Vitamin B12 (B12) is a generic term for all cobalamins biologically active in humans. It is a water-soluble vitamin( Reference Watanabe 1 , Reference Kozyraki and Cases 2 ), synthesised exclusively by micro-organisms( Reference Fang, Kang and Zhang 3 ) and its main sources are animal products( Reference Wong 4 ). Gastrointestinal absorption of B12 is complex( Reference Watanabe 1 ) and requires intact function of stomach, pancreas and terminal ileum( Reference Kozyraki and Cases 2 , Reference Wong 4 ). After absorption, B12 is converted into two coenzymes, adenosyl and methylcobalamin( Reference Guéant and Alpers 5 , Reference Giedyk, Goliszewska and Gryko 6 ). Adenosylcobalamin participates in the conversion of methylmalonyl-CoA to succinyl-CoA in mitochondria while methylcobalamin participates in the re-methylation of homocysteine to methionine( Reference Hannibal and Blom 7 ) in the cytoplasm. The recommended intake of B12 is 2·4 μg daily( 8 ) for adults and elders, and the British Committee of Hematology Standards suggests that serum levels of B12 < 148 pmol/l (200 pg/ml) would be sensitive enough to diagnose 97 % of individuals with B12 deficiency( Reference Hunt, Harrington and Robinson 9 , Reference Devalia, Hamilton and Molloy 10 ).
The main causes of B12 deficiency are vegan or vegetarian diet, diet poor in meat and dairy products, total or partial gastrectomy, ileum resection, use of metformin, drugs that block stomach acid and others( Reference Stabler 11 ). B12 deficiency is characterised by haematological and neurological effects, and manifestations range from mild to severe, including glossitis, fatigue, macrocytic anaemia and peripheral neuropathy. Furthermore, B12 deficiency is strongly related to hyperhomocysteinaemia, a great risk factor for CVD( Reference Hunt, Harrington and Robinson 9 , Reference Stabler 11 – Reference Ganguly and Alam 13 ).
An association between decreased serum levels of B12 and obesity has been observed in the general population by some authors( Reference Guven, Inanc and Kilinc 14 – Reference Guarnizo-Poma, Urrunaga-Pastor and Montero-Suyo 21 ). However, to date, no study has evaluated the association of serum B12 levels with excessive body weight using a ‘gold standard’ method to evaluate body adiposity such as dual-energy X-ray absorptiometry (DXA).
Kidney transplantation (KT) is the treatment of choice for most end-stage renal disease patients( 22 ). The goal in the management of kidney transplant recipients (KTR) is to avoid the risk of adverse events and comorbidities that may lead to graft failure or death, in particular obesity, infections, cancer and CVD( Reference Cimino and Snyder 23 – Reference Chan, Bosch and Jones 25 ). Diarrhoea is a common finding in KTR and can be related to the use of immunosuppressive drugs( Reference Aulagnon, Scemla and DeWolf 26 , Reference Shin and Chandraker 27 ), as the gastrointestinal tract is involved in the metabolism of several of these drugs, and adverse gastrointestinal events, occurring in more than 80 % of patients after KT, are attributed to its use( Reference Ekberg, Kyllonen and Madsen 28 ). Among the immunosuppressive drugs, mycophenolate mofetil (MMF) is extensively hydrolysed to mycophenolic acid by esterases in the stomach, small intestine, blood, liver and other tissues. There is evidence that patients using MMF frequently experience diarrhoea (from 15·6 to 32·5 %)( Reference Keown, Hayry and Mathew 29 ), which is associated with villous atrophy in the duodenum and erosive inflammation in the ileum( Reference Kamar, Faure and Dupuis 30 , Reference Maes, Dalle and Geboes 31 ), conditions that could favour B12 malabsorption.
Although B12 deficiency may favour CVD( Reference Ganguly and Alam 13 ), an important cause of mortality in KTR( Reference Jardine, Gaston and Fellstrom 32 ), at the present moment data about the prevalence of B12 deficiency in these patients are scarce. We can hypothesise that these patients may be at increased risk of B12 deficiency due to the recommendation to decrease the intake of animal protein before KT (during non-dialysis treatment)( Reference Wang, Kalantar-Zadeh and Fouque 33 ); the excessive weight gain that is common in KTR( Reference Chan, Bosch and Jones 25 ); and the use of MMF.
Therefore, the aim of the present study was to evaluate the prevalence of B12 deficiency in KTR and its association with B12 dietary intake, body adiposity and immunosuppressive regimen based on MMF.
Methods
This cross-sectional study followed the guidelines laid down in the Declaration of Helsinki, and all procedures were approved by the Committee on Ethics and Research of Pedro Ernesto University Hospital (CAAE: 38268714.0.0000.5259). Written informed consent was obtained from all participants.
We enrolled, prospectively, 368 unselected adults (18–70 years) with functional kidney grafts >180 d after transplantation. Exclusion criteria were actual or former use of B12 supplements, AIDS, cancer, autoimmune diseases, acute illness, amputation, liver failure, mental disorders and pregnant or lactating women.
Individuals who met the eligibility criteria and agreed to take part in the study were submitted to clinical, laboratory and nutritional evaluations. Data collected in the medical record included date of KT, type of graft donor and current use of immunosuppressive drugs. Blood sampling as well as the anthropometric measurements were taken from 07.00 to 09.00 hours after 12 h fasting and were performed within the period of 30 d in which dietary intake was evaluated. KTR were asked about lifestyle habits and the occurrence of diarrhoea on appropriate interviews. Patients who smoked at least one cigarette daily or those that stopped smoking within the previous 6 months were considered smokers. KTR who reported consumption of alcoholic beverages one or more times in the week were considered alcohol consumers. The habitual physical activity was evaluated by the Baecke questionnaire which assesses the physical activity in three subscales: at work, sports during leisure time and other physical activities during leisure time( Reference Baecke, Burema and Frijters 34 , Reference Florindo and Latorre 35 ).
Dietary intake
B12 dietary intake was assessed by three interviewer-administered 24 h dietary recalls (two weekdays and one weekend day). The first two 24 h recalls were obtained face-to-face and the last one through a telephone call. The 24 h recalls interval between the first and the last was 30 d.
The 24 h recalls were obtained by two dietitians trained to ask the patients to enumerate all the information about the food and drink they had consumed from midnight to midnight in the previous day, including the quantity.
Nutrient analysis of the 24 h recalls was performed using the software Food Processor Plus® (ESHA Research) and two Brazilian Food Composition Tables( 36 , 37 ). An average of the three recalls was used in the analysis. Adequate intake of B12 was considered when ≥2·4 μg/d( 8 ).
Laboratory parameters
The serum level of B12 was determined by chemiluminescent microparticle intrinsic factor assay using a commercial kit (Abbott) at the Laboratory of Nuclear Medicine at Pedro Ernesto University Hospital. This assay is designed to have a total CV ≤ 11 % for concentrations in the range of the low, medium and high controls.
Blood samples were also analysed to measure creatinine, urea, Hb, haematocrit, mean corpuscular volume, mean corpuscular Hb and mean corpuscular Hb concentration. These analyses were performed at the Pedro Ernesto University Hospital’s central laboratory. Serum urea and creatinine were determined by kinetic method. Creatinine was calibrated to IDMS: COBAS 6000 (Roche/Hitachi). The estimated glomerular filtration rate was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation( 38 ).
Anthropometric measurements
The anthropometric measurements were taken twice by two experienced dietitians, and mean values were used. The height was measured using a stadiometer, accurate to ±0·5 cm and the weight was obtained with a digital scale, accurate to ±0·1 kg (Filizola S.A.), with the patients wearing light clothing with no shoes and after emptying the bladder. The measure of the waist circumference (WC) was taken in the standing position midway between the lowest rib and the iliac crest, at mid-exhalation while the hip circumference was measured at the widest point over the hip/buttocks area with the tape parallel to the floor( 39 ). The neck circumference was measured in the midway of the neck between mid-cervical spine and mid-anterior neck, if palpable, just below the laryngeal prominence( Reference Onat, Hergenç and Yüksel 40 ).
BMI was calculated using the standard equation (kg/m2)( 41 ). Body adiposity index estimates body fat (%) using two anthropometric measurements (hip circumference and height) and was determined as described by Bergman et al. ( Reference Bergman, Stefanovski and Buchanan 42 ).
Dual-energy X-ray absorptiometry
The DXA procedure was performed by a trained technician using a GE Medical SystemsLunar® (Madison) with the patient in the supine position. The DXA system performs rectilinear scans over the length of the body. The scan begins at the top of the patient’s head and moves downward towards the feet. The program allows scanning up to 205 lines. During the scan, the source shutter opens to emit an X-ray beam. The software calculates fat mass, lean tissue and bone mineral mass. Fat-free mass is calculated as the sum of lean tissue plus bone mineral mass. Body composition was evaluated in total body and different sites, such as trunk. Visceral adipose tissue was estimated with the software CoreScan VAT( Reference Kaul, Rothney and Peters 43 ).
Immunosuppressive treatments
At the renal transplant outpatient clinic at Pedro Ernesto University Hospital, the most frequently used immunosuppressive regimens for long-term follow-up include a calcineurin inhibitor (cyclosporine or tacrolimus) or a mammalian target of rapamycin inhibitor (everolimus or sirolimus) in addition to an antimetabolite (MMF or azathioprine: AZA). All treatment regimens are administered in combination with steroids (prednisone 5 mg/d).
Statistical analysis
Sample size was determined based on a pilot study conducted by our group in which B12 deficiency was registered in 20 % of KTR( Reference Costa, Silva and Rosina 44 ). Then, considering that the number of KT outpatients in our service is 450, and a 95 % CI, the minimum sample size should be 160 patients.
A standard statistical package (STATA software, version 12.0; StataCorp) was used to perform statistics analysis. Normality was tested by the Shapiro–Wilk normality test. Continuous variables with normal distribution were expressed as mean values and standard deviations. Medians and interquartile ranges (IQR) were used to summarise variables with non-normal distribution. The individuals were stratified into two groups according to the levels of B12. KTR with values <200 pg/ml were allocated to the B12-deficient group and those with values ≥200 pg/ml to the B12-sufficient group( Reference Watanabe 1 ). The two groups were compared with the use of Student’s t test, Mann–Whitney test or χ2 exact test, as appropriate. The multiple logistic regression analysis was performed to assess the association of B12 deficiency with body adiposity. The accepted level of statistical significance was 5 %.
Results
A total of 368 KTR were interviewed, of which 227 met the eligibility criteria and agreed to participate in the study. Of these, 225 completed all evaluations and were included in statistical analyses. Mean age was 47·50 (sd 12·11) years (range 18–70 years), and 125 (56 %) were men. Mean transplant duration was 110·24 (sd 89·83) months (range 6–331 months), and mean B12 levels were 362·57 (sd 169·25) pg/ml (range 83–1042 pg/ml). The prevalence of B12 deficiency was 14 %.
The characteristics of the participants are shown in Table 1 according to B12 status. Mean corpuscular volume and mean corpuscular Hb were significantly higher among patients with B12 deficiency. The frequency of diarrhoea and MMF use was higher (without reaching statistical significance) in B12-deficient when compared with the B12-sufficient KTR (Table 1).
Table 1. Demographic and clinical characteristics and laboratory parameters of kidney transplant recipients according to vitamin B12 status
(Absolute values and percentages; medians and interquartile ranges (IQR); mean values and standard deviations)
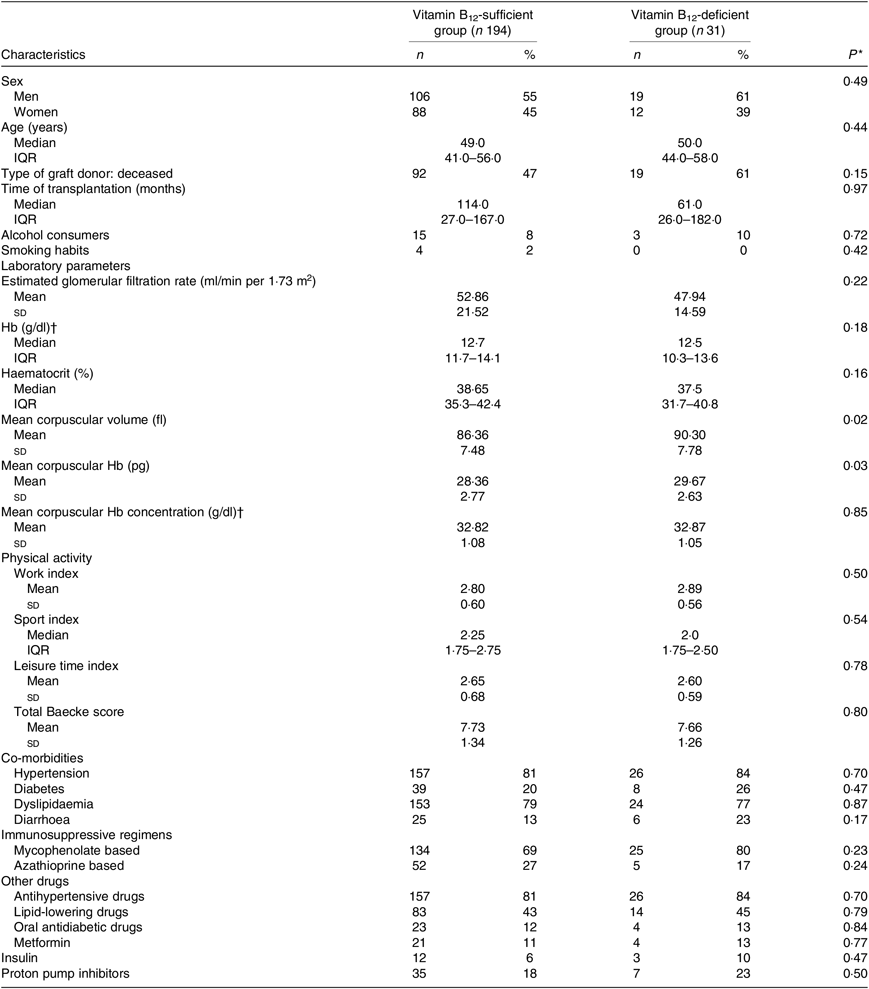
* Vitamin B12-sufficient group v. vitamin B12-deficient group (Student’s t test or Mann–Whitney test).
† To convert Hb and mean corpuscular Hb concentration in g/dl to g/l, multiply by 10.
The intake of B12 was significantly lower in participants with B12 deficiency (Table 2). B12 intake was considered adequate in 143 KTR (64 %) and sixteen patients out of them exhibited B12 deficiency.
Table 2. Dietary intake according to vitamin B12 status in kidney transplant recipients
(Medians and interquartile ranges (IQR))
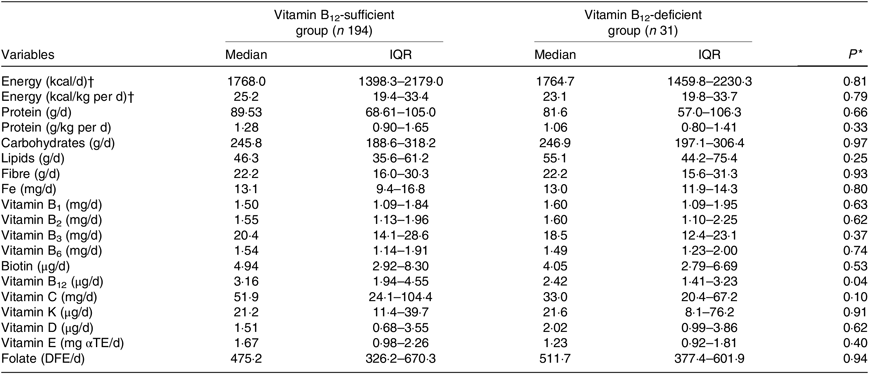
αTE, α-tocopherol equivalents; DFE, dietary folate equivalents.
* Vitamin B12-sufficient group v. vitamin B12-deficient group (Student’s t test or Mann–Whitney test).
† To convert energy in kcal to kJ, multiply by 4·184.
Considering participants of both sexes, individuals who had B12 deficiency exhibited higher values of WC. In the analysis conducted only in women, it was verified that those with B12 deficiency exhibited values of BMI, body adiposity index, WC, total body fat (% and kg) and trunk body fat (% and kg) significantly higher than women with B12 sufficiency (Table 3) even after adjustment for age, estimated glomerular filtration rate, time from transplantation, type of graft donor and physical activity (Table 4).
Table 3. Parameters of body adiposity according to vitamin B12 status in kidney transplant recipients
(Medians and interquartile ranges (IQR); mean values and standard deviations)
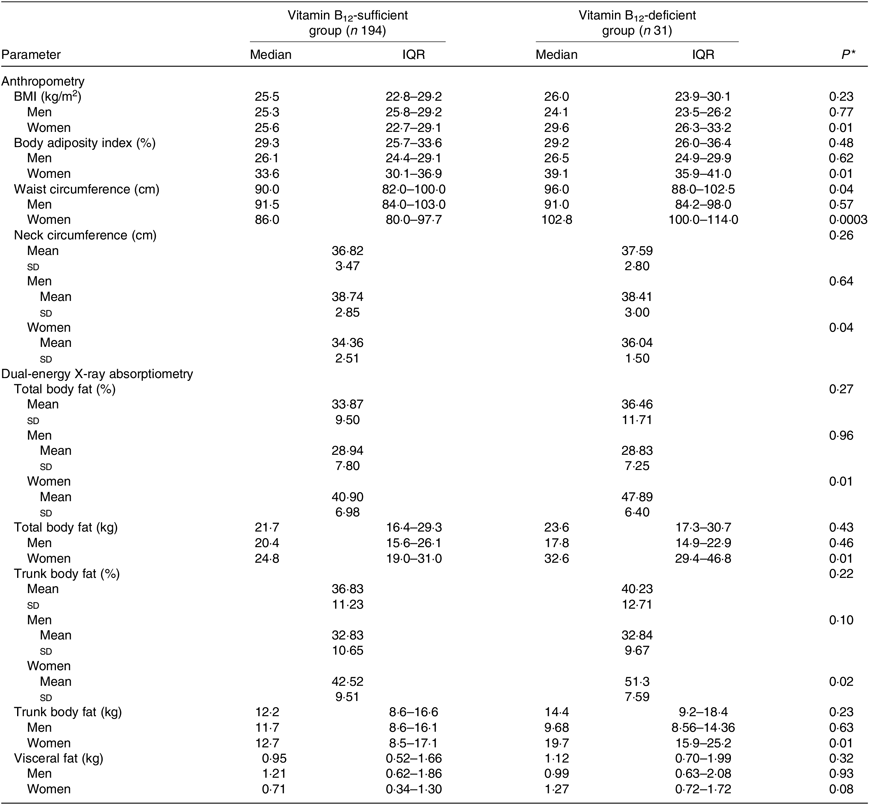
* Vitamin B12-sufficient group v. vitamin B12-deficient group (Student’s t test or Mann–Whitney test).
Table 4. Risk for vitamin B12 deficiency according to parameters of body adiposity in kidney transplant recipients
(Odds ratios and 95 % confidence intervals)
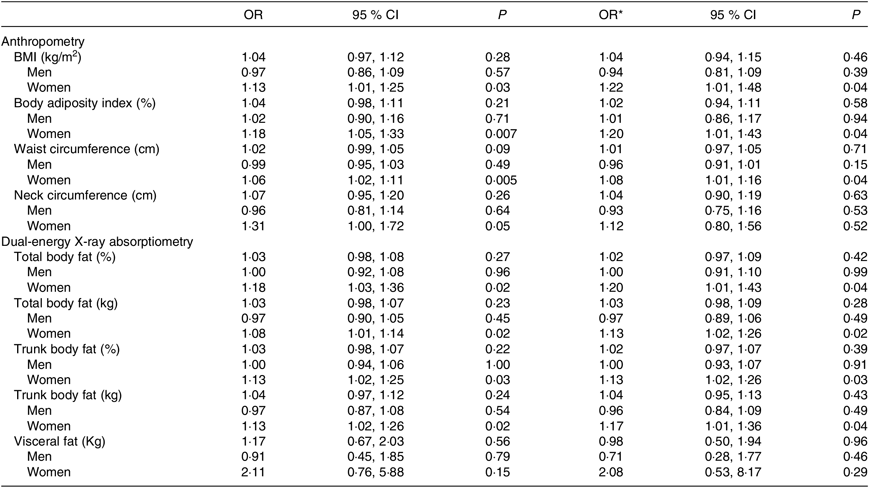
* Adjusted for age, estimated glomerular filtration rate, time from transplantation, type of graft donor and total Baecke score.
The frequency of B12 deficiency was not significantly higher in KTR using MMF (16 %) v. AZA (9 %) (P = 0·25) in the analysis considering all the participants included in the present study. However, in the analysis restricted to individuals who had adequate intake of B12 there was a significantly higher frequency of B12 deficiency in patients using MMF (17 %) v. AZA (2 %) (P = 0·01).
Discussion
To our knowledge, three studies have described the occurrence of B12 deficiency in KTR( Reference Födinger, Buchmayer and Heinz 45 – Reference Scott, Rogers and Weiner 47 ). Födinger et al. ( Reference Födinger, Buchmayer and Heinz 45 ) reported B12 deficiency in 8·9 % of 733 Austrians KTR using <160 pg/ml as the cut-off to determine the vitamin deficiency. Karakus et al. ( Reference Karakuş, Kanbay and Köseoglu 46 ) evaluating ninety KTR, who developed anaemia, found B12 deficiency in 40 % of the individuals with macrocytic and in 8·3 % of those with normocytic anaemia. Scott et al. ( Reference Scott, Rogers and Weiner 47 ) registered less than 1 % of both B12 and folic acid deficiency in 584 North American and Canadian KTR using the cut-off <200 pg/ml. In the present study, including KTR with a minimum of 6 months of transplantation, B12 deficiency prevalence (cut-off < 200 pg/ml) was 14 %.
Some authors have evaluated the dietary intake in KTR( Reference Heaf, Jakobsen and Tvedegaard 48 – Reference Osté, Corpeleijn and Navis 50 ), but only one study evaluated the intake of B12 ( Reference Heaf, Jakobsen and Tvedegaard 48 ), that was considered adequate in 94 % of the patients. In the present study, we found that a lower percentage of the participants (64 %) presented adequate B12 intake. Furthermore, the intake of protein presented a tendency to be lower in the B12-deficient group (Table 2) and was significantly lower in patients with inadequate v. adequate intake of B12 (median 71·5 (IQR 52·1–91·8) v. 94·4 (IQR 81·6–115·0) g/d, respectively, P < 0·0001) (data not shown). These findings suggest that B12 dietary intake needs to be carefully monitored in KTR, especially in those with lower protein intake.
We checked the within-subject consistency of dietary intake evaluated through the three 24 h recalls (data not shown). The dietary intake was similar for the same participant when we considered the two weekday recalls. However, as expected, the dietary intake evaluated through these two recalls differed from that assessed through the weekend day recall. The recalls obtained by telephone call compared with those obtained by face-to-face interviews did not present systematic difference. We believe that the use of trained interviewers contributed to the lack of difference. As described in the literature( Reference Posner, Borman and Morgan 51 , Reference Brustad, Skeie and Braaten 52 ), the dietary intake estimated through 24 h recalls collected by phone is comparable to the dietary intake that is collected face-to-face.
Although some studies have described the association between increased body adiposity and B12 deficiency in the general population( Reference Guven, Inanc and Kilinc 14 – Reference Allin, Friedrich and Pietzner 19 , Reference Guarnizo-Poma, Urrunaga-Pastor and Montero-Suyo 21 ), to our knowledge, the present study is the first to register this association in KTR. We observed that B12 deficiency was associated with increased central body adiposity (evaluated by WC) in the analysis including the whole cohort of KTR and with increased total and central body adiposity in the analysis including only women (considering both the anthropometric measures and DXA).
The mechanism by which individuals with excessive adiposity may exhibit decreased levels of B12 has not been completely elucidated. Guéant & Alpers( Reference Guéant and Alpers 5 ) and Garcia et al. ( Reference Garcia, Guéant-Rodriguez and Pooya 53 ) using an animal model demonstrated that the deficiency of methyl radical and other cofactors, such as B12, impairs the oxidation of the fatty acids, increases the stress on the endoplasmic reticulum and decreases the expression of sirtuin 1, a protein which plays a key role in molecular mechanisms of obesity. Li et al. ( Reference Li, Gueant-Rodriguez and Quilliot 20 ) proposed that the impaired conversion of methylmalonic acid (MMA) to succinyl-CoA, a B12-dependent process, may be associated with MMA accumulation, which could lead to an increase in lipogenesis, in addition to insulin resistance. In a study that included both in vivo and in vitro observations, Adaikalakoteswari et al.( Reference Adaikalakoteswari, Vatish and Alam 18 ) suggested that the B12 plays a role in epigenetic regulation by altering circulating microRNA during adipocyte differentiation that results in adipogenesis and adverse metabolic phenotype.
Since in the present study, a percentage of the individuals with adequate B12 intake presented B12 deficiency, we tested if immunosuppressive regimen including MMF was associated with the deficiency of B12 in these participants. We observed that in KTR with adequate B12 intake, the frequency of B12 deficiency was higher in patients using MMF than in those using AZA. This finding suggests that KTR using MMF may be at increased risk of B12 deficiency even if they present adequate B12 intake. However, in our opinion, additional studies are necessary to confirm this finding.
The evaluation of B12 status might include not only serum B12 but also markers of cellular B12, such as homocysteine and MMA( Reference Harrington 54 ). However, in individuals with renal dysfunction, these markers may be falsely increased, while the evaluation of serum B12 is not altered( Reference Harrington 54 – Reference Valente, Scott and Ueland 57 ). Considering that in the present study 68 % of the participants presented an estimated glomerular filtration rate <60 ml/min, we used solely serum B12 to evaluate B12 status.
The strengths of the present study include the evaluation of body adiposity with a ‘gold standard’ method (DXA). This is the first study to evaluate the prevalence of B12 deficiency in KTR and to observe an association between B12 deficiency with body adiposity and the use of MMF. As limitation, it is worth mentioning that the present study was conducted in only one centre and has a cross-sectional design not allowing to infer cause-and-effect relationships.
Conclusion
In conclusion, the prevalence of B12 deficiency in KTR was estimated as 14 % and was associated with reduced dietary intake of B12 as well as with higher adiposity, especially in women, and with the use of MMF in individuals with adequate B12 intake.
Acknowledgements
The authors express their sincere gratitude to Maria de Lourdes Guimarães Rodrigues, Débora Cristina Torres Valença, Bernardo Barreto da Silva Gaspar, Stephanie Giannini, Jessica Veiga Pires and Elisama de Moura Rodrigues Leite.
The present study was supported by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
K. S. S. P. contributed to the study conception and design; data collection, assembly, analysis and interpretation; manuscript drafting; and the approval of the final version of the manuscript.
M. R. S. T. K. contributed to the study conception and design; data collection, assembly, analysis and interpretation; manuscript drafting; and the approval of the final version of the manuscript.
M. S. C. contributed to the study conception and design; data collection, assembly, analysis and interpretation; manuscript drafting; and the approval of the final version of the manuscript.
K. T. C. R. contributed to data collection, assembly, analysis and interpretation; manuscript drafting; and the approval of the final version of the manuscript.
A. P. M. M. B. contributed to data collection, assembly, analysis and interpretation; manuscript drafting; and the approval of the final version of the manuscript.
M. I. B. S. contributed to the study conception and design; data analysis and interpretation; manuscript drafting; and the approval of the final version of the manuscript.
S. S. R. contributed to the study conception and design; data collection, assembly, analysis and interpretation; manuscript drafting; and the approval of the final version of the manuscript.
There were no conflicts of interest.







