Cystic fibrosis (CF) is the most common life-threatening autosomal recessive disorder in the Caucasian population, with an incidence of one in 3600 births in the Netherlands( Reference Slieker, Uiterwaal and Sinaasappel 1 ). CF is characterised by progressive pulmonary dysfunction. In addition, most patients have pancreatic insufficiency, which can lead to fat malabsorption and deficiencies of fat-soluble vitamins such as vitamin D( Reference O’Sullivan and Freedman 2 , Reference Turck, Braegger and Colombo 3 ). International CF nutritional guidelines therefore recommend age-group-specific vitamin D supplementation to maintain optimal serum 25-hydroxy vitamin D (25(OH)D) levels( Reference O’Sullivan and Freedman 2 , Reference Black and Scragg 4 ).
An association between serum 25(OH)D and pulmonary function (PF) was observed in the general population( Reference Black and Scragg 4 ), in paediatric patients with asthma( Reference Pojsupap, Iliriani and Sampaio 5 ) and in adults with chronic obstructive pulmonary disease( Reference Janssens, Bouillon and Claes 6 , Reference Ferrari, Schenk and Papadopoulou 7 ). However, whether this beneficial effect also applies to paediatric patients with CF is unknown, as the study results were inconclusive( Reference McCauley, Thomas and Laguna 8 ) or limited to a cross-sectional design( Reference Sexauer, Hadeh and Ohman-Strickland 9 , Reference Pincikova, Nilsson and Moen 10 ).
Furthermore, little is known about the daily practice of vitamin D intake, vitamin D supplementation and its association with serum 25(OH)D in paediatric CF patients, as previous studies lacked data on dietary vitamin D intake( Reference Green, Carson and Leonard 11 – Reference Pincikova, Sandberg and Hjelte 14 ), were limited by a cross-sectional design( Reference Simoneau, Bazzaz and Sawicki 12 , Reference Rovner, Stallings and Schall 15 ) or were conducted in the context of a trail in which the study sample was prescribed very high dosages of vitamin D, far above those used in current clinical practice( Reference Pincikova, Sandberg and Hjelte 14 , Reference Simoneau, Sawicki and Milliren 16 – Reference Shepherd, Belessis and Katz 19 ). We therefore set out to record the dietary vitamin D intake, vitamin D supplementation and the long-term relationship with serum 25(OH)D. In addition, we describe the association between serum 25(OH)D and PF in a large study sample of paediatric patients with CF during a 4-year follow-up.
Methods
Study sample
This retrospective study included Dutch children and adolescents with proven CF and proven pancreatic insufficiency who received medical care at the CF centre of the University Medical Centre Utrecht between January 2012 and March 2016. The diagnosis CF was confirmed by a positive sweat test and/or the presence of a known CF gene mutation on each cystic fibrosis transmembrane conductance regulator gene. Pancreatic insufficiency was defined by a documented history of fat malabsorption with a coefficient of fat absorption of <85 % and/or a faecal elastase of <15 μg/g stool or chymotrypsin activity <3 U/g per stool. Included patients had at least one serum 25(OH)D measurement obtained between January 2012 and March 2016 during the annual CF check-up. Excluded were one transplanted patient and two patients with serum 25(OH)D values above 200 nmol/l, as we suspected that these values were the result of measurement errors. Written informed consent was given by all patients or by parents or guardians of young patients. The study was performed according to the guidelines of the Medical Ethics Committee of the University Medical Centre Utrecht.
Dietary intake assessment
Yearly, patients were asked to complete a 3-d record of their food and beverage intake, consisting of two consecutive weekdays and one weekend day whenever possible. The dietary vitamin D intake was calculated for each assessment according to a standardised approach using the Dutch food composition table (2010) established by the Dutch Nutrition Centre.
The prescribed vitamin D supplements as documented in medical records were considered as supplementary vitamin D intake. The prescribed dosages were based upon the European Union guideline of 2002, which advices a daily supplementary vitamin D intake of 10–50 μg (400–2000 IU) for all ages( Reference Sinaasappel, Stern and Littlewood 20 ). In patients with an insufficient serum 25(OH)D level, the supplement dosage was adjusted, the increase being subject to the opinion of the physician in charge. Total vitamin D intake was calculated by adding dietary and supplementary intake.
The vitamin D intake (dietary intake, prescribed supplementation and total intake) was expressed as μg/d. Owing to the wide body weight range of the study sample (5·0–84·5 kg), we subsequently expressed the dietary, supplementary and total intake as μg/kg body weight per d.
Clinical measurements
Serum 25(OH)D levels in blood were routinely measured as part of the annual check-up and analysed by electrochemiluminescence sandwich immunoassay (Cabas E411; Roche) which was calibrated against National Institute of Standards and Technology standard material; performance was monitored daily using Lyphochek control material (Bio-Rad Laboratories). Mean CV% was within 8·7 %. Measurements were classified according to months with high (June–October) and low (November–May) UVB exposure( Reference Rovner, Stallings and Schall 15 ).
Height and weight were measured to the nearest 0·1 cm and 0·1 kg, respectively, during annual check-ups. Weight, height and BMI were compared with reference values using z-scores as calculated by specialised software of the Dutch Growth Foundation (Growth Analyser 4 RCT, 2010, Dutch Growth Foundation). Yearly, patients from 10 years of age onwards were screened for glucose tolerance by the modified oral glucose tolerance test (1·75 g/kg glucose, maximum dosage 75 g). An overnight fasting plasma glucose level and a 2-h post-prandial glucose level were measured. Those having glucose levels >11·1 mmol/l after oral glucose tolerance test, or fasting glucose >7·0 mmol/l were categorised as CF-related diabetes (CFRD)( Reference Moran, Pillay and Becker 21 ). CF-related liver disease (CFLD) was diagnosed according to the Colombo criteria( Reference Colombo, Russo and Zazzeron 22 ). Serum IgG, as a marker of chronic inflammation, was measured concurrently with serum 25(OH)D and expressed as g/l. The use of systemic corticosteroids in each year was recorded.
PF was assessed as forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) and expressed as a percentage of the predicted value for a given height, age and sex (% pred.) using the Global Lung Function Initiative reference values( Reference Quanjer, Stanojevic and Cole 23 ). For each child, the highest PF measured in the preceding calendar year was used, beginning at the age of 6 years. PF tests up to 2016 were included.
Statistical analysis
Categorical variables were examined using descriptive statistics. Continuous variables were assessed on normality and skewness. Due to repeated measures on individual patients in different years of age, children were stratified according to age year (year 0 = birth to <1 year, year 1 = 1 to <2 years, etc.) and measurements of dietary, supplemental and total vitamin D intake, intake per kg body weight and serum 25(OH)D were described accordingly.
To assess whether the initial measurements of total vitamin D intake and PF were related to serum 25(OH)D levels, children were categorised based on their serum 25(OH)D as having levels <50 nmol/l (deficient), levels between 50 and 75 nmol/l (sufficient) or >75 nmol/l (high sufficient), based upon current European Union guidelines in which serum 25(OH)D levels ≥50 nmol/l are considered sufficient( Reference Turck, Braegger and Colombo 3 ). Age, vitamin D intake (dietary, prescribed supplementation and total) and PF were compared cross-sectionally amongst the categorised serum 25(OH)D levels using one-way ANOVA or the Kruskal–Wallis test. To evaluate the effect of total vitamin D intake on serum 25(OH)D over time, linear mixed-effect regression models were used. These models allow inclusion of varying numbers of measurements per child, irregular observation times and missed observations. Age, sex, body weight and season were included as fixed effects. A random intercept and a random slope for age per child accounted for associations between measurements within children.
We also examined the longitudinal effect of serum 25(OH)D on PF (FEV1% pred. and FVC% pred.), using linear mixed-effect regression. Included were age, sex, z-score BMI, IgG, CFLD, CFRD, corticosteroid use and season as fixed effects and a random intercept and slope for age. Sample size was determined by the availability of data from the medical records. Exact effect sizes for longitudinal data are difficult to estimate and require many assumptions, so the current study presents a conservative estimate here. A sample size of 190 would provide 80 % power to detect a correlation of at least 0·20 between serum 25(OH)D and PF using a two-sided hypothesis test with a significance level of 0·05. Statistical analysis was performed using the Statistical Package for the Social Sciences Computer Software version 21 (IBM).
Results
Patient characteristics
Data of 190 paediatric patients (95 % Caucasian) were eligible for inclusion. In these patients, we obtained 545 measurements of serum 25(OH)D and 353, 532 and 346 measurements of dietary, supplementary and total vitamin D intake, respectively. A total of 408 measurements of PF were obtained in 157 children aged 6 years and older. Demographic and clinical characteristics of patients at the time of inclusion are described in Table 1.
Table 1 Demographical and clinical characteristics of 190 children and adolescents with cystic fibrosis at the time of inclusion (Medians and interquartile ranges (IQR); numbers of subjects and percentages)

25(OH)D, 25-hydroxy vitamin D; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; CFRD, cystic fibrosis-related diabetes mellitus; CFLD, cystic fibrosis-related liver disease.
* Percentage of predicted as calculated according to the Global Lung Function Initiative reference values( Reference Quanjer, Stanojevic and Cole 23 ).
† Low UVB months, November–May; high UVB months, June–October.
Descriptive baseline results
Median dietary intake at time of inclusion was 4·1 (interquartile range (IQR) 2·3–8·0)μg/d, supplementary intake was 10 (IQR 10–11·3)μg/d and total intake was 14·7 (IQR 12–21)μg/d. All remained relatively constant over the subsequent age-years (online Supplementary Table S1). Dietary, supplementary and total vitamin D intake are expressed as μg/kg per d, and serum 25(OH)D decreased with increasing age (Fig. 1).
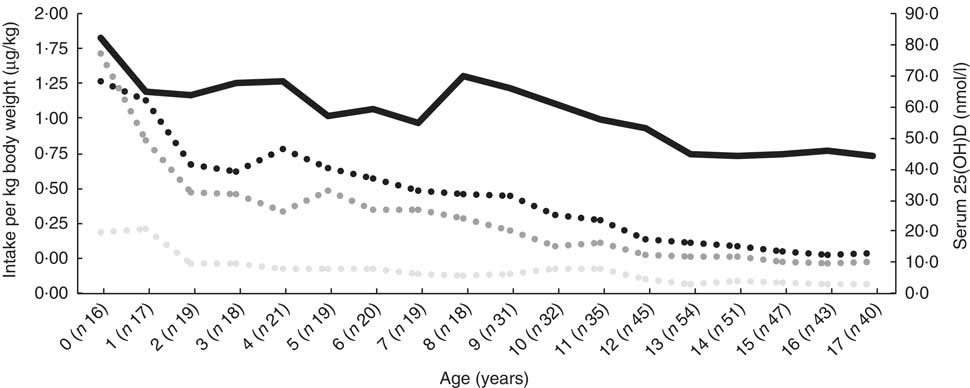
Fig. 1 Median serum 25-hydroxy vitamin D (25(OH)D) and dietary, supplementary and total vitamin D intake at the time of inclusion, expressed as μg/kg body weight per d in 190 patients with cystic fibrosis, stratified according to year of age. ![]() , Total vitamin D (μg/kg per d);
, Total vitamin D (μg/kg per d); ![]() , supplementary vitamin D (μg/kg per d);
, supplementary vitamin D (μg/kg per d); ![]() , dietary vitamin D (μg/kg per d);
, dietary vitamin D (μg/kg per d); ![]() , serum 25(OH)D.
, serum 25(OH)D.
A total of 76/190 (40 %) patients had deficient, 73/190 (38·4 %) patients had sufficient and 41/190 (21·6 %) had high-sufficient serum 25(OH)D levels at time of inclusion. The distribution of dietary vitamin D intake was comparable among the serum 25(OH)D classes (P = 0·170), while the distribution of supplementary and total vitamin D intake was significantly higher in classes with higher serum 25(OH)D levels (P = 0·010 and P = 0·018, respectively). Children in the lower serum 25(OH)D classes were significantly older (P = 0·000). Further, the distribution of FEV1% pred. and FVC% pred. did not differ among the serum 25(OH)D classes (P = 0·075 and P = 0·149, respectively).
Longitudinal analysis of vitamin D intake and serum 25-hydroxy vitamin D
Longitudinally, there was a significant relationship between total vitamin D intake and serum 25(OH)D (β = 0·02; 95 % CI 0·01, 0·03), which remained significant after correction for potential confounders (Table 2). In our study sample, on average, each 100 IU (2·5 μg) increase in vitamin D resulted in a 2 nmol/l (95 % CI 1·0, 3·0) increase in serum 25(OH)D. Further, each kg increase in body weight resulted in a 0·79-nmol/l decline (95 % CI –1·28, –0·29) in serum 25(OH)D. Serum 25(OH)D was significantly higher in months with high UVB (June–October) (β = 9·01 nmol/l; 95 % CI 4·84, 13·18). Age and sex were not significantly associated with serum 25(OH)D.
Table 2 Predictive factors of change in serum 25-hydroxy vitamin D expressed as nmol/l in 190 children and adolescents with cystic fibrosis, using a mixed effect regression model (Regression coefficients and 95 % confidence intervals)

Longitudinal analysis of serum 25-hydroxy vitamin D and pulmonary function
We observed a significant relationship between serum 25(OH)D and PF, expressed as FEV1% pred. and FVC% pred. after adjustment for age, sex, z-score BMI, IgG, CFLD, CFRD and corticosteroid usage. In our study sample, each 20-nmol/l increase in serum 25(OH)D resulted in an increase of 1·12 % (95 % CI 0·2, 2·04) of FEV1% pred. and 0·9 % (95 % CI 0·16, 1·64) FVC% pred. (Table 3). Furthermore, we found a negative association between PF and age and CFRD, while PF and BMI had a positive association.
Table 3 Predictive factors of respectively forced expiratory volume in 1 s (FEV1)% of predicted and forced vital capacity (FVC)% of predicted in 158 children and adolescents with cystic fibrosis, using a mixed effect regression model (Regression coefficients and 95 % confidence intervals)

25(OH)D, 25-hydroxy vitamin D; CFRD, cystic fibrosis-related diabetes mellitus; CFLD, cystic fibrosis-related liver disease.
Discussion
This longitudinal study in a large cohort of children and adolescents with CF, with a 4-year follow-up, showed a significant relationship between total vitamin D intake (dietary and supplemental intake) and serum 25(OH)D and between serum 25(OH)D and PF.
To the best of our knowledge, this is the first longitudinal study on serum 25(OH)D in paediatric patients with CF, including both dietary vitamin D intake and vitamin D supplementation dosages as prescribed in daily clinical CF care. In our study sample, and in accordance with other North-European CF populations( Reference Pincikova, Nilsson and Moen 10 ), the dietary vitamin D intake attributed one-third of the total vitamin D intake. In our study, the median dietary vitamin D intake remained fairly constant throughout the age-years, while serum 25(OH)D significantly decreased with age. We found no relationship between dietary vitamin D intake and serum 25(OH)D as reported previously( Reference Rovner, Stallings and Schall 15 ). However, total vitamin D intake was clearly related to serum 25(OH)D, indicating the importance of vitamin D supplementation to obtain and maintain adequate serum 25(OH)D.
Longitudinally, we found a relationship between total vitamin D intake and serum 25(OH)D. This is in line with a previous study in 360 adults with CF with a mean initial serum 25(OH)D of 47 nmol/l and a mean initial supplemental vitamin D intake of 16·2 μg/d (646 IU/d) in which an increase of 10–25 μg/d (400–1000 IU/d) or counselling led to high sufficient serum 25(OH)D values in 82 % of the subjects( Reference Stephenson, Brotherwood and Robert 13 ). Several intervention studies in paediatric CF patients, with serum 25(OH)D levels <75 nmol/l, found an increase in serum 25(OH)D to >75 nmol/l in 54–94 % of the study sample when prescribed extreme amounts of vitamin D supplements (1250 μg/week (50 000IU/week) up to 15 000 μg (600 000 IU) stoss therapy)( Reference Simoneau, Sawicki and Milliren 16 – Reference Shepherd, Belessis and Katz 19 ). In contrast to these findings, Hillman et al.( Reference Hillman, Cassidy and Popescu 24 ) found no extra effect of 50 μg (2000 IU) vitamin D/d supplementation in a small crossover trial in 15 paediatric CF patients. However, these patients had significantly higher serum 25(OH)D levels at baseline (median 83 nmol/l) than our study sample. It is questionable whether an increase in the already high serum levels can be expected, as previous studies have indicated that vitamin D intake has to increase exponentially, as higher serum 25(OH)D levels are to be reached( Reference Stephenson, Brotherwood and Robert 13 , Reference Zittermann, Ernst and Gummert 25 ).
Previous studies also reported an inverse association between age and serum 25(OH)D( Reference McCauley, Thomas and Laguna 8 , Reference Green, Carson and Leonard 11 , Reference Lansing, McDonald and Patel 26 – Reference Chavasse, Francis and Balfour-Lynn 28 ) in paediatric patients with CF, although no conclusive explanation was given. We found that children with higher levels of serum 25(OH)D were significantly younger, with the highest serum 25(OH)D levels in patients below the age of 4 years old. While having a fairly constant absolute vitamin D intake, the intake per kg body weight of patients below the age of 4 years was more than double compared with adolescents, leading to the assumption that body weight might influence serum 25(OH)D levels. Our longitudinal model indeed indicated that an increasing body weight is related to a decreasing serum 25(OH)D. This might be due to the dilution of vitamin D in larger body volumes( Reference Drincic, Armas and Van Diest 29 , Reference Pannu, Zhao and Soares 30 ) which occurs as children grow and weight increases( Reference Zittermann, Ernst and Gummert 25 , Reference Weishaar and Rajan 31 ). As increasing age was not sufficiently matched by a concurrent increase in (supplementary) vitamin D intake, a downward trend of serum 25(OH)D with age was observed.
To note, current CF guidelines provide recommendations for large age-groups (age ≤1 year, >1–10 years, 10 years onwards); although direct extrapolation of our results, which were based upon the 2002 guidelines, should be done with caution, children of different ages, and with large differences in body weight, still share the same recommendation. It might be more appropriate to use smaller age intervals or body weight, with yearly evaluation as described in the CF-specific guidelines( Reference Turck, Braegger and Colombo 3 ).
We and others found a significant relationship between serum 25(OH)D and PF in children and adolescents with CF, which remained after correcting for multiple confounders known to affect PF( Reference Sexauer, Hadeh and Ohman-Strickland 9 ). Several studies have addressed the relationship between vitamin D intake and PF although with conflicting results( Reference Sexauer, Hadeh and Ohman-Strickland 9 – Reference Simoneau, Bazzaz and Sawicki 12 , Reference Vanstone, Egan and Zhang 32 ). Cross-sectional studies in both paediatric and in mixed paediatric and adult populations found a relationship between FEV1% pred. and serum 25(OH)D( Reference Sexauer, Hadeh and Ohman-Strickland 9 – Reference Green, Carson and Leonard 11 ). The only previous longitudinal study in 130 paediatric patients with CF with a median follow-up of 4 years did not find a longitudinal relationship with PF( Reference McCauley, Thomas and Laguna 8 ). However, this study did not account for confounders that significantly affect the PF such as age, z-score BMI and CFRD, as we and others found these variables are indeed correlated with PF. Therefore, a positive effect of serum 25(OH)D on PF in the study by McCauley et al. ( Reference McCauley, Thomas and Laguna 8 ) may have been blurred.
A recent pilot study by Pincikova et al. ( Reference Pincikova, Sandberg and Hjelte 14 ) in sixteen patients (10 adults) did find a positive relationship between change in serum 25(OH)D and PF (FEV1 and FVC). Our study seems to indicate that this relationship is also present in paediatrics with CF.
Any beneficial effect of serum 25(OH)D on PF has been attributed to the immunomodulatory potentials of vitamin D( Reference Herscovitch, Dauletbaev and Lands 33 ). Metabolised serum 25(OH)D in airway epithelia exhibits anti-microbial effects by regulation of anti-microbial peptides( Reference Yim, Dhawan and Ragunath 34 ) and anti-inflammatory effects by pro-inflammatory cytokines( Reference McNally, Coughlan and Bergsson 35 ). This could reduce chronic pulmonary colonisation and thereby aid in the long-term preservation of PF( Reference Simoneau, Bazzaz and Sawicki 12 ).
Several limitations of this study can be mentioned. First, keeping food records might lead to alterations of the diet and to over- and/or under-reporting. Second, differences in seasonal variation in individuals might not be detected as the time of year in which the dietary intake recorded remained fairly constant over the study years. In addition, we followed the clinical practice and thereby did not measure adherence to vitamin D supplementation or accounted for missed dosages. Finally, as this study was retrospective in design, the causal effect of serum 25(OH)D on PF we found should ideally be reproduced in a prospective randomised trial.
Conclusion
In conclusion, in this large study sample, a significant relationship between total vitamin D intake (dietary and supplemental) and serum 25(OH)D and an inverse relationship between body weight and serum 25(OH)D were described in paediatric CF patients. In addition, higher serum 25(OH)D levels were associated with higher FEV1% pred. and FVC% pred. These findings suggest that vitamin D supplementation should increase with increasing body weight, as adequate serum 25(OH)D levels may contribute to the preservation of PF. Adjustments of the recommendations in which vitamin D supplementation increases with increasing weight, to maintain adequate serum 25(OH)D levels in children with CF, should be considered.
Acknowledgements
This research received no specific grant for any funding agency, commercial or not-for-profit sectors.
N. K. L. M. T. and J. W. W. contributed to the conception and design and coordination of the research, carried it out, analysed the data and drafted the manuscript; R. K. S. participated in the statistical analysis and critically revised the manuscript. R. H. J. H. participated in the design of the study and drafted the manuscript. C. K. v. d. E. critically revised the manuscript; all authors agree to be fully accountable for ensuring the integrity and accuracy of the work. All authors have read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518003021







