Scurvy is the clinical manifestation of severe vitamin C deficiency. Hippocrates (460 BC–370 BC) described scurvy in the ancient Greek army in men with leg pain and bleeding gums with gangrene(Reference Ralli and Sherry1). It is estimated that more than 2 million sailors died of scurvy between 1500 and 1800 AD, with common final causes of death including infection, bleeding and fractures.
Lind’s treatise on scurvy in 1753 described treating six pairs (twelve subjects) with potential remedies. Five pairs were given treatment with vinegar, cider, elixir of vitriol, mustard and garlic purges, or drinking two pints of seawater daily. These five treatments were ineffective. One pair received oranges and lemons and only these two subjects recovered(Reference Lind2). Vitamin C itself was not discovered until 1933, and Albert von Szent-Györgyi received the 1937 Nobel prize for this and other work.
In current times, vitamin C deficiency and scurvy are usually presumed to be rare in the absence of famine or eating disorders. However, we reported a case series of people with diabetes and poorly healing lower limb ulcers(Reference Christie-David and Gunton3) in whom there was prompt ulcer healing with vitamin C replacement. A report published during the period in which our study was conducted identified a 59 % rate of vitamin C deficiency in a high-risk foot ulcer clinic(Reference Brookes, Jaya and Tran4).
Foot ulceration can be defined as erosion of tissue or a breach in skin below the ankle. Conditions that increase the risk of foot ulceration include diabetes, peripheral vascular disease, any disease associated with sensory peripheral neuropathy and conditions affecting foot structure or architecture. Foot deformity, past foot ulcers and amputation are the most significant risk factors for future ulcers(Reference Boyko, Ahroni and Stensel5). These ulcers are often complicated by infection, including osteomyelitis, and/or impaired blood supply (vascular disease). Chronic foot ulcers carry a high risk of amputation.
Vitamin C is required for collagen formation and for proper function of the immune system to decrease and control infections. We hypothesised that vitamin C may improve foot ulcer healing and tested this with a randomised, double-blind, glucosamine-placebo-controlled trial of vitamin C supplementation in people attending the High-Risk Foot Clinic (now called Foot Wound Clinic) at a tertiary referral hospital.
Methods
This was a pragmatic, investigator-initiated, randomised, double-blind, inactive-placebo-controlled trial. No commercial support was provided for the trial, which was funded by a grant from the Research and Education Network of Westmead Hospital. Ethics approval was given by the Human Research Ethics Committee of Western Sydney Local Health District.
Patients
People were eligible to participate if they were adults who presented as a new patient to the High-Risk Foot Clinic at Westmead Hospital and had a current foot ulcer. No patients required exclusion for healing between booking their first clinic visit and attending. Other exclusion criteria were inability to give informed consent for cognitive issues (n 3 excluded) or language issues (0), and a decision being made at that first visit that the subject would proceed to amputation (n 1). One additional patient was excluded because their serum vitamin C level had been measured before clinic presentation and they were already being treated with vitamin C supplements. Two patients declined to consent after learning that a blood test was involved. Patient flow is detailed in the consort diagram (Fig. 3).
After signing informed consent, subjects completed a brief dietary survey and underwent a venipuncture for measurement of serum vitamin C. They were then randomised to vitamin C, 500 mg daily in a slow-release capsule, or the inactive comparator which was identical-appearing glucosamine sulphate capsules (1000 mg). The vitamin C was a commercial product made by Blackmores purchased from the local pharmacy. The slow-release formulation was chosen because it is safer in people with renal impairment, giving lower risk of oxaluria and also, because it is not flavoured/chewable as most vitamin C formulations are, it was easier to obtain a matching appearing control. Glucosamine was a commercial product made by BioOrganics also purchased from the local pharmacy. Neither Blackmores nor BioOrganics had any role in the trial design, funding, analysis or preparation of this report. These medications were delivered to the trials pharmacy at Westmead Hospital for dispensing.
Patients were recruited from January 2018 to March 2019. Numbers of subjects were estimated using PowerStat (Vanderbilt), α 0·05, power 0·8, assuming a 25 % sd in ulcer healing and a 40 % improvement in ulcer size with vitamin C. This recommended seven patients per group. The trial was stopped at sixteen subjects.
Randomisation and treatment
Randomisation was carried out by computerised random number generation (Excel), and the clinical trials pharmacists dispensed 4 weeks of the assigned medication. Patients were instructed to take one tablet daily and continue normal clinical attendance and all usual care as determined by the High-Risk Foot Clinic Team. Only the clinic trials pharmacist had access to the treatment assignment information, all other staff and patients were blinded.
At our institution, the serum vitamin C result takes approximately 4 weeks to return. The vitamin C result was available to the treating team in the clinic from that time. When the first 28 d of medication was completed, if the patient was vitamin C deficient, they were dispensed both vitamin C and glucosamine tablets by the clinical trials pharmacist for the second 28 d. If the baseline serum vitamin C was normal, they continued their original medication for a further 28 d. This was done so that all deficient people were offered treatment with vitamin C while original treatment assignment remained blinded. As stated above, all other care was provided as ‘usual care’ at the clinic; there were no other study interventions.
Outcome measures
The primary endpoint of the study was percentage ulcer healing at 8 weeks (percentage reduction compared with initial ulcer volume). Ulcer size was estimated using a silhouette 3D camera, or if unavailable (e.g. at home visits), by measuring ulcer dimensions. Ulcers were presumed to be ‘punched out’ at uniform depth for this purpose. If ulcers became larger, percentage ulcer healing was negative. The closest visit to 8 weeks was used for the primary endpoint. This occurred at a median of 63 d for the glucosamine group and 55 d for the vitamin C group. This time point was chosen based on clinic experience and data indicating that early heal is a good predictor of final healing(Reference Sheehan, Jones and Caselli6,Reference Sheehan, Jones and Giurini7) . People who underwent amputations before 8 weeks (n 2) had the ulcer size at the date of amputation used for their 8-week value for the primary endpoint.
Complete ulcer healing was considered to have occurred when the epithelium was intact (i.e. 100 % healing with no ongoing drainage). People who underwent amputations were considered to not have healed ulcers. Secondary outcomes included time to 50 % ulcer healing, time to complete ulcer healing and rate of healing of ulcers. Data were collected for amputation rates, which were a pre-specified secondary outcome; although when we planned the study, we considered that it was not powered to assess this outcome.
Serum vitamin C measurement
The blood test request forms included instructions to wrap samples in foil and place on ice immediately. Serum vitamin C was measured following precipitation of proteins, followed by separation on a reverse-phase HPLC column (lab-packed LiChrosorb RP-18 (5 micron) 150 × 3·0 mm) and measurement using an amperometric (BAS) electrochemical detector. Intra-assay CV at a vitamin C level of 45 μmol/l is 6·9 % and at 87 μmol/l is 6·5 %. The detection limit is 5 μmol/l, and the assay is linear to at least 200 μmol/l. Normal range is 40–100 μmol/l. People with undetectable levels returned a result of <5 and 4 μmol/l was used for analysis.
Statistical analysis
SPSS version 21 or GraphPad Prism version 8 were used for analysis. Analysis was by intention to treat. Where people had amputations, their ulcer was considered to not have healed and the ulcer size prior to amputation was used. Where values were not normally distributed (e.g. percentage ulcer healing) as indicated by skewness values >1 (calculated in SPSS), non-parametric testing was used. Two tailed P-values were used. A P-value of <0·05 was considered statistically significant.
Results
Demographic and baseline characteristics of the trial subjects are shown in Table 1. Nine people were randomised to control and seven to vitamin C. Four subjects in each group had known vascular disease, and four subjects in each group had diabetes mellitus. All subjects had at least one of vascular disease, diabetes, neuropathy or deformed foot architecture. There was a wide age range, with the younger adult patients (18–44, n 3) all having long-standing type 1 diabetes.
Table 1. Baseline characteristics of trial subjects*
(Mean values and standard deviations; median values and 95 % confidence intervals)
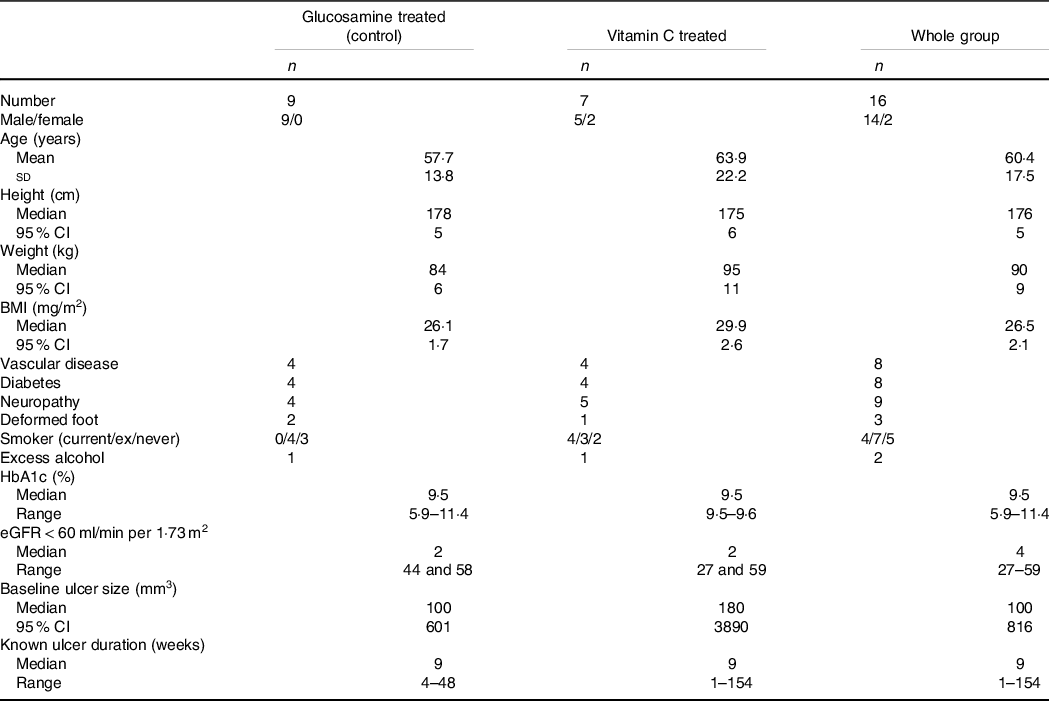
* Excess alcohol was considered ≥2 standard drinks per d. Those two people reported consuming ≥4 standard drinks daily. HbA1c is reported for people with known diabetes.
Baseline ulcer size was non-significantly larger in the people assigned to vitamin C and was not normally distributed (Fig. 1(a), note log-scale for y-axis). Baseline vitamin C levels were not measured in two people as they did not attend the venipuncture service after consenting to participate in the study, and being randomised (one control, one vitamin C) and having medications dispensed. They were considered to be vitamin C sufficient for intention-to-treat purposes. Median vitamin C in the study population was 30·5 μmol/l (interquartile range 4–52). Eight of the sixteen subjects were vitamin C deficient, with four subjects having undetectable levels (Fig. 1(b)). Four people in each treatment group had baseline deficiency. The shaded grey box indicates the normal range.
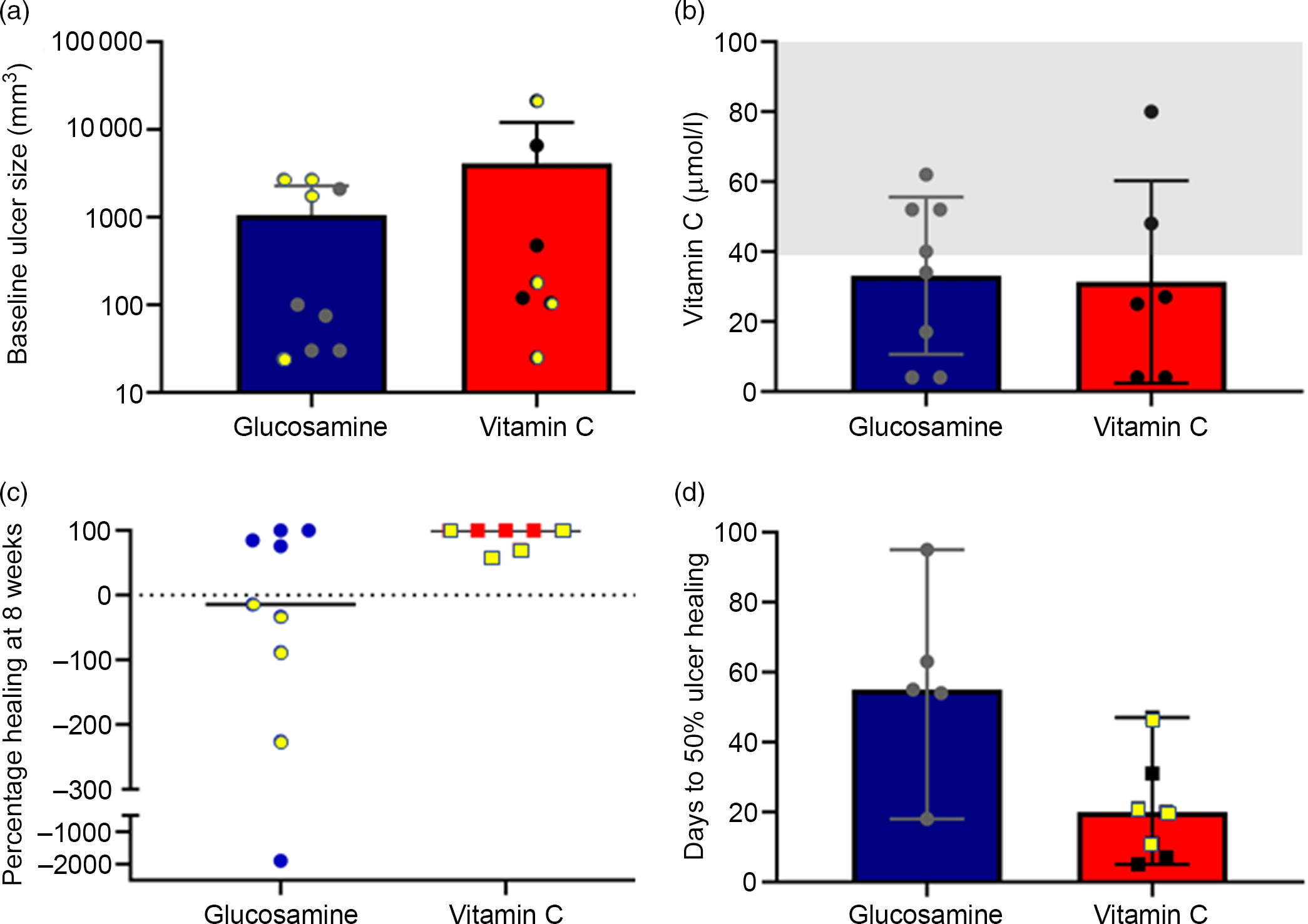
Fig. 1. Baseline ulcer and vitamin C, and healing of ulcers. (a) Baseline ulcer size. Note log-scale for y-axis. Individual values are shown. Symbols with yellow centres indicate people with vitamin C deficiency. (b) Baseline vitamin C levels. The shaded area indicates the normal range. (c) Percentage healing at 8 weeks (% reduction in ulcer volume). 100 % indicates complete healing. Negative values indicate enlarged wounds compared with baseline. Symbols with yellow centres indicate people with baseline vitamin C deficiency. (d) Days from baseline visit to 50 % reduction in ulcer volume. Symbols with yellow centres indicate people with baseline vitamin C deficiency.
The primary endpoint, as pre-specified in the clinical trial registry, was percentage ulcer healing at 8 weeks (percentage reduction in ulcer volume). This was significantly better in people in the vitamin C group at median 100 % compared with –14 % in the glucosamine group (P = 0·041, Fig. 1(c), yellow-filled symbols indicate people who were deficient at baseline). Median time to 50 % ulcer healing was significantly faster in the vitamin C group at a median of 20 d compared with a median of 48 d in the five of nine subjects who achieved 50 % ulcer healing in the glucosamine group (Fig. 1(d), P = 0·028).
Kaplan–Meier analysis of 50 % healing showed significantly improved results in the vitamin C group (Fig. 2(a), P = 0·004, deficient-at-baseline subjects have yellow symbols).
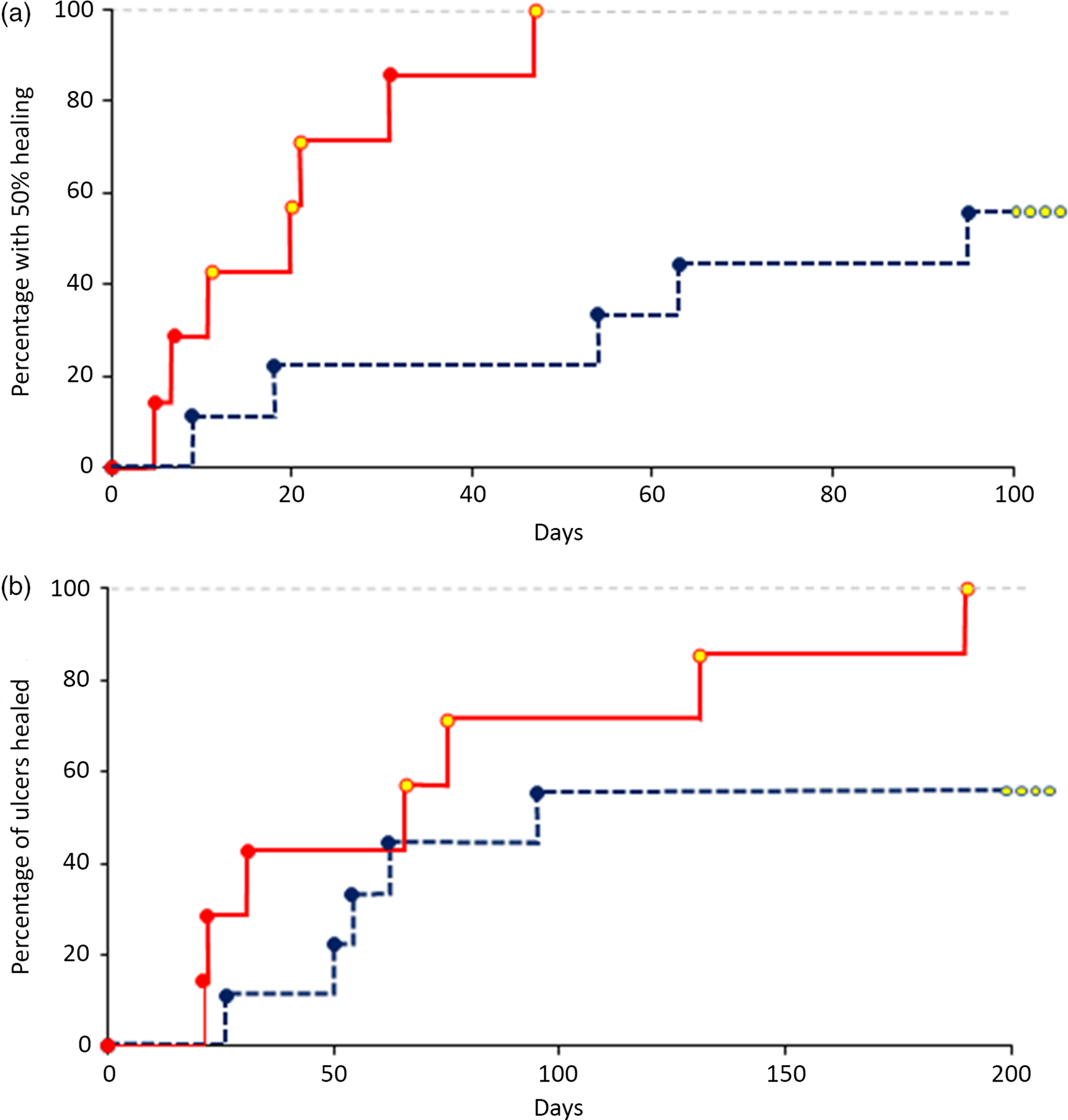
Fig. 2. Ulcer healing rates. (a) Percentage of people with 50 % ulcer healing compared with baseline volume (P < 0·01). Yellow symbols indicate people with baseline vitamin C deficiency. (b) Percentage of people with completely healed ulcers. Four subjects in the control group did not achieve ulcer healing. ![]() , glucosamine;
, glucosamine; ![]() , vitamin C.
, vitamin C.
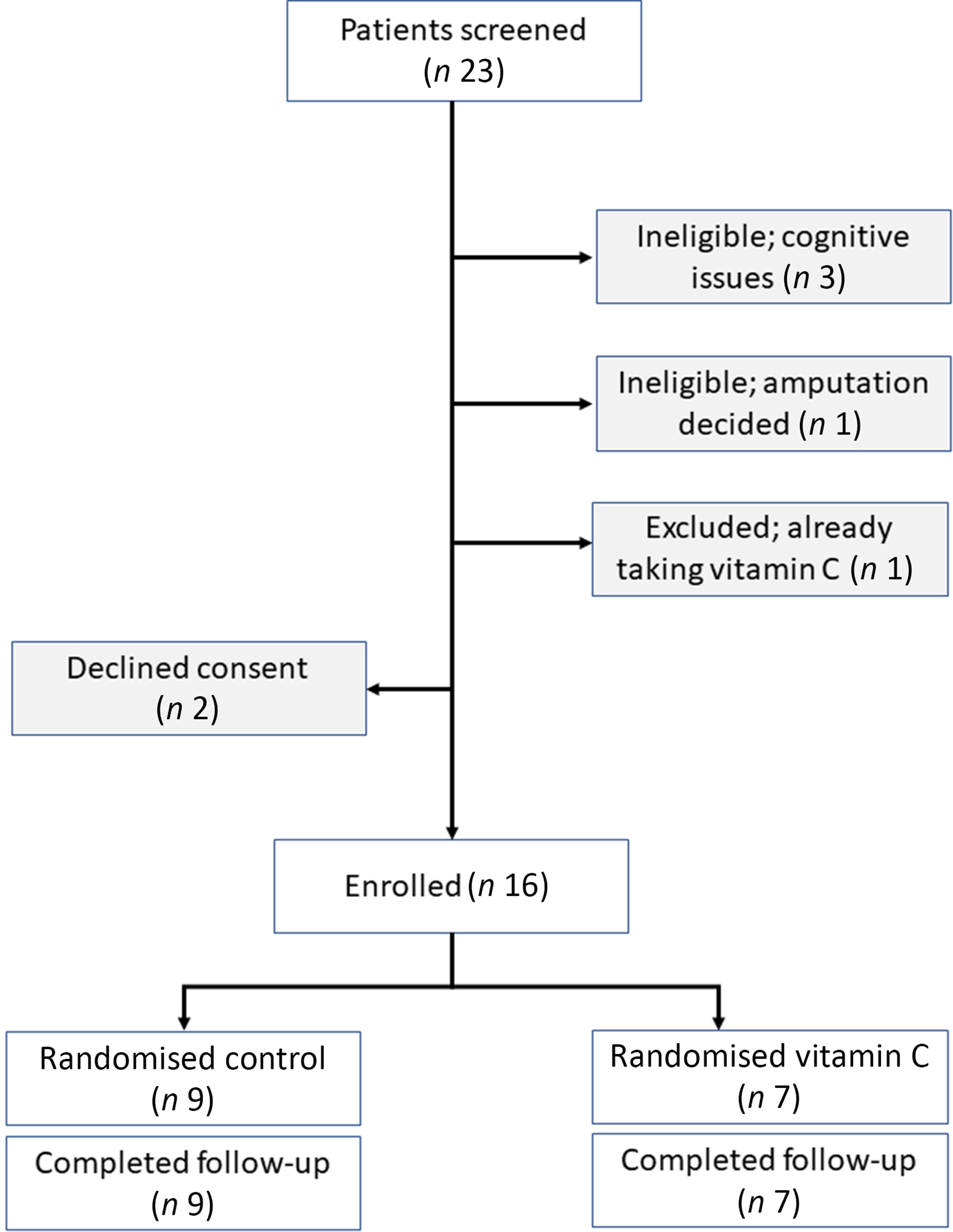
Fig. 3. Consort diagram.
All seven vitamin C subjects went on to complete ulcer healing at a median of 77 d (range 21–190 d), Fig. 2(b). Five of nine subjects in the glucosamine group went on to complete ulcer healing at a median of 77 d (range 26–146 d).
Serum vitamin C was positively correlated with serves of cooked vegetables eaten per d (r 0·558, P < 0·05). Unexpectedly, neither reported fruit nor fruit juice intake correlated with vitamin C. As expected, meat, fish, nut and water consumption did not correlate with vitamin C. Baseline ulcer size was negatively correlated with baseline serum vitamin C (r –0·622, P < 0·05 by Spearman testing). In the whole group, ulcer size at first and second follow-up visits also correlated negatively with vitamin C (r –0·656 and –0·754, respectively, both P < 0·05). The relationship between follow-up ulcer size and baseline vitamin C disappeared in the vitamin C group but strengthened in the glucosamine group (r –0·735 (P < 0·05) and –0·927 (P < 0·01), respectively).
Discussion
Guidelines in Australia for treatment of diabetes-related foot complications are overdue for review(8). Similar guidelines for people without diabetes are not available, but overall the European Wound Management Association guidelines describe wound care and the International Working Group on the Diabetic Foot guidelines describe prevention, assessment and interventions. In general, people without diabetes are treated similarly. Management for both groups includes pressure off-loading (removing physical pressure from the wound) which speeds wound healing(Reference Bus, van Deursen and Armstrong9) and protects from further injury. For people with no contraindications (such as impaired vascular supply or active infection), this should be in the form of a non-removable, full-contact individualised boot or cast. Appropriate, removable footwear should be used for people with vascular compromise or infection.
For people with impaired vascular supply, improving blood flow when possible assists healing(8). There is some evidence that hydrogel dressings may assist in selected wounds(Reference Hinchliffe, Valk and Apelqvist10). In 2018, a multi-centre randomised controlled trial (RCT) of topical epidermal growth factor spray showed significant benefit in diabetes-related foot ulcers(Reference Park, Han and Hong11). Ulcers in people without diabetes were not studied.
Most other strategies have not proven beneficial(Reference Hinchliffe, Valk and Apelqvist10). Many were not tested in RCT or the results were inconclusive. International Working Group on the Diabetic Foot updated their previous review in 2016 and concluded that: ‘with the possible exception of negative pressure wound therapy in postoperative wounds(Reference Armstrong and Lavery12), there is little published evidence to justify the use of newer therapies. Analysis of the evidence continues to present difficulties in this field as controlled studies remain few and the majority continue to be of poor methodological quality(Reference Hinchliffe, Valk and Apelqvist10,Reference Game, Apelqvist and Attinger13) ’.
Perhaps surprisingly, there are no trials showing that improved blood glucose control in people with diabetes hastens ulcer healing(Reference Fernando, Seneviratne and Tan14). Since high blood glucose levels are detrimental to immune cell function, it is still reasonable to aim for glucose levels <11 mmol/l (200 mg/dl). In addition, long-term better glycaemic control decreases the risks of amputations, so this should be the aim for people with foot ulcers because they are at increased risk of amputation(Reference Fernando, Seneviratne and Tan14).
The remainder of the treatment recommendations is necessarily primarily based on expert opinion and recommends debridement, control of exudate (fluid leak from the wound) and consideration of patient comfort and costs to guide dressing selection(Reference Bergin, Gurr and Allard15,Reference Lazzarini, van Netten and Fitridge16) . The 2019 International Working Group on the Diabetic Foot guidelines state on page 164 in relation to wound healing interventions, ‘do not use interventions aimed at correcting the nutritional status (including supplementation of protein, vitamin and trace elements, pharmacotherapy with agents promoting angiogenesis)’.
In the USA, there was a decrease in non-traumatic lower limb amputations in people with diabetes between 2000 and 2009, but this worsened by 50 % in the subsequent years to 2015(Reference Geiss, Li and Hora17). It is of particular concern that the increases in amputation rates were greatest in young and middle-aged adults (18–44 and 45–64 years). In 2010, the USA reported rates of amputations in people with diabetes were about three times higher than Australia, per head of population(Reference Carinci, Massi Benedetti and Klazinga18). In 2015, there were approximately 115 000 non-traumatic lower limb amputations in the USA in people with diabetes(Reference Geiss, Li and Hora17). These figures may be underestimated as minor amputations may be performed in the outpatient setting. In Australia, there are approximately 8000 lower limb amputations yearly. Most of these are non-traumatic, below the ankle amputations and relate to foot ulcers(Reference Dillon, Fortington and Akram19,20) . As stated in a ‘call to action’ editorial in the Medical Journal of Australia, this is one amputation about every 3 h(Reference Bergin, Alford and Allard21).
In the present study, vitamin C treatment improved ulcer healing in the High-Risk Foot Clinic subjects. This was a pragmatic trial with few exclusion criteria; patients had to be able to give written informed consent and not be planned for amputation on their first visit. We excluded one additional person who had pre-clinic testing of their vitamin C, was deficient and was already taking supplements (consort diagram, Fig. 3). With the intention of making the results as widely applicable as possible, the High-Risk Foot Clinic staff were instructed to continue to give usual standard of care in all other regards. All investigators were blinded to treatment assignment, so all therapeutic decisions were made according to usual care.
Most animals can synthesise vitamin C, but humans, primates, guinea pigs and bats do not have the necessary rate-limiting enzyme. This makes study of scurvy difficult, because animal models are expensive, ethically challenging and/or unfamiliar to most researchers. Data show improved wound healing in guinea pigs treated with vitamin C(Reference Krámer, Fillios and Bowler22), but the published human trial data do not answer the question of whether vitamin C is useful for healing foot ulcers.
One RCT studied forty-nine people undergoing elective surgery for tattoo removal. They were supplemented with both vitamin C and pantothenic acid. The trial found no differences in healing(Reference Vaxman, Olender and Lambert23). However, these people had no reason to expect problems with wound healing. The wounds were clean, electively created surgical non-foot wounds, rather than injury-related non-healing foot wounds which are the type seen at foot ulcer clinics.
A more relevant model to foot ulcers is pressure sores. A study of sixteen people with pressure sores tested three treatment groups: (1) control, (2) addition of high protein/energy supplements and (3) high protein/energy supplements + arginine + vitamin C + Zn supplements daily(Reference Desneves, Todorovic and Cassar24). Group 3 had the fastest ulcer healing. The patient groups all had low baseline Zn levels, so Zn supplementation has a likely benefit which is not possible to separate from vitamin C in the present study design. A second RCT of twenty surgical patients with pressure sores found significantly improved ulcer healing in the vitamin C group(Reference Taylor, Rimmer and Day25). In contrast, a randomised trial of eighty-eight nursing home residents with normal baseline nutrition did not find a benefit of vitamin C supplementation for pressure ulcers(Reference ter Riet, Kessels and Knipschild26).
A review of the literature for ‘foot ulcer’, ‘randomised or randomised’ and ‘vitamin C or ascorbate or ascorbic’ only identified two randomised studies of foot ulcers involving vitamin C. One randomised Iranian study used topical kiwifruit application(Reference Mohajeri, Safaee and Sanei27), and it reported significantly better ulcer healing in the kiwifruit group. Their proposed mechanisms for healing were vitamin C and actinidin, which is a proteolytic agent in kiwifruit. Baseline vitamin C was not reported.
Ulcers in a randomised study of people with leprosy (a relevant model because of the associated neuropathy) were treated with media conditioned with topical amniotic membrane stem cells and supplemented with nothing, vitamin C or vitamin E. There was no control group and all study groups showed significant healing. In both the vitamin C and E groups, there was 100 % ulcer healing. The vitamin E group healed fastest(Reference Prakoeswa, Natallya and Harnindya28). This suggests benefits of vitamin C and vitamin E in people with leprosy.
The study presented here appears to be the first report of a RCT of vitamin C alone for treatment of foot ulcers.
Synthesis of mature collagen, a critical structural protein in skin-healing, requires vitamin C(Reference Mandl, Szarka and Banhegyi29) which is needed for hydroxylation of the synthesised collagen chains. Appropriate hydroxylation is required for formation of the proper triple-helix structure of mature collagen.
In addition to the need for vitamin C for collagen formation, ascorbic acid is also needed for normal immune function. Osteomyelitis is a common reason for amputations in people with chronic foot ulcers. Inadequate vitamin C nutrition may therefore encourage development of osteomyelitis in the absence of skin integrity. Many of the sailors in centuries past who died from scurvy experienced bone fractures. It is interesting to speculate that lack of vitamin C may further predispose people to osteomyelitis by impairing bone repair.
The present study has limitations, especially relating to the small sample size. The consistent results across the study endpoints, biological plausibility and a recent report(Reference Brookes, Jaya and Tran4) finding similar rates of vitamin C deficiency in a high-risk foot service increase the likelihood that these results are correct. However, with small numbers, our study could not identify a cut-off point for vitamin C, that is, a level above which supplementation is not beneficial. It was also not able to identify any subgroups that benefited either more or less from supplementation. In addition, the numbers are too few to conduct an economic analysis.
Work on vitamin C trials is unlikely to be funded by industry, due to the low cost and ready availability of this vitamin. These additional studies will probably require larger-scale funding from not-for-profit grant funders, such as NIH or NHMRC.
Vitamin C is cheap, and at 500 mg/d of slow-release supplements, it is very safe. If supplementation prevents only one amputation per ten patients with chronic foot ulcers who would otherwise eventually undergo amputation, treating all patients might prevent more than 10 000 amputations per year in the USA alone. In addition, as time to 50 % ulcer healing was significantly shorter in people receiving vitamin C, it is likely that costs of running the service and costs to patients would be lower in people treated with vitamin C.
We recommend consideration of vitamin C supplementation, preferably with a slow-release form, in all people attending for chronic foot ulcers who do not have exemplary dietary fruit and vegetable intake. Ideally, given its very cheap cost to health services, and high likelihood of favourable cost outcomes, this would be provided free to patients, to improve compliance.
Acknowledgements
This work was funded by the Research and Education Network of Westmead Hospital. J. E. G. receives funding from NHMRC Program Grant APPID 1149976 Complexity in Nutrition.
The study was designed by J. E. G., C. M. G., L. B. and V. F. Patients were recruited by C. M. G. and T. L. and vascular patients were reviewed by M. V. Data were collected from medical records by T. L. and J. E. G. All authors assisted with manuscript preparation and review. The study was approved by the Westmead Human Research Ethics Committee and all participants gave written, informed consent. We would like to thank Ms Olivia Wroth and Dr Andrew Dwyer for proofreading and helpful comments on the paper.
The authors declare that there are no conflicts of interest.







