 cells/kg per d-treated HKH group. The Bcl-2 expression level was increased in the HKL group, but not significantly in the HKH group.

Fig. 4. Myocardial cell survival signalling pathway. (a) The western blot analysis of protein expression of the IGF1R signalling pathway. (b) The normalised protein expression of p-IGF1R/β-actin. (c) The normalised protein expression of p-PI3K/β-actin. (d) The normalised protein expression of p-Akt/β-actin. (e) The normalised protein expression of Bcl-2/β-actin. (f) The normalised protein expression of p-Bad/β-actin. N=normal control; HKL, group of hamsters fed the high-fat diet with normal water and heat-killed Lactobacillus reuteri GMNL-263 5×108 cells/kg per d via oral gavage; HF, group of hamsters fed a high-fat diet only with normal water; HKH, group of hamsters fed the high-fat diet with normal water and heat-killed L. reuteri GMNL-263 2·5×109 cells/kg per d via oral gavage. * P<0·05 compared with the normal group, *** P<0·001 compared with the normal group, **** P<0·05 compared with the HF group, ***** P<0·001 compared with the HF group.
Discussion
Dynamic microbial communities significantly affect human health, including the cardiovascular system( Reference Ettinger, MacDonald and Reid 13 ). In CVD, the most investigated application for probiotic therapy is the reduction of serum LDL-cholesterol. Increased LDL-cholesterol levels is a major risk factor for CVD( Reference Grundy 14 ). Previously, Shi et al.( Reference Shi, Li and Miyazawa 15 ) used heat-inactivated Lactobacillus gasseri treatments and reduced metabolic syndrome symptoms in high-fat and high-salt diet-fed Sprague–Dawley rats. Similarly, L. reuteri can reduce serum cholesterol by interrupting lipid acid and bile acid conjugation. In addition, using heat-killed L. reuteri GMNL-263 treatments still can slightly decrease serum LDL-cholesterol in high-fat diet-fed hamsters, as shown in Table 1. The BSH activity of heat-killed L. reuteri GMNL-263 remains unclear. The mammalian intestine harbours a great number of bacteria (approximately 1014 bacteria)( Reference Qin, Li and Raes 16 ). Supplementing a specific amount of probiotics, such as GMNL-263 bacteria, might change the gut microbiota environment and affect serum cholesterol.
Serum LDL-cholesterol was only somewhat reduced in the GMNL-263 treatment plus high-fat diet groups, but the heart functions were improved and are shown in Table 2. In 8 weeks, the GMNL-263 2·5×109 cells/kg per d treatment improved the EF from 85·71 % in the high-fat diet-only hamster hearts to 91·81 % and the %FS from 46·93 to 57·92 %. In the heart section investigations, the GMNL-263 treatments also improved the myocardial disarray (Fig. 1).
Our previous work indicated that increased expression of Fas ligand and its receptor Fas leads to cardiomyocyte apoptosis through the release and activation of caspase-8 from FADD( Reference Deng, Lee and Kuo 17 ). In this study, the GMNL-263 treatments reduced the Fas ligand expression and downstream apoptosis-inducing signalling proteins (Fig. 2 and 3). Further, HSP27 is thought to be inhibitory against Fas-induced apoptosis( Reference Charette, Lavoie and Lambert 18 – Reference Cherng, Huang and Kuo 20 ). Interestingly, the GMNL-263 treatments in the high-fat diet-fed hamsters increased HSP27 in a dose-dependent manner. This result is similar to the other strains of probiotic bacteria, such as Lactobacillus paracasei, L. plantarum, Lactobacillus brevis and Bacillus subtilis, in their host( Reference Reilly, Poylin and Menconi 21 – Reference Okamoto, Fujiya and Nata 24 ). Recently, a study revealed that selenium-enriched probiotics might increase the HSP27 and HSP70 mRNA levels( Reference Gan, Ren and Chen 25 ). However, the mechanism behind the GMNL-263 treatments increasing the HSP27 expression levels and the role of HSP27 in hyperlipidaemia need further investigation.
After the 8-week experiment, there was no significant difference in the IGF1R-associated cell survival signalling pathway between the control group and the HF group (Fig. 4). However, serum cholesterol-lowering effects might increase the p-IGF1R and downstream p-PI3K, p-Akt, Bcl-2 and p-Bad reactivation in the GMNL-263 treatment groups.
In conclusion, supplementary heat-killed L. reuteri GMNL-263 can slightly reduce serum cholesterol. The moderate anti-hyperlipidaemia effects of GMNL-263 may reactivate the IGF1R/PI3K/Akt cell survival pathway and reduce Fas-induced myocardial apoptosis in high-fat diet-fed hamster hearts.
Acknowledgements
This study was supported in part by the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002).
The authors’ contributions were as follows: W.-J. T., W.-W. K., C.-H. K., Y.-L. Y. and C.-Y. H. designed the experiments; W.-J. T., C.-Y. S., Y.-H. C., T.-J. H. and C.-Y. H. analysed the results; and W.-J. T., V. V. P., Y.-H. C. and C.-Y. H. prepared and edited the manuscript. All authors read and approved the final manuscript.
The authors declare no conflicts of interest.









