Type 2 diabetes is characterised by deranged metabolism and inappropriate hyperglycaemia, resulting from defects in the secretion and cellular action of insulin. Treatments aimed at enhancing β-cell function and reducing insulin resistance are therefore key to improving metabolic control and retarding the development of diabetic complications. At least 250 million individuals worldwide suffer diabetes and it is estimated that by 2030 this number will double(1). Diabetes is one of the top five most significant diseases in the developed world. Based on WHO recommendations, anti-diabetic agents of plant origin are important for use in traditional medicine(2). Man has long turned to plants as a source of readily available and innovative medicines(Reference Day, Bailey and Flatt3). It is estimated that at least 75 % of the world's population relies significantly on plant medicines(Reference Weragoda4). These considerations have led to a renaissance of nutritional, clinical and scientific interest in the potential of plant treatments from across the world for diabetes therapy(Reference Gray and Flatt5–Reference Hannan, Ali and Rokeya7). Recent studies have explored the insulinotropic effects of aqueous extracts of traditional plants on perfused pancreas and similar effects were observed by in vitro testing of clonal pancreatic β-cell lines(Reference Hannan, Marenah and Ali6, Reference Hannan, Ali and Rokeya7).
The fruit of Terminalia bellirica has been used in traditional medicine for the treatment of anaemia, asthma, cancer, colic, constipation, diarrhoea, dysuria, headache, hypertension, inflammation and rheumatism(Reference Duke, Bogenschutz-Godwin and Ducelliar8). Several studies have shown that the fruit contains termilignan, thannilignan, 7-hydroxy-3′,4′-(methylenedioxy) flavone, anolignan B, gallic acid, ellagic acid, β-sitosterol, arjungenin, belleric acid, bellericoside and cannogenol 3-O-β-d-galactopyranosyl-(1 → 4)-O-α-l-rhamnopyranoside(Reference Nandy, Podder and Sabu9–Reference Khan and Gilani10).
T. bellirica has many reported medicinal applications. The fruits of T. bellirica together with T. chebula and Embilica officinalis have been used traditionally for the treatment of diabetes and have been investigated for their anti-diabetic and antioxidant activities(Reference Sabu and Kuttan11). These studies have demonstrated that oral administration of a single dose of T. bellirica extract (100 mg/kg body weight) reduced blood glucose in normal and alloxan-treated (120 mg/kg) diabetic rats within 4 h. Furthermore, daily administration of extract for up to 11 d significantly reduced glucose from day 7 onwards(Reference Sabu and Kuttan11). Aqueous extracts of the bark or fruits of T. bellirica have also been used for a variety of ailments in humans, including diarrhoea, leucoderma, impotence, bronchitis, cold, cholera, respiratory tract infections, anaemia, haemorrhoids, eye infections, as a brain tonic and as a diuretic to remove kidney stones(Reference Chopra, Nayar and Chopra12–Reference Sharma, Chhangte and Dolui14).
The aim of the present study was to investigate the anti-diabetic actions of T. bellirica fruit extract on insulin secretion and glucose uptake at the cellular level. Furthermore, possible effects on protein glycation and starch digestion were examined in vitro.
Materials and methods
Plant material preparation
Dried fruits of T. bellirica (Gaertn.) Roxb. (family: Combretaceae) were procured from a commercial supplier in Delhi, and available in Europe (batch T/002080) from Top-Op (Foods) Ltd (Stanmore, Middlesex, UK). For in vitro work, a decoction was prepared by bringing 25 g/l of material to the boil in water. Once boiling, the suspension was removed from the heat and allowed to infuse over 15 min. The suspension was filtered (Whatman no. 1 filter paper) and the volume adjusted so the final concentration was 25 g/l. Samples, 1 ml, of the filtered plant solution were brought to dryness under vacuum (Savant Speed-vac; Savant Instrumentation, Inc., Farmingdale, NY, USA). Dried fractions were stored at − 20°C until required. Fractions were reconstituted in incubation buffer for subsequent experiments as required.
Insulin secretion
Insulin release was determined using monolayers of BRIN-BD11 clonal pancreatic cells(Reference McClenaghan, Barnett and Ah-Sing15). BRIN-BD11 cells were grown in Roswell Park Memorial Institute (RPMI) 1640 tissue culture medium containing 11·1 mm-glucose, 10 % fetal calf serum and antibiotics (50 000 IU penicillin–streptomycin/litre), and maintained at 37°C in an atmosphere of 5 % CO2 and 95 % air. At 24 h before acute experiments, cells were harvested and seeded in twenty-four-well plates at a density of 1·0 × 105 cells per well. Following overnight attachment, the culture medium was removed and cells were preincubated for 40 min at 37°C with 1 ml Krebs–Ringer bicarbonate buffer supplemented with 1·1 mm-glucose and 1 % bovine serum albumin. Subsequent test incubations were performed for 20 min at 5·6 mm-glucose using a similar buffer supplemented with the aqueous plant extract and the agents indicated in the figures. Samples were stored at − 20°C for subsequent insulin RIA(Reference Flatt and Bailey16). Cell viability was assessed after 20 min incubation using a modified neutral red assay as described previously(Reference Mathews, Flatt and Abdel-Wahab17).
Adipocyte differentiation and cellular glucose uptake
3T3-L1 fibroblasts obtained from the American Type Culture Collection (Manassas, VA, USA) were used to determine glucose uptake(Reference Frost and Lane18). Cells (passages 5–10) were seeded in twelve-well plates at a density of 1·0 × 105 cells per well, maintained at 37 ± 2°C with 5 % CO2 and fed every 2 d with Dulbecco's modified Eagle's medium supplemented with penicillin (50 U/ml), streptomycin (50 μl/ml) and fetal bovine serum (10 %, v/v). Adipocyte differentiation was initiated as described in detail elsewhere by the addition of insulin (1 μg/ml), 0·5 mm-3-isobutyl-1-methylxanthine and 0·25 μm-dexamethasone(Reference Mathews, Flatt and Abdel-Wahab17). Before acute tests, cells were incubated in serum-free Dulbecco's modified Eagle's medium for 2–3 h to establish basal glucose uptake. Cellular glucose uptake was determined for 15 min at 37°C using Krebs–Ringer bicarbonate buffer supplemented with 3H-labelled 2-deoxyglucose (18·5 kBq (0·5 μCi)/well), 50 mm-glucose, insulin and other test agents as indicated in the figures. Hexose uptake was terminated after 5 min by three rapid washes with ice-cold PBS, after which cells were detached by the addition of 0·1 % SDS and subsequently lysed. Scintillation fluid was added to solubilised cell suspensions and mixed thoroughly. Radioactivity was measured on a Wallac 1409 Scintillation Counter (Wallac, Turku, Finland).
Starch digestion
To assess in vitro starch digestion, 100 mg soluble starch (Sigma-Aldrich, St Louis, MO, USA) was dissolved in 3 ml distilled water in the absence and presence of plant extract or acarbose (50 μg/ml) (Bayer AG, Leverkusen, Germany) as a positive control. Then 40 μl of 0·01 % heat-stable α-amylase (from Bacillus leicheniformis; Sigma-Aldrich) was added. After incubation at 80°C for 20 min, the mixture was diluted to 10 ml and 1 ml was incubated with 2 ml of 0·1 m-sodium acetate buffer (pH 4·75) and 30 μl of 0·1 % amyloglucosidase from Rhizopus mold (Sigma-Aldrich) for 30 min at 60°C. Glucose released was measured on the Analox GM9 glucose analyser (Analox Instruments, London, UK).
Protein glycation
A simple in vitro system was employed to assess protein glycation based on the use of insulin as a model substrate(Reference O'Harte, Hørjup and Flatt19). In brief, 100 μl human insulin (1 mg/ml) was incubated in 10 mm-sodium phosphate buffer (pH 7·4) with 220 mm-d-glucose, plant extract or aminoguanidine (positive control) for 24 h. Sodium cyanoborohydride was added and the reaction was stopped by the addition of 0·5 m-acetic acid. Glycated and non-glycated insulin was separated and quantified using reversed-phase HPLC(Reference O'Harte, Hørjup and Flatt19).
Statistical analysis
All results are expressed as mean values with their standard errors for a given number of observations (n). Groups of data were compared statistically using the unpaired Student's t test. Results were considered significant if P < 0·05.
Results
Insulin secretion studies
Insulin release from BRIN-BD11 cells was increased significantly in a dose-dependent manner by the aqueous extract of T. bellirica over the concentration range (0·5–10 mg/ml) (Fig. 1). Furthermore, l-alanine (10 mm) was used as a positive control and it significantly enhanced insulin secretion at 5·6 mm-glucose (7·3 (sem 0·3) v. 1·2 (sem 0·2) ng/106 cells per 20 min (control); P < 0·001; n 8). Cell viability over 20 min incubations with the plant extract was unchanged at concentrations up to 5 mg/ml and was decreased by 10 % at 10 mg/ml (data not shown). The extract enhanced insulin secretion in the absence and presence of 16·7 mm-glucose. Both diazoxide (300 μm) and verapamil (50 μm) abolished T. bellirica (0·5 mg/ml)-induced insulin secretion (Fig. 2). Furthermore, the extract did not significantly increase insulin secretion in depolarised cells nor in cells stimulated with 200 μm-tolbutamide or 200 μm-glibenclamide (Fig. 2). In addition, the increase of the insulin release in depolarised cells (30 mm-KCl) was significantly less in the presence of the plant extract. The insulin-secretory activity of T. bellirica was abolished in the absence of Ca (Fig. 3).

Fig. 1 Effect of Terminalia bellirica extract (■) compared with 5·6 mm-glucose (control) (□) on insulin release. Values are means of eight separate observations, with standard errors represented by vertical bars. Mean value was significantly different from that of the control treatment: ** P < 0·01, *** P < 0·001.

Fig. 2 Modulation of Terminalia bellirica extract-induced insulin secretion by established stimulators and inhibitors of β-cell function. (□), Control; (■), T. bellirica (0·5 mg/ml); G5·6, 5·6 mm-glucose; G16·7, 16·7 mm-glucose. Values are means of eight separate observations, with standard errors represented by vertical bars. Mean value was significantly different from that of the G5·6 control in the presence or absence of the plant extract: * P < 0·05, ** P < 0·01, *** P < 0·001. Mean value was significantly different from that of the respective incubation in the absence of the plant extract: † P < 0·05, ††† P < 0·001. ‡‡‡ Mean value was significantly different from that of the 16·7 mm-glucose condition in the presence or absence of the plant extract (P < 0·001).

Fig. 3 Effects of Terminalia bellirica extract on insulin release in the presence of 1·28 mm-Ca2+ (□) and in the absence of Ca2+ (■). Values are means of eight separate observations, with standard errors represented by vertical bars. *** Mean value was significantly different from that of the control in the presence of 1·28 mm-Ca2+ only (P < 0·001). ††† Mean value was significantly different from that of the respective compound in the presence of 1·28 mm-Ca2+ (P < 0·001).
Insulin action
The aqueous extract of T. bellirica (1 mg/ml) enhanced the basal [3H]deoxyglucose uptake in a similar magnitude to 10− 9 m-insulin (P < 0·05; Fig. 4). The combined actions of the extract and insulin further exceeded the effects of either alone (P < 0·001; Fig. 4).
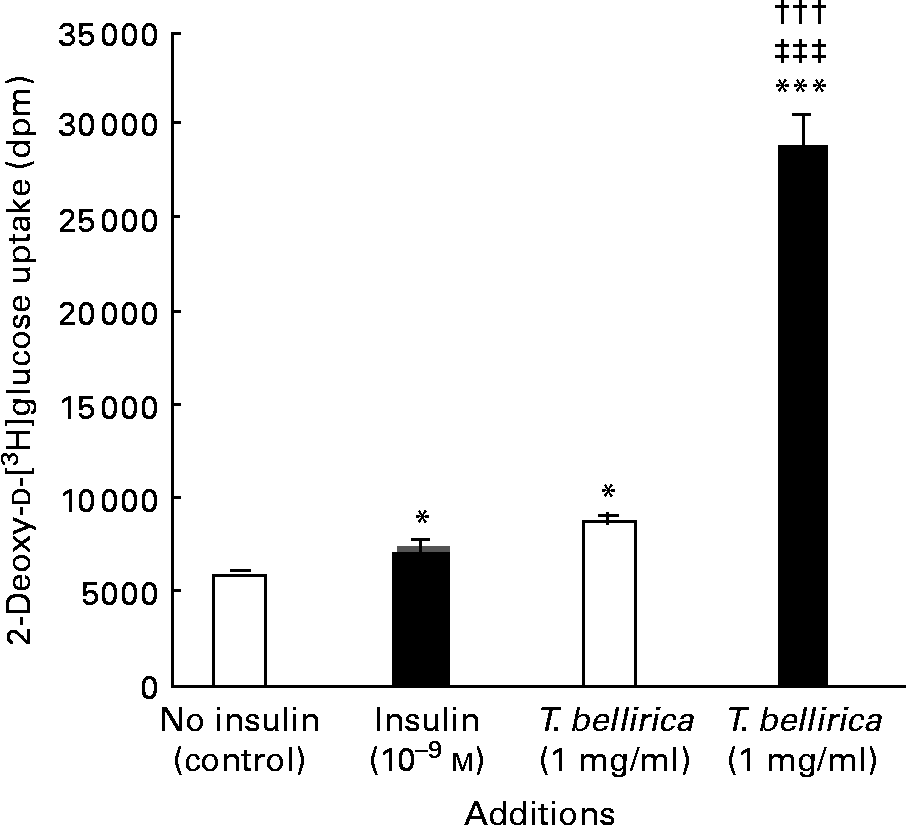
Fig. 4 Effects of Terminalia bellirica extract and the presence of 10− 9m-insulin (■) or the absence of insulin (□) on 2-deoxy-d-[3H]glucose transport. Values are means of four separate observations, with standard errors represented by vertical bars. Mean value was significantly different from that of the no-insulin (control) condition: * P < 0·05, *** P < 0·001. ††† Mean value was significantly different from that of the T. bellirica incubation without insulin (P < 0·001). ‡‡‡ Mean value was significantly different from that of the 10− 9 m-insulin condition (P < 0·001).
Starch digestion
Incubation with the aqueous extract (10–50 mg/ml) resulted in a 10–78 % decrease in enzymic liberation of glucose from starch (Fig. 5). Using acarbose (1 mg/ml) as a positive control, glucose liberation from starch was inhibited by 95 % (4·7 (sem 0·5) % glucose liberated compared with 99·6 (sem 1·6) % for control; P < 0·001).

Fig. 5 Effects of Terminalia bellirica extract on starch digestion. Values are means of three separate observations, with standard errors represented by vertical bars. *** Mean value was significantly different from that of the plant extract-absent condition (P < 0·001).
Glycation studies
The aqueous extract of T. bellirica exerted a partial concentration-dependent inhibition of insulin glycation (Fig. 6). At 25 and 50 mg/ml glycation was inhibited by 42–66 %. Aminoguanidine (50 mm) inhibited glycation by 75 % in this system.
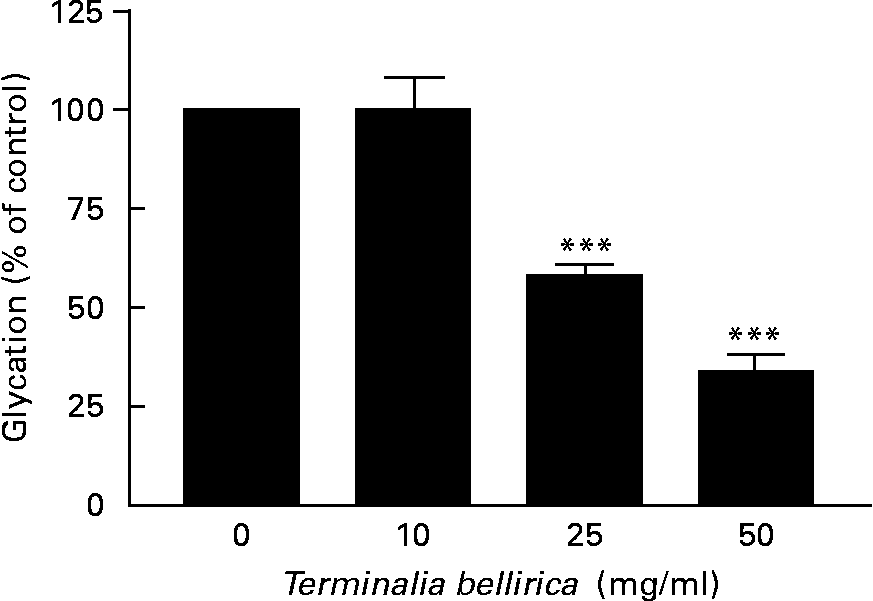
Fig. 6 Effects of Terminalia bellirica extract on protein glycation. Values are means of three separate observations, with standard errors represented by vertical bars. *** Mean value was significantly different from that of the plant extract-absent condition (P < 0·001).
Discussion
The aqueous extract of T. bellirica stimulated insulin secretion concentration-dependently from BRIN-BD11 cells at levels of 0·5 mg/ml and above. Cellular viability was not affected, confirming that the insulinotropic effect was not due to simple leakage of insulin from the cells. Depletion of extracellular Ca2+ or incubation with either verapamil (inhibitor of voltage-gated Ca2+ channels) or diazoxide (opener of K-ATP channels)(Reference Dunn and Peters20) inhibited the stimulatory effect, suggesting the importance of Ca2+ uptake in the mode of action of the plant constituents. Consistent with this view, the stimulatory effects of agents that indirectly depolarise the cell by inhibiting K-ATP channels (the sulfonylureas tolbutamide and gilbenclamide) were not affected by the T. bellirica extract. However, the aqueous extract of T. bellirica partially attenuated the elevation in insulin secretion induced by the direct effect of the membrane-depolarising agent KCl.
In addition to the prominent β-cell-stimulatory effects, T. bellirica enhanced cellular glucose transport in differentiated 3T3-L1 adipocytes. At 1 mg/ml, its stimulatory actions were similar to those of insulin (10− 9 m) alone. In addition, the combined actions of the extract and insulin exceeded the effects of either agent alone. The synergistic actions of insulin and T. bellirica extract on the enhancement of glucose uptake warrant further investigation to establish the dose–response relationship of these effects. Additional studies of the insulin-signalling pathway and effect of plant extract components are needed to assess the possible novelty of the mechanisms involved.
Using a simple in vitro test, consisting of the digestive enzymes α-amylase and α-glucosidase, the potential of T. bellirica to retard starch digestion was evaluated by its effect on glucose liberation. In this system, the established α-glucosidase inhibitor acarbose completely inhibited glucose liberation at the dose of 50 μg/ml. In contrast, the T. bellirica extract produced a significant 10–50 % reduction in starch digestion at concentrations of 10 mg/ml and above. Components of the extract responsible for this effect are unknown but it is noteworthy that several alkaloid compounds, including castonospermine from the seeds of Castanosperum australe, have established α-glucosidase-inhibitory action(Reference Day, Bailey and Flatt3). Moranoline (1-deoxynojirimycin), of which the therapeutic agent miglitol is a derivative, was originally isolated from mulberry root bark (Mori cortex)(Reference Yoshikuni21, Reference Kurihara, Fukami and Kusumoto22). Several hydroxyflavonoid compounds have also been isolated from marjoram leaves, each exhibiting glucosidase-inhibitory activity(Reference Kawabata, Mizuhatak and Sato23).
In the final series of experiments, the ability of the T. bellirica extract to inhibit protein glycation was assessed using insulin as a model substrate(Reference O'Harte, Hørjup and Flatt19). It is also potentially interesting that insulin is normally glycated in vivo and that glycated insulin has reduced biological activity(Reference Abdel-Wahab, Barnett and O'Harte24, Reference Hunter, Boyd and O'Harte25). This raises the possibility that inhibition of protein glycation may have benefits in addition to serving as a prophylactic for diabetic complications. In this simple system, the classical inhibitor aminoguandine decreased insulin glycation by 80 %. Similarly, T. bellirica inhibited glycation by 42–66 % at 25–50 mg/ml. The effective concentration is substantially greater than that needed to influence the secretion and action of insulin, and therefore is less likely to reflect an important element of the anti-diabetic properties of this plant.
In conclusion, the present study has shown that an aqueous extract of fruit of T. bellirica stimulated both the secretion and action of insulin as well as inhibited starch digestion and protein glycation. The ability of plant constituents to influence these parameters in vivo depends entirely on soluble active principle(s) being absorbed via the gut. Further work is required to assess this and to isolate and characterise the active components to bring forward potential new agents for diabetic therapy.
Acknowledgements
The present study was supported by the University of Ulster Research Strategy Funding.
All authors have contributed to the conception and design of the experiments. V. K. and Y. H. A. A. W contributed to the experimental research. P. R. F. and Y. H. A. A. W contributed equally to the supervision of the research, analysis and preparation of the paper.
There is no conflict of interest to declare by any of the authors.








