Conjugated linoleic acids (CLA), a group of linoleic acids with conjugated double bonds, have received considerable attention for their potential to regulate energy expenditure, inflammation and oxidative processes in animals(Reference Shen, Chuang and Martinez1,Reference Chen, Yang and Ross2) . Studies in different animal models have shown that CLA was incorporated into tissues and modulated lipid metabolism in liver, adipose tissue and muscle(Reference Kim, Kim and Whang3,Reference Shen and McIntosh4) . CLA, especially the t10c12 isomer, was reported to exert its de-lipidating effects by decreasing fat storage in mature adipocytes and therefore reducing adipocyte size(Reference Choi, Kim and Han5,Reference Yeganeh, Taylor and Tworek6) , related to the decreased expression of hepatic genes involved in fatty acids synthesis and the increased expression of lipolysis genes(Reference Koronowicz, Banks and Szymczyk7,Reference Wang, Wang and Zhang8) , or by reducing the differentiation of preadipocytes to mature adipocytes via inhibiting the transcriptional level of adipocyte protein and PPARγ (Reference Chen, Tang and Zhang9–Reference Fleming, Eckert and Denisenko11). Therefore, consuming CLA was considered as a potential strategy for preventing the development of diabetes and obesity. In layers, lipid accumulation in the liver and abdomen could induce fatty acid haemorrhagic syndrome and lead to the significant degradation of egg production and reproduction performance(Reference Trott, Giannitti and Rimoldi12–Reference Wen, Yan and Zheng14). Therefore, CLA has attracted wide attention in the fields of poultry science and nutrition, for its effect on reducing abdominal fat accumulation in broiler chickens and laying hens and decreasing the total cholesterol (TC) concentration in the liver and egg of laying hens(Reference Wang, Wang and Zhang8,Reference Ramiah, Meng and Sheau Wei15,Reference Royan, Meng and Othman16) . Besides, supplementing CLA in poultry diet has also been suggested as a way to obtain CLA-enriched meat and egg product(Reference Ramiah, Meng and Sheau Wei15,Reference Kumari Ramiah, Meng and Ebrahimi17) . However, controversial effects of CLA supplementation on hepatic lipid metabolism in different animal models have been reported, probably due to the type of isomers, proportions and levels of CLA, the difference of experimental animal strains, and the fatty acid composition and fat content of diet(Reference Shinn, Gilley and Proctor18–Reference Mennitti, Oliveira and Morais20).
Maternal effects refer to parental phenotypes having a direct influence on offspring phenotype and have been studied extensively over the past several decades in animals due to their economic importance in domestic mammals(Reference Chen, Tang and Zhang9). Alterations in maternal nutrition may affect certain physiological and biochemical functions in the offspring(Reference Pillai, Sereda and Hoffman10,Reference Fleming, Eckert and Denisenko11) . In rats, maternal CLA was demonstrated to reduce lipogenesis, prevent high-fat diet-induced liver steatosis and reverse the metabolic dysfunction and impair insulin sensitivity induced by maternal high fat in adult offspring(Reference Lavandera, Gerstner and Saín21–Reference González, Lavandera and Gerstner23). In avian, as oviparous animals, maternal nutrition was transferred to the egg yolk and newly hatched chicks, which further altering the chick growth when the maternal nutrition was assimilated both before and after hatching(Reference Tanvez, Amy and Chastel24,Reference Lv, Fan and Song25) . Previous study reported that supplementing CLA in laying hens diet increased the incorporation of CLA isomers in yolk sac of eggs, which further incorporated high level of CLA in liver, plasma, adipose and brain tissue in chick offspring and decreased the total fat accumulation and hepatic TAG content in chick offspring(Reference Cherian, Ai and Goeger26,Reference Liu, Zhang and Yan27) . But, the addition of CLA to low-fat laying hens diet might result in non-preferential CLA incorporation into yolk sac and reduced hatchability of fertile eggs, which may be related to the increased ratio of SFA:MUFA or the severely interfered transference of lipid from yolk sac to embryo(Reference Muma, Palander and Nasi28–Reference Leone, Worzalla and Cook30). Even though maternal CLA could reduce the lipid accumulation in offspring, there is limited information about the appropriate supplemental level of CLA in breeder hens diet at recommended fat levels, or the metabolic effect of maternal CLA on modulating lipid metabolism in chick offspring. In this sense, the rational use of CLA in poultry should be further studied.
Our previous studies have demonstrated that supplementing CLA in maternal diet regulated the development and lipid metabolism of chicks during embryonic period(Reference Fu, Zhang and Yao31). As the development of newly hatched chicks entirely depends on the nutrients deposited in the yolk sacs for the first 7 d post-hatch, it is unclear that whether the incorporated CLA in yolk sac could further been translated into physiological and metabolic features of post-hatch chicks, and programme altered lipid metabolism in hatchling. Therefore, the aim of the present study was to investigate the effect of maternal CLA on the development and lipid metabolism of offspring chicks during 7 d post-hatch. Previous studies showed that the egg production rate might decrease when the addition level of CLA exceeds 2 %(Reference Shang, Wang and Li32,Reference Kim, Hwangbo and Choi33) . Moreover, we previously showed that 1·0 % CLA supplementation in Arbor Acres female broiler breeders reduced the fertilisation rate and the egg hatchability(Reference Fu, Zhang and Yao31), which may related to the difference of fatty acid composition and transportation in yolk sac(Reference Leone, Worzalla and Cook34). Therefore, we supplemented 0·5 % CLA, a lower dose, in Hy-Line Brown breeder hens diet due to the consideration for health addition level of CLA in breeder hens. The development production and egg quality of hens were also detected, and the fatty acid composition in yolk and the liver of chicks post-hatch were also conducted. As CLA was reported to exhibit strong antioxidant capacity in hens hepatocytes(Reference Nakamura and Omaye35–Reference Qi, Wang and Yue37), some parameters related to the activity of antioxidative enzyme were detected in the liver of chick offspring. The results may provide a basis for bioactive supplementation of matrilineal diets that regulate the growth and metabolism of offspring chickens.
Material and methods
Materials
CLA containing 81 % pure CLA (cis-9, trans-11 = 36·0 %, trans-10, cis-12 = 41·7 %, other isomers = 3·3 %) was purchased from Aohai Biologic Limited Company.
Animals and experimental design
All procedures and experiments in this work were approved by the Institutional Animal Care and Use Committee of Shandong Academy of Agricultural Sciences (SAAS-2019-026). Experiments were performed on Hy-Line Brown breeder hens and their offspring at a commercial farm (Jinan, China) under standard conditions.
Ninety 36-week-old Hy-Line Brown breeder hens (2·10 (sd 0·11) kg body mass) were housed in wire cages (with 1 bird placed in one cage, 36 cm × 25 cm × 39 cm) and exposed to a 16L:8D illumination cycle, cared in accordance with animal welfare regulations. The maize–soyabean meal-based breeder diet is supplied by Poultry Institute of Shandong Academy of Agricultural Sciences and formulated to meet the nutritional requirements of hens according to the National Research Council(38) (Table 1). All hens were allotted 120 g of feed at 06.00 hours every day and provided water ad libitum throughout the experiment. After a 2-week adaptation period, equal numbers of hens were divided into one of two dietary treatment groups and fed breeder diets containing 0 or 0·5 % CLA for 8 weeks (control (CT) group or CLA group, respectively), artificially inseminated using seminal fluid from male breeders (36-week-old male Arbor Acres breeder broilers). To equalise the concentration of total fat in both diets and meet the assigned CLA additions, 0·62 % of the CLA replaced 0·62 % of the soyabean oil (w/w). Each groups included five replicates of nine birds per replicate. The number and the mass of eggs in each replicate were recorded at the end of every week.
Table 1. Ingredients and the analysed and calculated chemical composition of the experimental diets
(Percentages)
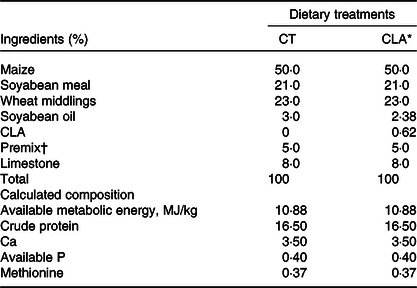
CT, control group; CLA, conjugated linoleic acid.
* CLA represents hens fed with 0·5 % CLA (with 0·62 % CLA mixture substituted for equivalent soyabean oil) in basal diet.
† Supplied with the following nutrients per kg of diet: protein, 280 g; Met, 28 g; dicalcium phosphate, 160 g; vitamin A, 27 mg; vitamin D3, 50 μg; vitamin E, 13·33 mg; vitamin K3, 1 mg; vitamin B1, 1·20 mg; vitamin B2, 5·80 mg; vitamin B6, 2·6 mg; vitamin B12, 0·012 mg; niacin, 66 mg; biotin, 0·10 mg; pantothenic acid, 10 mg; folic acid, 0·7 mg; Cu, 80 mg; Fe, 80 mg; Mn, 100 mg; Zn, 75 mg and ethoxyquin, 5 g.
At the end of the 6th week, forty-five eggs were randomly collected from CT or CLA groups especially to determine egg quality. Egg weight, Haugh unit, albumen height and yolk colour were measured using an Egg Multi Tester (EMT-7300, Tohoku Rhythm Co., Ltd). Eggshell strength on the vertical axis was measured by an Instron 3360 apparatus. The egg shape index was calculated by height/diameter. After breakout, the albumen and yolk were separated and weighted. Eggshell was weighted after adhering albumen was removed with slow flowing tap water and left was dried at room temperature for 1 h. Eggshell thickness was measured at the sharp, blunt ends and equator after removing the shell membranes using a micrometer and calculated with the average eggshell thickness.
During the last 2 weeks, 450 eggs from the different groups were randomly collected (with ten eggs per hen) and placed into an electric forced-draft incubator at 37·5 (sd 0·5) °C and 60 % relative humidity with intermittent rotation. On day 7 of incubation, the eggs were candled with incandescent light, and the infertile eggs were numbered and removed from the incubator. On day 22 of incubation, infertile eggs and dead germs were determined and removed. The fertility rates and hatchability rates of the eggs from different groups were determined. The fertility rate was the proportions of fertile eggs to total eggs set for incubations, and the hatchability rate was the proportions of hatched chicks during 21 d of incubation to total eggs set for incubation. According to the treatment on the maternal generation, chicks were divided into two groups (CT and CLA) with six replicates of twenty birds each. The chicks were housed in a standard house for 7 d, with free access to water and diet (basal maize–soyabean meal with 21·5 % crude protein and 12·37 MJ/kg of metabolisable energy)(Reference Zhao, Lin and Jiao39).
Sample collections
On days 1, 3, 5 and 7 post-hatching, two hatchling chicks per replicate (twelve birds per group) were randomly selected and weighted after 12 h fasting. Blood samples were obtained for the wing vein blood with a heparinised syringe. Plasma was obtained after centrifuged at 400 g for 10 min and stored at −20 °C for further analysis. After bleeding, the birds were killed using pentobarbital anaesthesia. Liver, subcutaneous adipose tissue (SAT) and residual yolk sacs were collected, or massed, and stored at −80 °C for further analysis.
Measurement of serum biochemical indices
Serum TAG, TC, leptin, insulin and adiponectin levels were assayed using commercial assay kits (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer’s protocol.
Fatty acid composition in residual yolk sac and liver
The fatty acid composition in residual yolk sac and liver were determined and performed using freeze-dried powder as previously described(Reference Fu, Zhang and Yao31). Briefly, yolk sac and hepatic fatty acids were extracted twice from a mixture of chloroform and methanol (2:1 vol/vol) and transesterified to methyl esters with BF3-methanol solution (13 %) as previously described(Reference Xu, Harvey and Pavlina40). The fatty acid methyl esters were analysed using direct transesterification by GC with a Hewlett-Packard HP6890 GC System, installed with an Agilent HP-88 chromatographic column (100 m × 0·25 mm × 0·20 μm) and equipped with a flame ionisation detector. The GC conditions were as follows: 260 °C injector temperature; 270 °C detector temperature; He carrier gas; 1:50 split ratio; temperature programme set for 100 °C for 5 min, followed by an increase in 5 °C/min to 240 °C and then maintained for 30 min. Peaks were identified by a composition for retention times with those of the corresponding standards from Sigma-Aldrich.
Measurement of biochemical indices in liver
The liver samples were homogenised in 0·25 mol/l sucrose, 1 mmol/l dithiothreitol and 1 mmol/l EDTA at pH 7·4. The cytosolic fractions of chick offspring liver were obtained by centrifugation at 10 000 g for 1 h at 4 °C and used for quantification of fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC) activities according to the methods of Lavandera et al. (Reference Lavandera, Gerstner and Saín21) and Wang et al. (Reference Wang, Li and Song41) and expressed as mU/mg of protein, where 1 mU was 1 nmol NADH consumed (ACC) and 1 nmol NADPH consumed (FAS) per minute. Liver samples were homogenised in a buffer (pH 7·4) containing 0·25 M sucrose, 1 mM EDTA and 10 mM Tris–HCl. Homogenate was centrifuged at 700 × g for 10 min at 4 °C, and supernatant fluid was centrifuged again at 12 000 × g for 15 min at 4 °C. The carnitine palmitoyltransferase-1a (CPT1a) activity was assayed in the liver mitochondrial fraction by the method of Bieber et al. by subtracting the initial rate of formation of CoA of d(+)-carnitine from the initial rate of formation of CoA of l(-)-carnitine. The CPT1a activities were expressed as mU/mg protein (1 mU = 1 nmol CoA/min). Protein content was determined using bovine serum albumin as standard by the Lowry method(Reference Lowry, Rosebrough and Farr42). To avoid the loss of enzyme activity, the activities of FAS, ACC and CPT1a were measured immediately after the tissues were collected.
The malondialdehyde, glutathione peroxidase and total superoxide dismutase were determined using commercial assay kits (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer’s protocol.
Quantitative real-time PCR analysis
Total RNA was isolated from liver tissue using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. Reverse transcription and quantification of mRNA were performed as previously described(Reference Fu, Liu and Gao43). Primer 5.0 software (Primer-E Ltd.) was used to design primers for exon–intron junctions. Primer sequences are listed in Table 2. Real-time PCR (Applied Biosystems 7500 Real-time PCR System; Applied Biosystems) was performed according to the following protocol: 95 °C for 10 s; 40 cycles of 95 °C for 5 s and 60 °C for 40 s. A standard curve was plotted to calculate the efficiency of the PCR process. PCR of β-actin was used to normalise and quantify the mRNA levels of the target genes using the comparative CT method (2-ΔΔCT)(Reference Livak and Schmittgen44). Specificity of the amplification products was verified by melting curve analysis. All samples were run in duplicate.
Table 2. Gene-specific primers of related genes
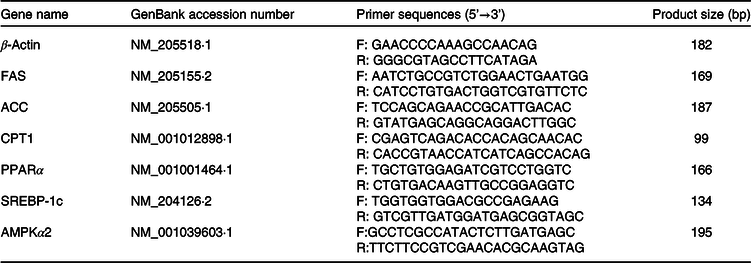
FAS, fatty acid synthase; ACC, acetyl-CoA carboxylase; CPT, carnitine palmitoyltransferase; SREBP-1c, sterol regulatory element-binding protein-1c; AMPKα2, AMP-activated protein kinase.
Statistical analyses
Values were expressed as mean values and standard deviations. The statistical analysis was performed by Student’s t test, using the Statistical Analysis Systems statistical software package (Version 8e, SAS Institute). Statistical significance was set to P < 0·05.
Results
Egg production performance and egg quality of breeder hens
As previous studies showed that the addition level of CLA in diet might decreased the egg production of laying hens(Reference Shang, Wang and Li32,Reference Kim, Hwangbo and Choi33) , we detected the effect of 0·5 % CLA supplementation on egg production performance or egg quality. As shown in Table 3, CLA supplementation had no statistical influence on the body or egg mass, or egg production rate of breeder hens (Table 3, P > 0·05). There were no significant differences on Haugh unit, albumen height, shell strength, shell thickness, egg shape index, shell mass and albumen mass between the CT and CLA groups (Table 4, P > 0·05). However, the CLA treatment had darkened yolk colour (P < 0·0001) and increased yolk and relative yolk mass (P = 0·0278 and 0·0159, respectively). Furthermore, CLA supplementation of maternal diets had no influence on the fertility rate (P > 0·05) or the hatchability of their eggs (Table 5, P > 0·05).
Table 3. Growth and egg production performance of hens
(Mean values and standard deviations)

CT, control group; CLA, conjugated linoleic acid.
* CLA represents hens fed with 0·5 % CLA (with 0·62 % CLA mixture substituted for equivalent soyabean oil) in basal diet.
Table 4. Effect of CLA supplementation on egg quality of hens (n 45) (Mean values and standard deviations)
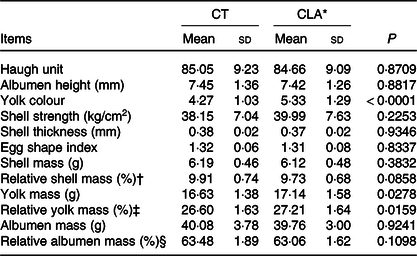
CT, control group; CLA, conjugated linoleic acid.
* CLA represents hens fed with 0·5 % CLA (with 0·62 % CLA mixture substituted for equivalent soyabean oil) in basal diet.
† Relative shell mass, shell weight/egg mass.
‡ Relative yolk mass, yolk sac weight/egg mass.
§ Relative albumen mass, albumen weight/egg mass.
Table 5. Effect of CLA supplementation on fertility and hatchability of hatching eggs
(Mean values and standard deviations)
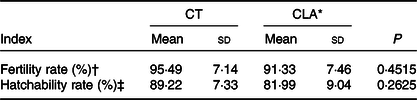
CT, control group; CLA, conjugated linoleic acid.
* CLA represents hens fed with 0·5 % CLA (with 0·62 % CLA mixture substituted for equivalent soyabean oil) in basal diet.
† Values are the proportions of fertile eggs to total eggs set for incubation (n 200).
‡ Value are the proportions of hatched chicks during 21 d of incubation to total eggs set for incubation (n 200).
Body mass, liver mass, adipose tissue deposition and yolk sac absorption efficiency of early chick offspring
Feeding breeder hens with 0·5 % CLA significantly increased the body mass of the chick offspring compared with that of the chicks from CT groups at day 1 (Fig. 1(a), P < 0·05). Meanwhile, the liver mass was increased at days 1, 3, 5 and 7 (Fig. 1(b), P < 0·05). Residual yolk sac mass was not significantly different between the CT and CLA groups (Fig. 1(c), P > 0·05). CLA treatment decreased the SAT deposition of chicks from the CLA group at days 1, 3, 5 and 7 (Fig. 1(d), P < 0·05) compared with the CT group.
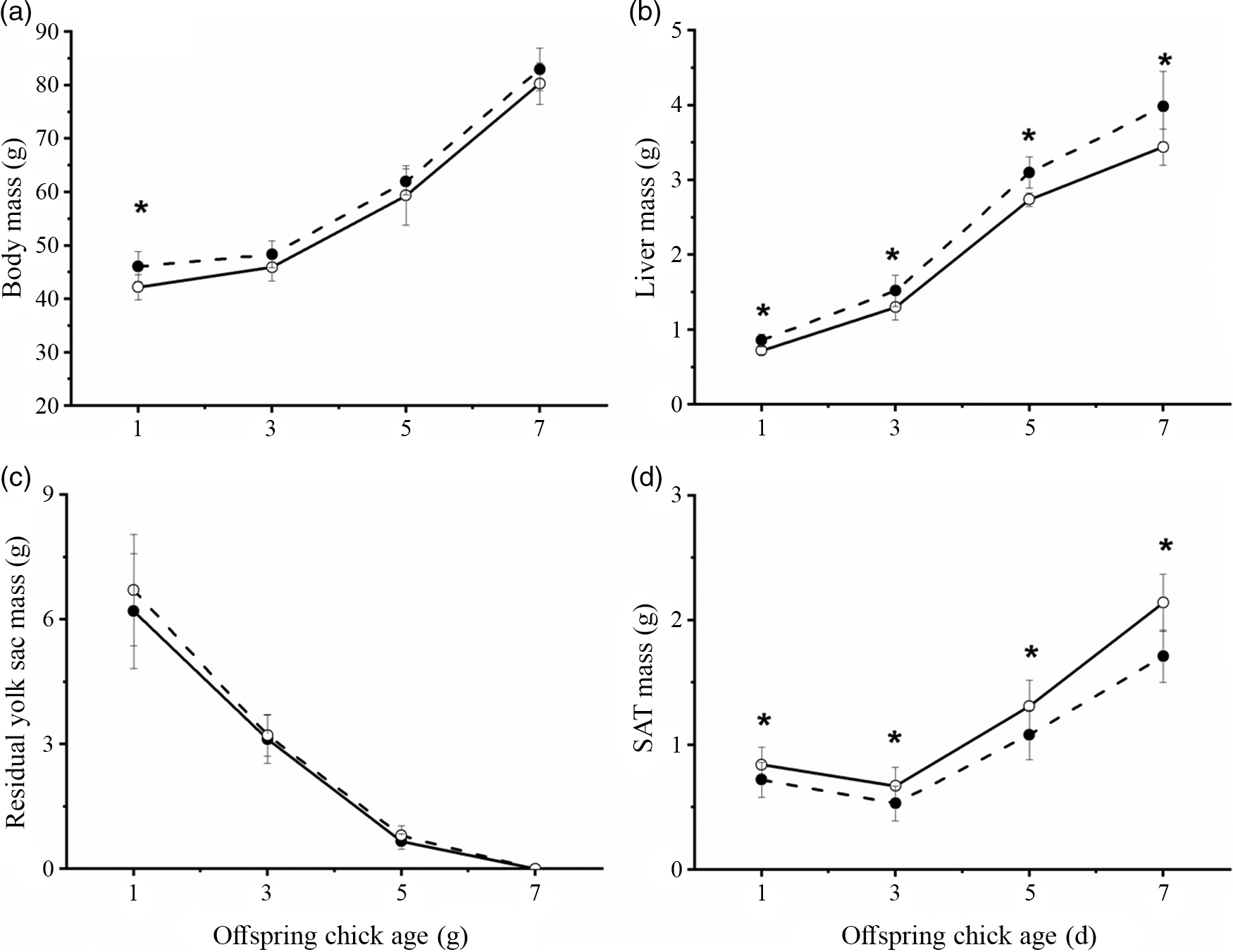
Fig. 1. CLA supplementation in breeder diets influences the body mass (a), yolk absorption (b), liver weight (c) and subcutaneous adipose tissue weight (d). n 8. *P < 0·05. CT, control group; CLA, conjugated linoleic acid group. Each column represented the mean and standard deviation of results. *P < 0·05, compared with control. ![]() , CT;
, CT; ![]() , CLA.
, CLA.
Lipid metabolic indexes in serum of chick offspring
The serum TAG, TC and insulin levels in the CLA group were decreased compared with the CT group at days 1, 3, 5 and 7 (Table 6, P < 0·05). On the other hand, the serum leptin levels in chicks from CLA group were decreased during the first 3 d post-hatch (P < 0·05), and the adiponectin contents were increased in the CLA group at days 1, 3 and 7 after hatching (Table 6, P < 0·05).
Table 6. Effect of maternal CLA diet on serum parameters of offspring chicks (n 8)
(Mean values and standard deviations)

CT, control group; CLA, conjugated linoleic acid; SAT, subcutaneous adipose tissue.
Percentage of conjugated linoleic acid, SFA, PUFA and MUFA in residual yolk sac and liver
The percentage of two bioactive CLA isomers (c9t11 and t10c12), SFA, PUFA and MUFA in residual yolk sac and liver of early chick offspring was detected during the first 7 d post-hatch and expressed as percentage of total fatty acid methyl esters (Table 7). In CLA group, both c9t11-CLA and t10c12-CLA isomers were incorporated in yolk sac and the liver of chick offspring (P < 0·05). Interestingly, with the development of chicks, the relative content of both CLA isomers in yolk sac was increased, until the yolk sac was totally absorbed by chicks at day 7. While the content of both c9t11- and t10c12- CLA isomers in liver was decreased with the development of chicks, with undetected at day 7. In addition, the proportion of SFA in the residual yolk sac was significantly increased in the CLA group before the yolk was totally absorbed by chicks, accompanied by the decreased proportion of PUFA and MUFA compared with that from the CT group (P < 0·05), except for that of PUFA at 3 d (P > 0·05). Meanwhile, the hepatic SFA content in chicks from CLA group was significantly increased at days 1, 3 and 7 (P < 0·05), and the hepatic PUFA content was significantly decreased at days 3, 5 and 7 (P < 0·05). There was no significant difference for the hepatic MUFA concentration in chicks between CLA and CT groups (P > 0·05), except that at day 1 post-hatch (P < 0·05).
Table 7. Effect of maternal CLA diet on percentage of CLA (% of total fatty acid methyl esters) in yolk sac, and liver of offspring chicks (n 8)
(Mean values and standard deviations,)

CT, control group; CLA, conjugated linoleic acid; ND, not detected.
Hepatic lipid metabolism in chick offspring
The hepatic TAG and TC levels of chick offspring were significantly decreased in CLA group at days 1, 3, 5 and 7 (Fig. 2(a), P < 0·05). The hepatic TAG levels were mostly regulated by lipogenesis and β-oxidation. The FAS and ACC activities in chick offspring liver were diminished, and the CPT1a activity was increased in the CLA group (Fig. 2(b), P < 0·05).
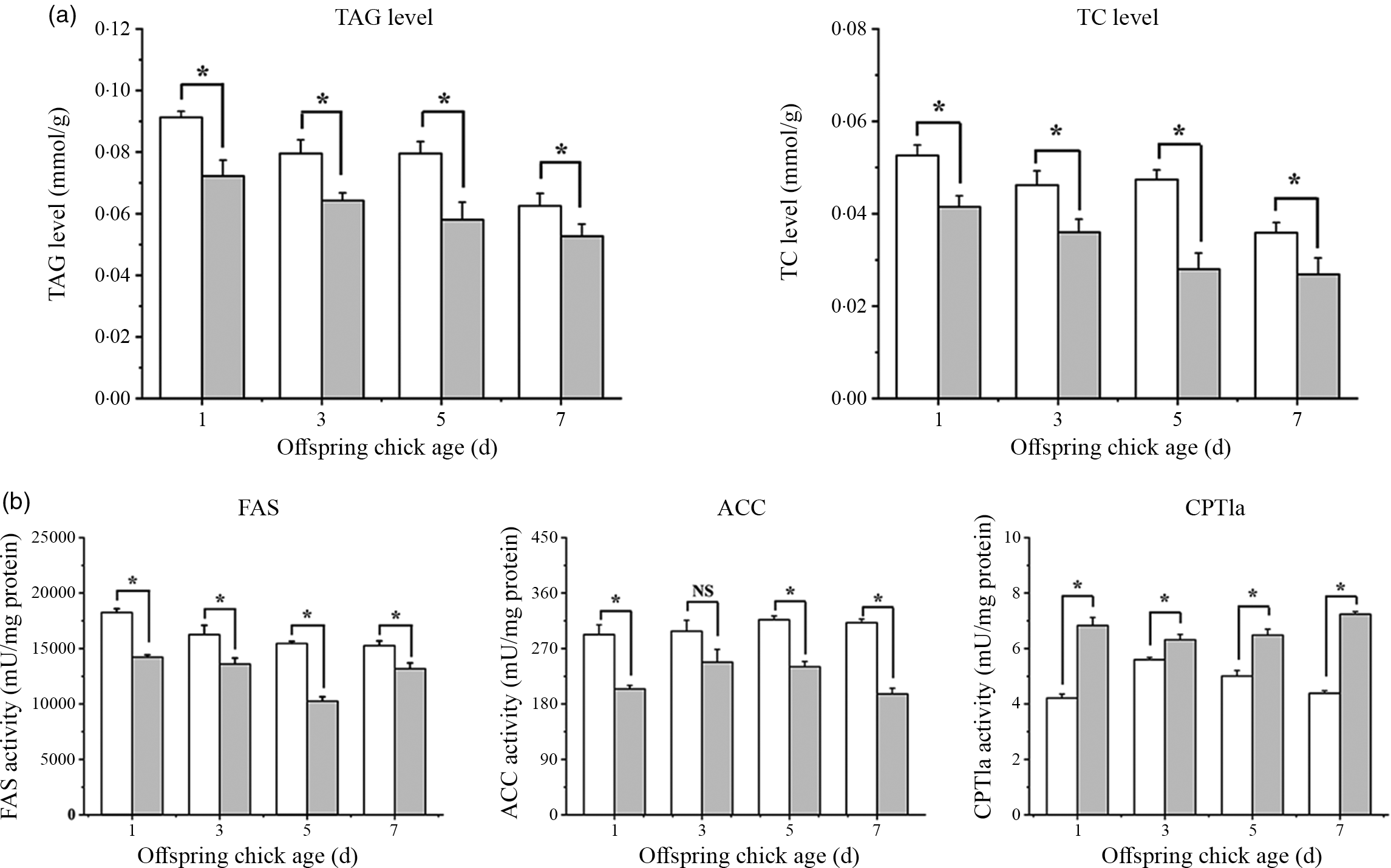
Fig. 2. CLA supplementation in breeder diets regulates lipid metabolism in chick offspring liver. (a) TC and TAG levels (mmol/g protein) in liver. (b) Lipogenic enzymes (FAS and ACC, mU/mg protein) and β-oxidative enzyme (CPT1a, mU/mg protein) activity in liver (n 8). Each column represented the mean and standard deviation of results. *P < 0·05, compared with control. TC, total cholesterol; FAS, fatty acid synthase; ACC, acetyl-CoA carboxylase; CPT, carnitine palmitoyltransferase. ![]() , CT;
, CT; ![]() , CLA.
, CLA.
As the liver is known to be the primary site of fatty acid synthesis in birds(Reference Xu, Wang and Mao45), we further investigated the expression of key genes and transcriptional factors related to fatty acid biosynthesis and β-oxidation in the liver of chick offspring. The level of mRNA of CPT1, AMP-activated protein kinase α (AMPKα) and PPARα were increased in the liver of chicks from CLA group (Fig. 3(a)–(c), P > 0·05). The FAS, ACC1 and SREBP-1c gene expression was decreased in the liver of chicks from CLA group (Fig. 3(d)–(f), P > 0·05).
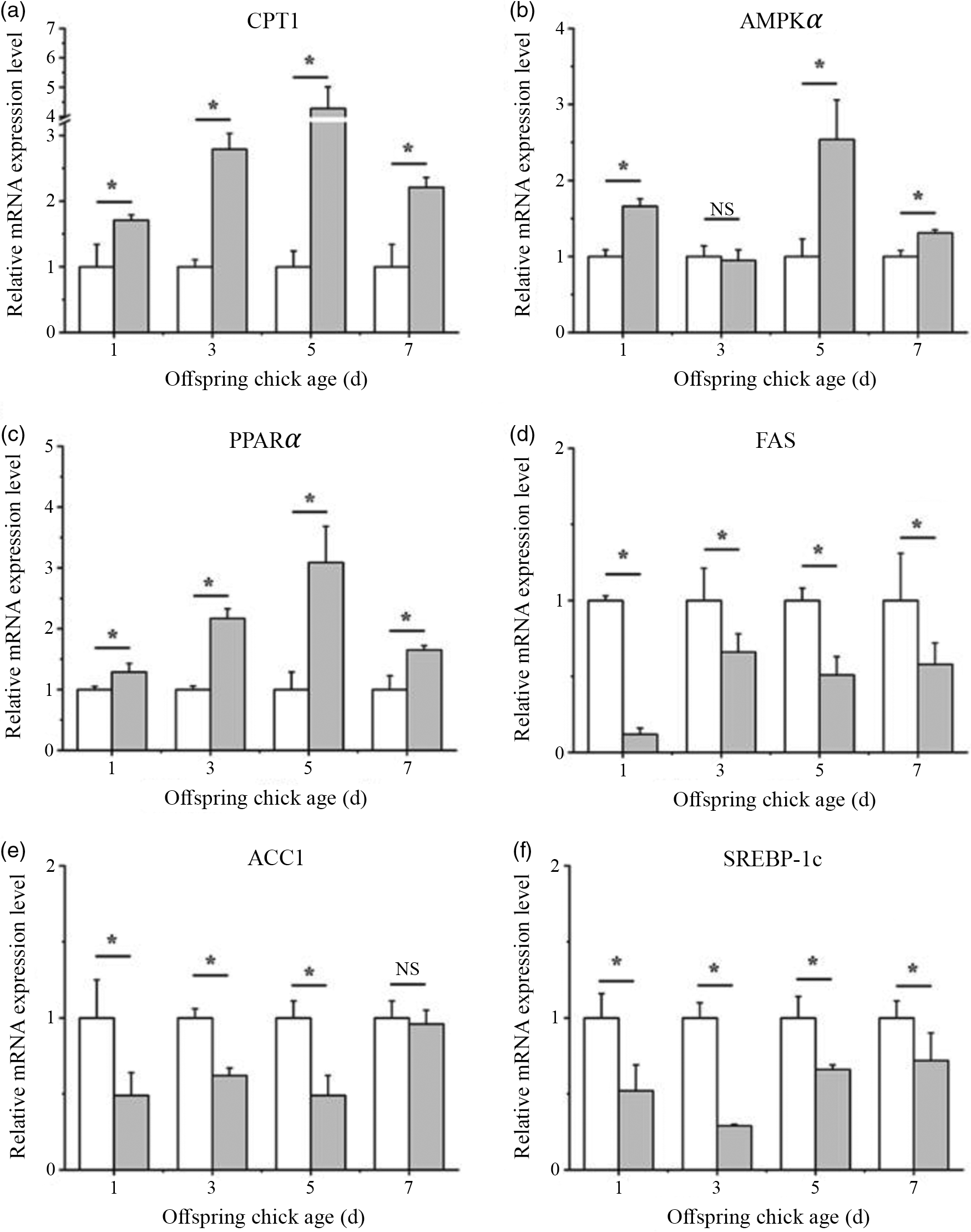
Fig. 3. CLA supplementation in breeder diets alters the hepatic mRNA levels of genes related to fatty acid metabolism in chick offspring. CPT, carnitine palmitoyltransferase (a); AMPKα, AMP-activated protein kinase (b); PPARα (c); FAS, fatty acid synthase (d); ACC, acetyl-CoA carboxylase (e); SREBP-1c, sterol regulatory element-binding protein-1c (f). Each column represented the mean and standard deviation of results. *P < 0·05, compared with control. n 8. ![]() , CT;
, CT; ![]() , CLA.
, CLA.
Hepatic antioxidant indexes of chick offspring
Consistent with prior reports that maternal CLA enhanced the antioxidant capacity of their chicks(Reference Jiang, Nie and Qu46), we demonstrated that during the early life of chicks, the activity of glutathione peroxidase and total superoxide dismutase in liver was higher and levels of malondialdehyde were lower (Table 8; P < 0·0001) in the CLA group than the CT group.
Table 8. Effect of maternal CLA diet on hepatic antioxidative capability of offspring chicks (n 8)
(Mean values and standard deviations)
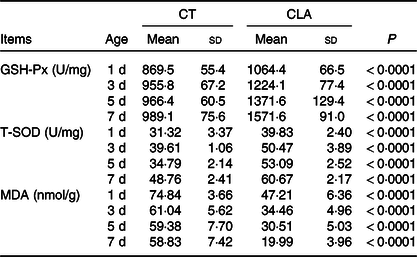
CT, control group; CLA, conjugated linoleic acid; GSH-Px, glutathione peroxidase; T-SOD, total superoxide dismutase; MDA, malondialdehyde.
Discussion
Over the last few decades, broilers were selected for genetic breeding based on rapid growth, meat production and carcass yield. With the increase in body mass, abdominal fat deposition also increased in broilers(Reference Moreira, Boschiero and Cesar47). The excess abdominal fat deposition reduced carcass lean yield, increased feed cost and reduced the qualities of the meat of broilers. CLA (especially t10,c12 isomer) is reported to reduce body lipid deposition and regulate hepatic lipometabolism, as well as the inflammatory response and antioxidative capability in animals, which refers to c9,t11 isomers(Reference Shen and McIntosh4). Furthermore, CLA is reported to have a potential key role in reducing the lipid deposits in offspring(Reference Lavandera, Gerstner and Saín21,Reference González, Lavandera and Gerstner23) . Our previous study found that maternal CLA reduced lipid deposition in developing chick embryo and newly hatched chicks(Reference Fu, Zhang and Yao31). Taking into account that the reduction in lipid deposition in developing chick embryo might also induce the metabolic changes of offspring during their developing progress, we aim to investigate the effects of maternal dietary treatment with CLA on the hepatic lipometabolism and SAT deposition of early chick offspring. In addition, some antioxidant enzyme activity was detected to investigate the effect of maternal CLA on antioxidant capacity of chick offspring.
Many investigations have shown that CLA may have beneficial effects on diabetes, obesity, the inflammatory response, atherosclerosis and glucolipid metabolism(Reference Reynolds, Loscher and Moloney48,Reference Kennedy, Martinez and Schmidt49) . Therefore, CLA has also attracted wide attention in the fields of poultry science and nutrition, for its effect on reducing abdominal fat accumulation in broiler chickens and laying hens, and decreasing the TC concentration in the liver and egg of laying hens(Reference Wang, Wang and Zhang8,Reference Ramiah, Meng and Sheau Wei15,Reference Royan, Meng and Othman16) . Besides, supplementing CLA in poultry diet has also been suggested as a way to obtain CLA-enriched meat and egg product(Reference Ramiah, Meng and Sheau Wei15,Reference Kumari Ramiah, Meng and Ebrahimi17) . However, controversial effects of CLA on production and development of laying hens have also been reported. Previous studies showed that CLA diet might decrease feed intake and the egg production of laying hens when the addition level of CLA exceeds 2 %(Reference Shang, Wang and Li32,Reference Kim, Hwangbo and Choi33) . In the present study, the 0·5 % CLA supplementation in diet did not affect the body mass and egg production performance of Hy-Line Brown breeder hens, which was consistent with previous studies involving use of similar levels of CLA supplementation(Reference Fu, Zhang and Yao31,Reference Cherian, Traber and Goeger50) . Therefore, the effect of CLA supplementation on egg production rate of laying hens is dependent on the dose of CLA in the diet. Kim et al. (Reference Kim, Hwangbo and Choi33) reported that high-dose CLA supplementation might result in disruption of homoeostasis of lipid metabolism in the liver, which further led to the decrease in the egg production and quality in laying hens.
In the present study, the results showed that dietary CLA supplementation had no significant influence on most of the characteristics of egg quality but dramatically darkened the yolk colour and increased the mass of yolk sacs, in agreement with prior studies(Reference Qi, Wu and Zhang36,Reference Kennedy, Martinez and Schmidt49) . This was related to the yolk water content and the movement of ions through the vitelline membrane that would been altered by changes in the lipid metabolism and fatty acid composition(Reference Aydin, Pariza and Cook51).
Classic studies demonstrated that CLA supplementation inhibited the activity of Δ-9 desaturase (also called stearoyl-CoA desaturase), an enzyme capable of converting SFA (C16:0 andC18:0) to MUFA (C16:1 and C18:1) in liver, and further increased the SFA:MUFA ratio in egg yolk(Reference Tous, Lizardo and Vilà52). Furthermore, the addition of CLA to low-fat laying hens diet (0·5–0·75 %) might result in non-preferential CLA incorporation into yolk sac and the severely interfered transference of lipid from yolk sac to embryo, and then reduced hatchability of fertile eggs(Reference Muma, Palander and Nasi28–Reference Leone, Worzalla and Cook30), while, in the present study, the egg fertilisation rate and the hatchability rate of fertile eggs were not influenced by maternal CLA treatment. This phenomenon might result from the soyabean oil supplementation in the CLA group, with 2·38 % soyabean oil and 0·62 % CLA-mix oil. Previous studies confirmed that the addition of plant oil, such as soyabean oil and maize oil, could recover the CLA-induced loss of hatchability(Reference Cherian, Ai and Goeger26,Reference Muma, Palander and Nasi28,Reference Fu, Zhang and Yao31) . It suggested that the presence of other fatty acids in diet might alter the effect of CLA on hatchability, while the specific fatty acids that play the main recovery role hinge upon further investigation. All of these findings established a safe and effective CLA-supplemented maternal diet, which has no negative influence on the egg production performance of hens.
The chick embryo is a common model system for research on the embryonic development of vertebrates(Reference Lv, Fan and Zhang53). Chick hatchling relies upon nutrients deposited in the egg, generally originating from the maternal diet(Reference Nasir and Peebles54). Our previous studies have confirmed the deposition of CLA isomers in yolk sac of the developing embryos via feeding hens with CLA-supplemented diet, which further resulted in influencing the hepatic fatty acid metabolism and fat deposition of embryos(Reference Fu, Zhang and Yao31). However, it is unclear that whether the incorporated CLA from yolk sac could further been translated into physiological and metabolic features of offspring, and program altered lipid metabolism in hatchling.
In the present study, the fatty acids composition in residual yolk of chick offspring was influenced by maternal CLA supplementation during the first 5 d post-hatch, with increasing the amount of SFA and decreasing the amount of PUFA and MUFA. As previous studies studied, this was conceivable that the effect of maternal CLA supplementation on fatty acid composition in yolk of chick offspring was mediated through inhibition of Δ-9 desaturase activity in hens liver and was attributable to the incorporation of CLA isomers. At hatch and through the first few days post-hatch, the chick contributes to absorb yolk material. The liver in newly hatched chicken is full of lipids which are absorbed from the yolk(Reference Liu, Zhang and Yan27,Reference Noble and Cocchi55) , followed by the transition from a lipid diet (yolk) to primarily a carbohydrate (standard ration) diet(Reference Latour, Devitt and Meunier56). Therefore, the regulated fatty acid composition in yolk by maternal CLA supplementation further influenced the absorption of fatty acids of embryo, which resulted to the similar fatty acids composition in the liver of chick offspring to that in yolk. These results were consistent with the previous findings(Reference Leone, Worzalla and Cook34). Upon absorption, CLA is converted into a conjugated C18:3 product by Δ-6 desaturase, and further elongated and desaturated into conjugated C20:3 and C20:4, but not catabolised via β-oxidation by hepatocytes in mice and rats(Reference Sebedio, Angioni and Chardigny57–Reference Berdeaux, Gnadig and Chardigny59). Therefore, the content of CLA isomers in the liver of chick offspring declines as age increased.
The maternal nutritional condition and fatty acid intake during pregnancy or lactation are critical factors that are strongly associated with normal fetal and postnatal development, which influence the modifications in fetal programming and in the individual risk for developing metabolic diseases throughout life(Reference Mennitti, Oliveira and Morais20). The chicks from CLA treatment group showed an increase in body mass and liver mass and a decrease in SAT deposition, which showed the effect of maternal CLA on body composition of chick offspring, especially the de-lipidative effect of maternal CLA on chick offspring. Plasma TAG levels are regulated by the hepatic steatolysis rate and the lipid metabolism status in adipose tissue and muscle. In the present study, the TAG levels in liver and plasma of chick offspring were decreased by maternal CLA treatment. Previous studies with laying hens have confirmed these results and attributed t10c12-CLA’s de-lipidation effects in avians(Reference Wang, Wang and Zhang8). In vitro, t10c12-CLA increased leptin gene expression and inhibited expression of PPARγ and its downstream targets in newly differentiated adipocytes(Reference Brown, Boysen and Chung60). Similarly, we found that the level of serum leptin and adiponectin was increased and the serum insulin level decreased in the CLA group, which further confirmed the de-lipidation effect of maternal CLA on decreasing in glucose and fatty acid uptake and TAG synthesis.
For chickens, liver is the main site of de novo lipogenesis and was reported to be a key target for CLA(Reference Noble and Cocchi55,Reference Aydin, Pariza and Cook61,Reference Laliotis, Bizelis and Rogdakis62) . The present study revealed that dietary CLA supplementation decreases the liver mass in chick offspring during the early life, which was consistent with previous study(Reference Lavandera, Gerstner and Saín21). Furthermore, we observed a sustained reduction of TAG and TC levels in the liver of chick offspring in reaction to the supplementation of maternal dietary CLA, suggesting a decreased fat deposition of chicks from CLA group, presumably because the CLA supplementation elevated the metabolic rate of fat by inhibiting fatty acid synthesis and accelerating fatty acid oxidation(Reference West, Delany and Camet63). Similar results have been previously shown in animals fed CLA diets(Reference González, Lavandera and Gerstner23,Reference Fu, Zhang and Yao31,Reference Andreoli, Illesca and González64) . Changes of hepatic lipid metabolism directly influence not only TAG levels in liver but also the lipid accumulation in muscle and adipose tissue(Reference Richards, Proszkowiecweglarz and Rosebrough65). It was showed that dietary CLA supplementation elicited reductions in abdominal fat percentage in broilers(Reference Suksombat, Boonmee and Lounglawan66). Similarly, the present study revealed that CLA supplementation in breeder hens diet reduced SAT mass. These events indicate that the switch period in hepatic lipid metabolism has a significant impact on adipose tissue of starter chickens. CLA has been proposed to attenuate hepatic steatosis by modulating hepatic genes involved in lipogenesis and lipid oxidation(Reference Purushotham, Shrode and Wendel67). We found that CLA supplementation in hens diet inhibited lipogenesis in chicks by reducing the enzyme activities and/or mRNA production of FAS, ACC1 and SREBP-1c and activated lipolysis by increasing the production of CPT1, AMPKα and PPARα, consistent with the findings of Lavandera et al.(Reference Lavandera, Gerstner and Saín21) and Zhang et al. (Reference Zhang, Huang and Tian68). The change of lipometabolism-related gene expression, as well as transcription factor activities, further demonstrates an imbalance between lipogenesis and lipolysis and showed that CLA might programme the lipid metabolism driving to a prevention of lipid accumulation in SAT. Interestingly, this imbalance between lipogenesis and lipolysis in chick offspring post-hatch was accompanied by the incorporation of CLA isomers in liver. The concentration of CLA in chick liver was decreased with time going by. Whether this de-lipidation effect of maternal CLA on the lipid metabolism of adult chick offspring is unclear, further detection and verification need to be continued.
The beneficial effect of CLA on antioxidative activity has been demonstrated in different animal models(Reference Qi, Wang and Yue37,Reference Hanschke, Kankofer and Ruda69) . Oxygen free radical and lipid peroxidation reactions play an important role in body metabolism and reflect the body’s ability to maintain normal physiological progress. Free radicals result in damage to the cell membrane structure and function via the induction of free radical-dependent chain reactions(Reference Cheeseman and Slater70). Antioxidant enzymes, including superoxide dismutase and glutathione peroxidase, play important roles in antioxidative processes. In the present study, we observed that the activities of total superoxide dismutase and glutathione peroxidase increased in the liver of chicks from the CLA group. The activity of malondialdehyde was decreased in livers of chicks from the CLA group, consistent with the findings of Qi et al. (Reference Nakamura and Omaye35). These results indicate that maternal CLA enhances the lipometabolism in early chick offspring. Furthermore, this alteration may reduce lipid peroxidation and free radical effects on membranes.
We conclude that 0·5 % CLA supplementation in maternal diet has no significant influence on the development, egg quality and fertility of breeder hens. Furthermore, there is no effect on the egg quality produced by these hens. We observed that maternal CLA supplementation increased the CLA isomers incorporation in the liver of chick offspring post-hatch, accompanied by the decrease of lipogenesis and increase of lipolysis in the livers. As nutritional changes of maternal feed induce epigenetic modifications in the embryo and neonate, which may influence metabolic status during adulthood, more detailed mechanism by which maternal CLA in mediating offspring lipometabolism awaits further study.
Acknowledgements
This work was supported by the National Science Foundation of China (31902176), the Natural Science Foundation of Shandong Province (ZR2019BC005) and Major Scientific and Technological Innovation Project of Shandong Province (2019JZZY020602, 2019JZZY020611). We appreciate the linguistic assistance provided by TopEdit (www.topeditsci.com) during the preparation of this manuscript.
C.-Y. F. formulated the research questions, designed the study and wrote the article; Y. Z. conducted the study and performed the sample analyses; W.-B. W. interpreted findings and prepared the manuscript; T.-H. S. and X.-F. W. carried our collections and analytical determinations; P.-P. Y. assisted in the maintenance and killing of birds; X.-L. L. contributed to the design and planning of the study and discussed the manuscript.
The authors declare that there are no conflicts of interest.














