Inadequate nutrient intakes are common among children in low- and middle-income countries and have serious consequences for health, growth and development( Reference Ochola and Masibo 1 ). Micronutrient deficiencies in childhood have been found to impair growth, increase morbidity and mortality risk, reduce school achievement and decrease productivity in adulthood( Reference Caulfield, Richard and Rivera 2 ). Though substantial impairment of linear growth is known to occur among children under 2 years of age, height-for-age deficits continue to accrue if dietary intakes are insufficient to fuel catch-up or maintenance growth( Reference Victora, de Onis and Hallal 3 – Reference Friedman, Phillips-Howard and Mirel 5 ). Deficiencies in micronutrients, including Fe, Zn, vitamin B12 and folate, may impair brain development and cognitive function( Reference Khor and Misra 6 – Reference Gewa, Weiss and Bwibo 9 ). Children with low body mass are at increased risk for reduced muscular strength and work capacity, delays in maturation and lower bone density in adulthood( Reference Best, Neufingerl and van Geel 10 ).
Detailed dietary assessments are needed to develop strategies that prevent these compromises to child growth and development. However, there have been few recent studies of nutrient intakes or prevalence of intake inadequacy among early school-aged children in sub-Saharan Africa( Reference Ochola and Masibo 1 , Reference Vähätalo, Mikkilä and Räsänen 11 – Reference Onimawo, Ukegbu and Asumugha 17 ). Of the studies that have examined food and nutrient intakes in this population, most reported intakes observed during a single day or estimated a simple average of intakes over 2–3 d in a short period of time( Reference Vähätalo, Mikkilä and Räsänen 11 – Reference Semproli, Canducci and Ricci 14 , Reference Gewa, Murphy and Weiss 16 , Reference Onimawo, Ukegbu and Asumugha 17 ). Children’s dietary intakes in sub-Saharan Africa can vary by season and therefore repeat 24-h recalls or weighed dietary records across multiple seasons are crucial for accurately measuring usual intakes( Reference Mitchikpe, Dossa and Ategbo 15 , Reference Ferguson, Gibson and Opare-Obisaw 18 , Reference Hotz, Chileshe and Siamusantu 19 ). Repeat diet assessments are also needed to accurately estimate usual nutrient intakes by accounting for day-to-day variation( Reference Vähätalo, Mikkilä and Räsänen 11 – Reference Semproli, Canducci and Ricci 14 ). Estimation of usual intakes enables more accurate estimates of the prevalence of inadequacy( Reference Dodd, Guenther and Freedman 20 ). Finally, few of the studies used the probability approach recommended by the Institute of Medicine (IOM) (National Academies of Sciences, USA) for estimating prevalence of intake inadequacy( Reference Gewa, Murphy and Weiss 16 , 21 ).
This study described food and nutrient intakes and risk of intake inadequacy among 4–8-year-old children in rural Zambia. The objectives of the study were to describe usual diets and nutrient intakes of 4–8-year-old Zambian children over a period of 6 months; to estimate the likelihood of intake adequacy of fourteen nutrients; and to describe major sources of each nutrient.
Methods
Data for this study were collected as part of an efficacy trial of pro-vitamin A carotenoid biofortified maize, described by Palmer et al.( Reference Palmer, Siamusantu and Chileshe 22 ) This research was carried out in Mkushi District, Central Province, Zambia, a rural district with a mix of small-holder and large-scale farming. Children aged 4–8 years and not yet attending school were enrolled into geographic clusters of fifteen to thirty children. Eligible children were identified by a door-to-door census conducted in all towns and villages in northern Mkushi with sufficient estimated population density to form clusters with a diameter of ≤1 km. The biofortified maize trial enrolled 1226 children. Clusters were assigned by block randomisation to a treatment group receiving meals containing biofortified maize (twenty-five clusters), a control group receiving meals containing traditional, unfortified maize (twenty-five clusters) or a non-intervened group (fourteen clusters). The non-intervened group formed the observational arm of the trial, enabling assessment of usual child diet for comparison with the diets and nutritional status of the children assigned to receive meals. Children in the non-intervened group were not provided any food during the study; their families received a food package equivalent to the intervention’s food value at the end of the 6-month trial. This secondary analysis was restricted to evaluating dietary patterns among children in the non-intervened group of the efficacy trial (n 202). Children in the non-intervened group did not differ significantly from children in the other two groups by age, sex, education of the head of household or household ownership of durable goods (data not shown).
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Institutional Review Board at the Johns Hopkins Bloomberg School of Public Health (Baltimore, USA) and the Ethics Review Committee of the Tropical Disease Research Centre (Ndola, Zambia). Verbal informed consent was obtained from every child’s primary caregiver. Verbal consent was witnessed and formally recorded.
Data were collected on a monthly basis over the 6-month efficacy trial (August 2012–April 2013), yielding a total of seven survey rounds over three agro-ecological seasons (late post-harvest, early lean and late lean seasons). Household socio-economic and demographic data were collected by questionnaire administered to the primary caregiver of the enrolled child during the baseline survey. Child height and weight were measured at baseline using a portable stadiometer (ShorrBoard; Weigh and Measure LLC) and flat scale (Model 874; Seca). Height-for-age, weight-for-age and BMI-for-age were calculated using the WHO growth standards( 23 , 24 ). Dietary intake data were collected in all seven monthly survey rounds, using a tablet-based 24-h recall described by Caswell et al.( Reference Caswell, Talegawkar and Dyer 25 ) Data were not collected on Sundays due to high rates of religious service attendance in this population. Therefore, the 24-h recall data reflect dietary intakes on 6 d of the week, excluding Saturdays. Clusters were randomly assigned to a day of week for the first survey round and subsequent scheduling ensured that each cluster was visited at least once on all 6 d of the week over the following survey rounds.
The primary caregiver, accompanied by the child, was asked to recall all foods and drinks the child consumed between waking the previous day and waking the day of the interview. For most foods, the respondent was asked to describe the amount the child consumed by indicating the closest match among five portions of a similar food shown in a photo booklet. For other foods, the respondent was asked to report the number of food units the child consumed. The tablet tool used a modified multiple-pass method, first prompting the respondent to describe foods as they were consumed sequentially through the day, with time-of-day specific prompts to aid recall. In the second pass, a picture chart memory aid was used to check for missed foods. In the final pass, the interviewer asked the respondent to review the child’s day, probing for any occasions on which the child may have consumed foods not yet recalled. All food description details – including detailed description, added ingredients, portion size and where the child obtained the food – were collected for each recalled food in the first pass the food was recalled.
All data analysis was performed using SAS 9.4. Simple descriptive statistics were used to report distributions of household-level descriptors and child age and sex.
Observed nutrient intakes
Observed nutrient intakes were calculated from the 24-h recall data using a standard database of recipes and a food composition table of local foods, as described previously( Reference Caswell, Talegawkar and Dyer 25 ). We used a database of standard local recipes developed for a 2009 survey of dietary intakes among women and children under 5 years of age in rural Zambia (HarvestPlus, unpublished results). Where needed, we modified recipes by removing ingredients or substituting similar main ingredients, retaining relative proportions of the other ingredients. Additional recipe information was collected using focus groups with local women. We compiled a local food composition table based primarily on a food composition table developed by HarvestPlus in their 2009 survey (HarvestPlus, unpublished results), adding food composition data from the Zambia Food Composition Tables published by the Zambian National Food and Nutrition Commission, the US Department of Agriculture National Nutrient Database for Standard Reference and several other regional and international food composition tables( 26 – Reference Hotz, Lubowa and Sison 31 ). Portion weights were estimated based on the weight of food in the portion size photograph selected by the respondent and a density adjustment, or by a weight per unit for foods recorded by number of units consumed. Weights of ingredients in mixed foods were calculated by multiplying the portion weight by each ingredient’s fraction in the standard recipe. Nutrient contents of foods were calculated by multiplying ingredient or unmixed food weights by nutrient contents from the food composition table, and the nutrient contents of all foods reported in the 24-h recall were summed to estimate observed intake of each nutrient.
Usual nutrient intake distributions
Because nutrient intakes can vary widely from day to day, even where diets are monotonous, intake on a single day or averaged over 2–3 d may yield an inaccurate estimate of usual intake( Reference Dodd, Guenther and Freedman 20 ). Therefore our objective in this analysis was to describe usual, daily intake over time, rather than intake on a single day or simple average over several days. Day-to-day variation inflates the variance of the intake distribution, creating bias in estimates of the prevalence of inadequacy. Appropriate statistical models can reduce the excess variance, thereby describing the distribution of usual nutrient intakes( Reference Dodd, Guenther and Freedman 20 ).
We estimated the usual nutrient intake distributions of energy and 13 macro- and micronutrients using the National Cancer Institute (NCI) methods described by Tooze et al. ( Reference Tooze, Kipnis and Buckman 32 ) for estimating intake distributions of foods or nutrients consumed on a daily basis. Because vitamin B12 is found only in fortified or animal source foods which are consumed infrequently in this population, the observed intake of vitamin B12 was zero on many of the recall days in our study. Therefore we used an expansion of the NCI method for estimating usual intakes of vitamin B12 as a function of both amount consumed and probability of consumption( Reference Tooze, Midthune and Dodd 33 ). To estimate distributions of the percent of energy from protein, carbohydrates and fat, we used the bivariate approach described by Freedman et al.( Reference Freedman, Guenther and Dodd 34 ) We used SAS macros provided by NCI for running the estimation procedures( 35 ).
We estimated usual intake distributions over all included children and by age group (under 5 years v. 5 years or older). We also examined usual intake distributions by sex and did not find meaningful differences (data not shown). In these and subsequent food and nutrient intake analyses, we used the observed intake data from all recalls for which the included child was not reported ill on the day covered by the dietary recall interview. When performing the usual intake distribution estimation procedures, we controlled for whether the day covered by the recall was a market day because exploratory analyses indicated a trend toward lower intakes of energy and nutrients on market days. In the overall distribution, we also controlled for age and sex.
Probability of inadequacy
We calculated probability of inadequacy overall and by age group using the usual intake distributions produced by the NCI method. For most micronutrients with normal requirement distributions, we used the estimated average requirement (EAR) and CV published by the IOM to calculate the probability of inadequacy( Reference Otten, Hellwig and Meyers 36 ). For Zn, we used the EAR and CV for diets with low Zn bioavailability provided by the International Zinc Nutrition Consultative Group (iZiNCG)( 37 ). For each percentile of the usual nutrient intake distribution, we calculated the probability of inadequacy as the percent of the requirement distribution falling to the right of the percentile’s median intake (SAS PROBNORM statement). The probabilities of inadequacy were averaged over all percentiles to arrive at the probability of inadequacy for each nutrient. To calculate probability of inadequacy of Fe, we adjusted the percentile values of the Fe requirement distribution published by the IOM to reflect the 10 % Fe bioavailability of a high phytate, low meat diet estimated by the World Health Organization( Reference Otten, Hellwig and Meyers 36 , 38 ). The probability of inadequacy for each percentile of the usual intake distribution was assigned as the average of the requirement distribution percentiles above and below the percentile’s median intake. For example, an intake of 8·88 mg Fe was assigned a probability of inadequacy of 35 % because it falls between the 60th and 70th percentiles of the requirement distribution. To describe adequacy of macronutrient intakes, we calculated the percent of children with intakes above and below the Acceptable Macronutrient Distribution Ranges (AMDR) recommended by the Institute of Medicine( Reference Otten, Hellwig and Meyers 36 ).
Food consumption patterns and contribution to nutrient intakes
To describe food consumption patterns and contributions to nutrient intakes, we grouped unmixed foods, drinks and disaggregated ingredients of mixed dishes into fifty-two food types. To determine the food types used in this analysis, we first classified foods by major food groups (vegetables, fruits, meats, etc.). Within each major food group, commonly consumed foods were retained as individual food types, and infrequently consumed foods were aggregated by similarity. For example, among vegetables, tomatoes are frequently consumed so were assigned their own food type code, but green beans are infrequently consumed so were assigned to the aggregate food type for other non-leafy vegetables. The number of times a child consumed each food type during each 24-h recall was counted and averaged over all included recalls to describe the average number of times the food type was consumed per day. The average quantity consumed, in grams dry weight, was calculated as the average serving size among all instances of a food type being consumed.
For energy and each nutrient, we calculated the percent of total intake provided by each of the fifty-two types of foods. We first summed the contents of all foods of a given type consumed in each 24-h recall. This sum was divided by the total observed intake from the same 24-h recall to obtain the percent of total intake provided by each food. These percentages were averaged across all included 24-h recalls to describe the usual sources of each nutrient.
Results
Of the 202 children in the initial sample, two children were excluded because they only completed one round of dietary data collection and were reported ill in that round, resulting in a sample size for these analyses of 200 children. The total number of children per round for whom a recall was completed was 200 at baseline, and 182, 179, 172, 181, 175 and 190 in five sequential monitoring surveys and final follow-up, respectively. The number of children reported ill was highest in the fourth monitoring survey (January 2013, fifty-four ill children), and ranged from 17 to 38 in the other six survey rounds. The total number of included recalls per survey round ranged from 125 to 177. All estimates of dietary intake relate to apparently healthy days of children based on caregiver’s report that the child was not ill.
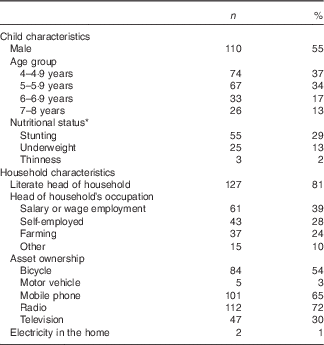
The mean age at baseline of children included in this analysis was 5·5 (sd 1·2) years. Child and household characteristics are presented in Table 1.
Table 1 Baseline characteristics of children (n 200) and households (n 157) participating in the non-intervened arm of a biofortified maize efficacy trial, Mkushi, Zambia, 2012 (Numbers and percentages)
Median usual energy intake was 6422 kJ/d (1535 kcal/d) (Table 2). Children under five had lower energy intakes than children 5 years and older. Older children had higher intakes of protein and fat, and slightly higher carbohydrate intakes, than younger children. Most micronutrient intakes were similar across age groups, with the exceptions of Fe and vitamin C, which were higher among older children than among younger children.
Table 2 Usual nutrient intakes over 6 months and prevalence of nutrient intake inadequacy overall and by age, among 4- to 8-year-old children (n 200), participating in the non-intervened arm of a biofortified maize efficacy trial, Mkushi, Zambia, 2012–2013 (Medians and 25th to 75th percentiles)
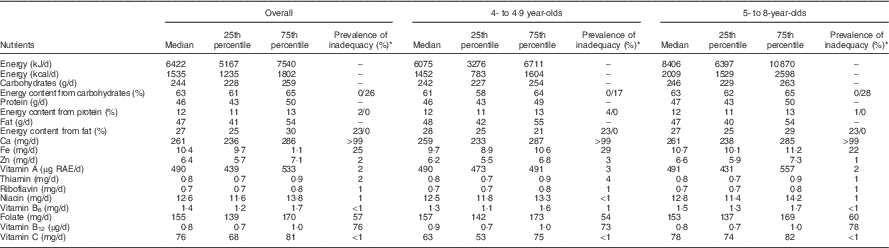
RAE, retinol activity equivalent.
* For carbohydrates, protein and fat, prevalence of inadequacy is shown as percent under/over the Acceptable Macronutrient Distribution Range.
Children were at highest risk of inadequate intakes for Fe, folate, vitamin B12 and Ca (Table 2). The estimated prevalence of inadequate Ca intake was nearly 100 %. The estimated prevalence of inadequate intakes of Fe, folate and vitamin B12 was 25, 57 and 76, respectively. The prevalence of inadequate intake was <3 % for Zn, vitamin A, thiamin, riboflavin, niacin and vitamin B6 and vitamin C. Estimates of prevalence of inadequacy were similar between the two age groups, except for folate and vitamin B12, which were slightly higher among older children than among younger children, and for Fe, which was higher among younger children. The percentage of children falling outside the AMDR for protein and fat showed little difference by age group. Children under five were less likely to have high carbohydrate intakes than older children.
Vegetable oil, tomatoes and maize were each consumed an average of three times/d, and main ingredients in common side dishes, such as rape leaves, small fish, pumpkin leaves and beans, were consumed 0·3–0·6 times/d, on average (Table 3). Foods consumed in the largest quantities included maize, mango, fritters or scones, bread, other fruits and cassava. Maize was the main contributor to intakes of energy, protein, carbohydrates, Fe, Zn and most of the B vitamins (Table 4). Commonly consumed side dish ingredients were important sources of nutrients. Vegetable oil accounted for 51 % of fat intakes. Small fish were the main source Ca and vitamin B12, and were among the top five contributors to intakes of energy, protein, fat and all micronutrients except vitamin C and folate. Rape leaves were an important source of vitamin A, vitamin C and Ca in the diets of children.
Table 3 Number of servings per day and quantity consumed per serving of twenty-five most frequently consumed foods among 4- to 8-year-old children participating in the non-intervened arm of a biofortified maize efficacy trial (1071 observation days among 200 children), Mkushi, Zambia, 2012–2013 (Mean values and standard deviations)
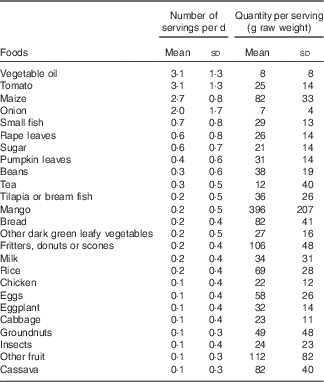
Table 4 Foods contributing to energy and nutrient intakesFootnote * among 4- to 8-year-old children (n 200) participating in the non-intervened arm of a biofortified maize efficacy trial, Mkushi, Zambia, 2012–2013
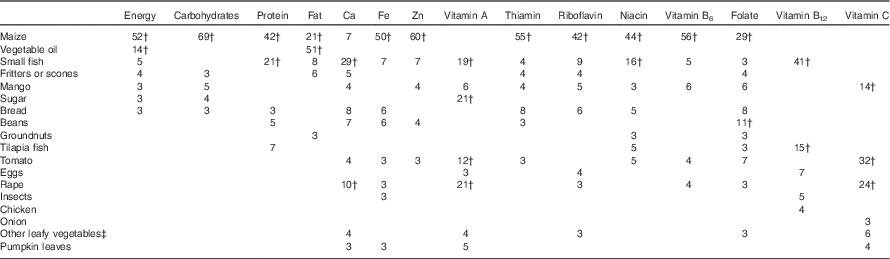
* Only foods contributing, on average, at least 3 % of total intakes are shown.
† Foods contributing 10 % or more of total intakes.
‡ Any leafy vegetables other than rape leaves, cabbage or pumpkin.
Discussion
Using dietary recalls collected over 6 months, we have described the usual nutrient intakes of apparently healthy rural Zambian children, identified Ca, Fe, folate and vitamin B12 as nutrients with high prevalence of inadequate intake and described key foods and food sources of each nutrient.
The median energy intakes in this study population were similar to those reported for children of the same age in Mexico and in Kenya and exceeded the energy requirements estimated by the Food and Agriculture Organization and World Health Organization( Reference Mwaniki and Makokha 13 , Reference Gewa, Murphy and Weiss 16 , Reference Flores, Macias and Rivera 39 – 41 ). Our estimate of usual energy intakes among 4–4·9-year-old children in Mkushi district, Zambia, 6075 kJ/d (1452 kcal/d), is slightly lower than the usual energy intake of 6389 kJ/d (1527 kcal/d) reported for 4–5-year-old children in Mkushi and Nyimba districts of Zambia in 2009( Reference Hotz, Palaniappan and Chileshe 42 ). We also observed higher protein and fat intakes than those reported for preschool children in the 2009 survey( Reference Hotz, Chileshe and Siamusantu 19 ). These differences may be attributable to differences between study sites, ages of included children or seasonal timing of data collection( Reference Hotz, Chileshe and Siamusantu 19 , Reference Hotz, Palaniappan and Chileshe 42 ). Though one quarter of children in this study had high carbohydrate intakes, nearly all children consumed fat and protein within the AMDR( Reference Otten, Hellwig and Meyers 36 ). Risk of inadequate energy intake in this study population appears low, though additional factors such as high prevalence of parasitic infection and high physical activity levels may increase energy requirements above those cited( 41 ). The rate of thinness in this population was very low, supporting a conclusion that energy intakes are adequate. Total protein intakes also appear adequate, though protein quality may be limited.
Though macronutrient intakes appear adequate, we found that children in this population are at high risk of inadequate Ca and vitamin B12 intakes, with additional risks of Fe and folate inadequacy. These results are largely corroborated by other dietary surveys conducted in the region. In the previous survey of 4–5-year-olds in Mkushi and Nyimba districts of Zambia, rates of Ca and Zn inadequacy were similar to what we observed, Fe inadequacy was more prevalent, and rates of vitamin B12 and folate inadequacy were lower but still of concern. Similar probability of inadequate Fe intake and lower but still substantial risks of Ca, vitamin B12 and folate inadequacy were reported among rural Kenyan first graders, and low Ca intakes were found among 5–8-year-old children in another study in rural Kenya( Reference Semproli, Canducci and Ricci 14 , Reference Gewa, Murphy and Weiss 16 ). Risks of inadequate Ca and folate intakes, and a lesser risk of Fe inadequacy have been reported for 1–9-year-old South African children( Reference Steyn, Nel and Labadarios 12 ).
Other dietary studies among school-aged children in this region reported risks of inadequate intakes that we did not observe. Higher risks of inadequate Zn, vitamin A, vitamin C or potassium intakes were found among rural Kenyan children, and risk of inadequate niacin intake was reported for South African children( Reference Steyn, Nel and Labadarios 12 , Reference Semproli, Canducci and Ricci 14 , Reference Gewa, Murphy and Weiss 16 ). The discrepancies observed between these studies and ours may be due to differences in context, age group or methods. Zambia has a national policy requiring fortification of sugar with vitamin A. Though children consumed sugar less than once per day, sugar contributed 21 % of total vitamin A intakes, on average, and may help explain why we found low prevalence of vitamin A intake inadequacy. Most of the previous studies used single-day or averaged intakes rather than modelling usual nutrient intakes( Reference Steyn, Nel and Labadarios 12 , Reference Semproli, Canducci and Ricci 14 , Reference Gewa, Murphy and Weiss 16 ). Two used the Recommended Nutrient Intakes from the WHO rather than the Dietary Reference Intakes from the IOM used here( Reference Steyn, Nel and Labadarios 12 , Reference Semproli, Canducci and Ricci 14 ). Finally, different methods and assumptions were used when accounting for the bioavailability of Fe and Zn( Reference Steyn, Nel and Labadarios 12 , Reference Semproli, Canducci and Ricci 14 , Reference Gewa, Murphy and Weiss 16 , Reference Hotz, Palaniappan and Chileshe 42 ). The choice of requirement based on bioavailability assumptions can have a marked impact on estimated prevalence of inadequacy( Reference Hotz, Palaniappan and Chileshe 42 ). That inadequate Ca intakes emerge as a consistent problem despite varying methods suggests that this is likely a substantial public health nutrition problem among children across the region.
Our findings of inadequacy of Fe, folate, vitamin B12 and Ca intakes in this population are consistent with findings from nutritional biomarker and dietary supply studies. A recent review estimated a 29 % prevalence of Fe deficiency among African school-aged children. Of the 58 % prevalence of anaemia among Zambian children under five, about 20 % would respond to Fe supplementation( Reference Best, Neufingerl and van Geel 10 , 43 ). In rural Kenya, 38 % of primary school children in a school-based feeding trial had low plasma vitamin B12, though only 1 % had low plasma folate( Reference McLean, Allen and Neumann 44 ). A nationally representative survey in Cameroon reported that 8 % of preschool-aged children had low plasma folate and 30 % had low plasma vitamin B12 Reference Shahab-Ferdows, Engle-Stone and Hampel (45 ). However, using dietary assessment, the same study found that 39 % of non-breast-feeding preschool-aged children had inadequate folate intakes, which more closely matches our findings( Reference Shahab-Ferdows, Engle-Stone and Hampel 45 ). Using national food supply and demographic data, the estimated risk of Ca deficiency is 80 % across Africa and 100 % in Zambia( Reference Kumssa, Joy and Ander 46 ).
Inadequate intakes of vitamin B12, Fe and folate imply serious risks to the children in this population. Insufficiencies of these nutrients are associated with risk of impaired cognitive function, and with anaemia and its consequences( Reference Nyaradi, Li and Hickling 7 , Reference Balarajan, Ramakrishnan and Özaltin 47 ). Deficiencies of vitamin B12 and folate during gestation and childhood have been associated with lasting deficits in cognitive development, and micronutrient supplementation trials among school-aged children have shown positive effects on memory( Reference Khor and Misra 6 , Reference Nyaradi, Li and Hickling 7 , Reference Louwman, van Dusseldorp and van de Vijver 48 ). In a school-based feeding trial in rural Kenya, children with higher Fe intakes had greater increases in problem-solving scores and children with higher vitamin B12 intakes had greater increases in scores on a test of attention and recall( Reference Gewa, Weiss and Bwibo 9 ). Deficiencies of Fe, vitamin B12 and folate are among the nutritional causes of anaemia, which can cause fatigue, cognitive impairment and reduced work capacity( Reference Balarajan, Ramakrishnan and Özaltin 47 ). The observed inadequate intakes of Ca may compromise bone growth and accrual of bone mineral density, particularly if Ca inadequacy extends into puberty( Reference Ross, Taylor and Yaktine 49 ).
The risks of micronutrient inadequacy we observed in this population may be attributed to the monotonous, predominantly plant-based diet. Maize contributed over half of total energy content consumed by 4–8-year-old, rural Zambian children and most of the foods consumed frequently and in largest quantities were plant foods. Previous studies among school-aged children in low- and middle-income countries have similarly shown that their diets include high consumption of cereals and starchy roots and tubers and limited consumption of animal source foods( Reference Ochola and Masibo 1 , Reference Ferguson, Gibson and Opare-Obisaw 18 ). Despite this finding, several foods that were occasionally consumed are good sources of the nutrients for which kids are most deficient. Small, whole fish are a good source of Ca, Fe and vitamin B12 and were consumed at a rate of 0·66 servings/d. They were among the top sources of energy, protein and fat, and several micronutrients, including vitamin B12 and Ca. However, they were consumed in small portions, averaging 29 g raw weight per serving. Similarly, beans, groundnuts and dark green leafy vegetables are good sources of folate that were commonly consumed but in small portions.
Because nutrient-rich vegetables, beans and fish are widely available but underutilised, dietary diversification programmes, including nutrition education and programmes to improve household access to nutrient-rich foods, should be evaluated for impact and feasibility in rural Zambia. Fe- and Zn-biofortified beans, which have been shown to improve Fe status among Rwandan women, are now available in Zambia (E Simpungwe, HarvestPlus, unpublished results), and expanded access could provide needed Fe in children’s diets( Reference Haas, Luna and Lung’aho 50 ). Ca remains a significant concern as the amount of available in Zambia’s national food supply is insufficient to meet the dietary requirements of its population, so dietary diversification strategies alone will not be sufficient for reducing the prevalence of Ca inadequacy( Reference Kumssa, Joy and Ander 46 , Reference Joy, Ander and Young 51 ). Given the importance of maize in the diet, fortification of maize with Ca has been suggested as a strategy for decreasing the nation-wide risk of Ca inadequacy in Zambia( Reference Kumssa, Joy and Ander 46 ). Maize fortification with other micronutrients is also being consider by policymakers( Reference Fiedler, Afidra and Mugambi 52 ). However, rural Zambians commonly consume maize milled at small-scale, local mills, making coordination of an effective national fortification policy logistically and financially challenging( Reference Fiedler, Afidra and Mugambi 52 ). Despite the challenges to maize fortification, the importance of sugar as a contributor to total vitamin A intakes shows the positive role fortification policy can have in filling gaps in diets that might otherwise have higher risk of inadequacy. Point-of-use fortification with multiple micronutrient sprinkles, shown to reduce anaemia, Fe deficiency and vitamin A deficiency among children under two and preschoolers, could also be considered for school-aged children( Reference De-Regil, Suchdev and Vist 53 – Reference Suchdev, Ruth and Woodruff 56 ).
Our study has several important strengths. We collected 24-h dietary recall data on a monthly basis over 6 months, yielding up to seven recalls per child and capturing data on dietary intakes over three agricultural seasons. Further, we used these repeat measures to estimate usual nutrient intake distributions before assessing probability of inadequacy. Modelling usual intake distributions reduces the variance inflation due to day-to-day changes in intake, thereby reducing bias in the estimated prevalence of inadequacy. These strengths distinguish this study from previous assessments of diet among school-aged children in sub-Saharan Africa. However, our data do not cover the full year, and dietary intakes in the harvest season might have influenced our estimates had they been available. Other potential sources of error in our intake estimates include the use of portion size photos in the 24-h recall and use of standardised local recipes. An additional limitation is that our study area and sampling design were chosen for a food-based intervention rather than a nationally representative survey. We describe diet and nutrient intakes among apparently healthy children, which may affect comparison of our results to other dietary surveys. We did not have sufficient data on amino acid composition to incorporate protein quality in our description of protein adequacy. Finally, though we used Fe and Zn requirements set by the IOM and iZiNCG to reflect low bioavailability diets, we may still be underestimating the prevalence of inadequacy of these nutrients, if Fe and Zn bioavailability from the rural Zambian diet are lower than assumed.
The heavily plant-based diet of 4–8-year-old rural Zambian children places them at risk for anaemia, impaired cognitive development and reduced bone growth due to deficiencies of Fe, folate, vitamin B12 and Ca. Foods providing these nutrients, such as small, whole fish, beans and leafy vegetables, are consumed infrequently and in small quantities. Further research into strategies to improve the year-round availability, affordability and provision of micronutrient-rich foods to children in this population is urgently needed to safeguard their health, growth and development.
Acknowledgements
The authors thank Christopher Chibuye, Rose Mwanza, Ngosa Molobeka, and Edward Mupotola in Mkushi District for their support and acknowledge the contributions of Brian Dyer and Rolf Klemm at Johns Hopkins University on the dietary recall methodology. The authors thank Fabiana Moura, Mourad Moursi and Abdelrahman Lubowa at HarvestPlus for providing local food composition and standard recipe tables. Finally, the authors thank the children and families of Mkushi, Zambia who participated in this study.
This work was funded by HarvestPlus, with support from the United Kingdom Department for International Development, and Global Affairs Canada. Additional support was provided by the Sight and Life Global Nutrition Research Institute at Johns Hopkins University. None of the funders had a role in the design, analysis or writing of this article.
B. L. C. contributed to conception and design, conducted the dietary and statistical analyses, interpreted results and drafted the manuscript. S. A. T. assisted with dietary and statistical analyses, contributed to analysis and interpretation, and edited and critically revised the manuscript. W. S. managed field work and critically revised the manuscript. K. P. W. provided scientific guidance, contributed to conception and design, contributed to analysis and interpretation and critically revised the manuscript. A. C. P. designed the efficacy trial protocol, managed field work, contributed to analysis and interpretation and edited and critically revised the manuscript.
None of the authors has any conflicts of interest to declare.






