Weight loss in the absence of disease or surgical intervention can only occur as the result of a chronic negative imbalance between energy intake (EI) and energy expenditure (EE). Although apparently simple, energy balance regulation is a dynamic process that requires a better understanding for evidence-based and realist interventions( Reference Hall, Heymsfield and Kemnitz 1 ).
Metabolic and behavioural compensations have been observed in response to diet and/or exercise interventions designed to induce changes in the energy balance components( Reference King, Caudwell and Hopkins 2 – Reference Knuth, Johannsen and Tamboli 4 ). It is recognised that weight loss induced from a negative energy balance reduces over time, as energetic demands are attenuated, mitigating an indefinite exposure to energy balance deficit( Reference Hall, Sacks and Chandramohan 5 ). Although metabolic adaption to weight loss occurs( Reference Hall, Sacks and Chandramohan 5 , Reference Thomas, Martin and Heymsfield 6 ), behavioural compensations, that is compensation for an energy balance intervention through behaviour changes, may also occur. Indeed, behavioural compensation resulting from creating a negative energy balance includes a reduction in voluntary EE and/or an increase in EI in the absence of a strict control( Reference Melanson, Keadle and Donnelly 7 ). To better clarify voluntary EE, important concepts should be addressed, such as physical activity EE (PAEE) that represents the overall energy expended to move the body, further divided into structured physical activity (PA) (exercise) or non-exercise activity thermogenesis (NEAT). Non-exercise PA (NEPA) refers to the physical motion of the body in activities that do not pertain to volitional exercise, including all activities of daily living (fidgeting, maintaining posture and ambulation), whereas NEAT defines the EE associated with these activities( Reference Levine 8 ). However, the role of NEPA and/or NEAT on compensation from exercise and/or diet-induced weight loss is less well understood.
A recent systematic review with meta-analysis indicated no mean changes in NEPA during exercise training( Reference Fedewa, Hathaway and Williams 9 ). However, the authors reported that session duration, intervention length, age and sex influenced changes in NEPA during exercise training( Reference Fedewa, Hathaway and Williams 9 ). Washburn et al.( Reference Washburn, Lambourne and Szabo 10 ) indicated that more data from adequately powered trials using objective measurements are required to improve the understanding of the effects of exercise-induced weight loss on NEAT and NEPA. Measurement of EI and EE, including all components of EE, objective measurement of PA and accurate measurement of changes in body energy stores, must be included in such studies.
Relatively few studies have investigated the effect of energy restriction on free-living NEPA and NEAT, possible owing to the cost and burden of measuring PA accurately in participants’ habitual environment. Furthermore, many of the findings are contradictory. No changes in posture allocation (time spent reclining or sitting v. standing or ambulating) were observed when obese people lost weight( Reference Levine, Lanningham-Foster and McCrady 11 ), whereas other studies only found trends towards decreases in PA among non-obese weight-reduced men( Reference Heyman, Young and Fuss 12 , Reference Velthuis-te Wierik, Westerterp and van den Berg 13 ). In contrast, other groups found a decrease in PA and corresponding EE during an energy restriction diet, with inclusion of exercise training( Reference Racette, Schoeller and Kushner 14 , Reference Redman, Heilbronn and Martin 15 ). Three randomised controlled trials (RCT) under the Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy (CALERIE) study found that energy restriction significantly decreased NEAT, but not NEPA( Reference Martin, Das and Lindblad 16 ).
Dhurandhar et al.( Reference Dhurandhar, Kaiser and Dawson 17 ) provided a systematic review with meta-analysis using a mathematical modelling approach concluding that there is substantial compensation in both dietary and exercise interventions designed to induce weight loss. The authors identified a possible range of behavioural and metabolic compensations that can be very difficult to quantify, but which may reduce the expected amount of weight loss after a given intervention( Reference Dhurandhar, Kaiser and Dawson 17 ). The extent to which this compensation is due to changes in NEPA or NEAT is unclear. There is insufficient evidence to definitively answer the question of whether diet or exercise-induce weight loss leads to compensatory reductions in NEAT and NEPA, as a result of increases in sedentary behaviour, decreases in overall PA or both. So far no systematic review has covered both exercise and diet, and their combined and independent effects on compensatory activity. The aim of this systematic review is to describe the effects of diet and/or exercise energy balance interventions on behavioural compensation in NEPA and/or related decreases in NEAT of free-living adults.
Methods
Criteria for study eligibility: studies and participants
In this review, articles reporting changes in compensatory behaviours occurring during or as a result of diet and/or exercise interventions, designed to intervene in one or more components of the energy balance equation, were retrieved. To be included, studies had to fulfil all of the following criteria: (1) adult samples (>18 years), regardless of sex; (2) n>10 participants; (3) an intervention period of at least 1 week; (4) be published in English language; (5) include objective measures of total EE (TEE) and/or PA (doubly labelled water (DLW), indirect calorimetry, accelerometer (ACC), pedometer, inclinometer); and (6) be a clinical trial. In turn, studies involving participants taking medication or having diseases/conditions known to affect metabolism/weight (cancer, thyroid disease, diabetes, bariatric surgery, pregnancy, total parenteral nutrition, HIV/AIDS, organ transplant, Prader–Willi Syndrome, polycystic ovary syndrome, chronic obstructive pulmonary disease or acute illnesses, such as infections or traumatic injury) were excluded. The current review is registered on PROSPERO (PROSPERO 2017 CRD42017052635).
Information sources and search strategy
A comprehensive search of peer-reviewed articles published until 1 March 2017 (including online ahead of print publications) was conducted in the following electronic databases: Pubmed, PsycINFO, Embase, CINAHL, Cochrane Library, ERIC and SPORTDiscus. Searches included all meaningful combinations of the following sets of terms: (i) terms concerning the population of interest (e.g. adults, obese, overweight); (ii) terms concerning the intervention(s) of interest (e.g. diet or energetic restriction, PA or exercise, weight or body fat loss/change, behaviour change or lifestyle intervention); (iii) terms representing the outcomes of interest (e.g. NEPA, spontaneous PA, NEAT, compensatory response/behaviour); and (iv) terms concerning the study design (e.g. trial, experimental, treatment). A complete list of search strategies can be obtained from the authors, whereas a search strategy example for Pubmed is provided as an additional file (online Supplementary material SI). Other sources included manual cross-referencing of literature cited in prior reviews and retrieved studies, and hand-searches of the content of key scientific journals.
Study selection and data processing
All abstracts identified from the literature searches were screened for potential inclusion eligibility by one author (P. B. J.). Of all abstracts, duplicates were removed and twenty-three added from other sources. In all, seventy-five full-text articles were retrieved, and thirty-six met all inclusion criteria and were included in the present review (Fig. 1). A data extraction form was developed, based on the PRISMA statement for reporting systematic reviews( Reference Liberati, Altman and Tetzlaff 18 ). Data extraction was conducted by two authors (P. B. J. and E. V. C.) and included information about the article (e.g. authors, year), participants (e.g. demographics, BMI), study design, intervention characteristics (e.g. aim, length, follow-up, arms), outcome measures and main results.
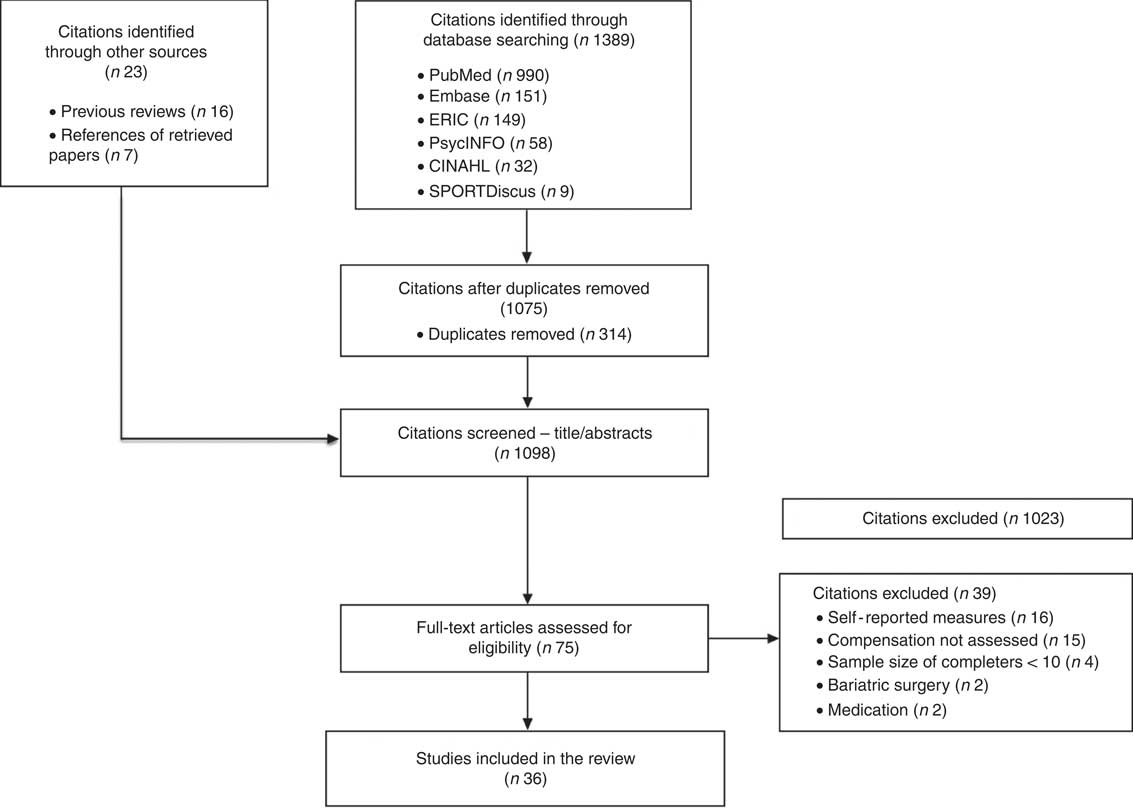
Fig. 1 Flow diagram.
The articles were grouped by study design as RCT (Table 1), randomised trials (RT, Table 2) and non-randomised trials (NRT, Table 3), whereas in the results text, articles were further presented by intervention type: diet-only, exercise-only and combined diet and exercise. Across studies, heterogeneity was observed in various parameters, including (i) study characteristics (sample size, completion rate, trial length, with or without behavioural intervention, methodology for NEPA/NEAT); (ii) participant characteristics (sex, age, BMI, ethnicity, activity level); (iii) diet (degree of energy restriction) or exercise prescriptions (mode, frequency, intensity, duration); (iv) assessment of NEPA/NEAT (ACC, heart rate (HR), activity diary, indirect calorimetry, DLW); and (v) main outcomes (compensation or non-compensation in NEPA and related energy expenditure, NEAT). If the outcome measure was PA assessed through activity monitors, then NEPA was used. If the outcome was non-exercise EE measured using DLW or assessment from accelerometry or other methods, NEAT was used. When PAEE was referred to as NEAT, an assumption that volitional exercise during the intervention was not performed was made. This terminology was used consistently throughout the manuscript to adequately differentiate these two concepts/outcomes. Considering this heterogeneity, a meta-analysis was found inappropriate. Results based on the extracted data were instead synthesised and presented grouped by study design (Tables 1–3) and intervention type (in the text).
Table 1 Randomised controlled trials (ten studies)
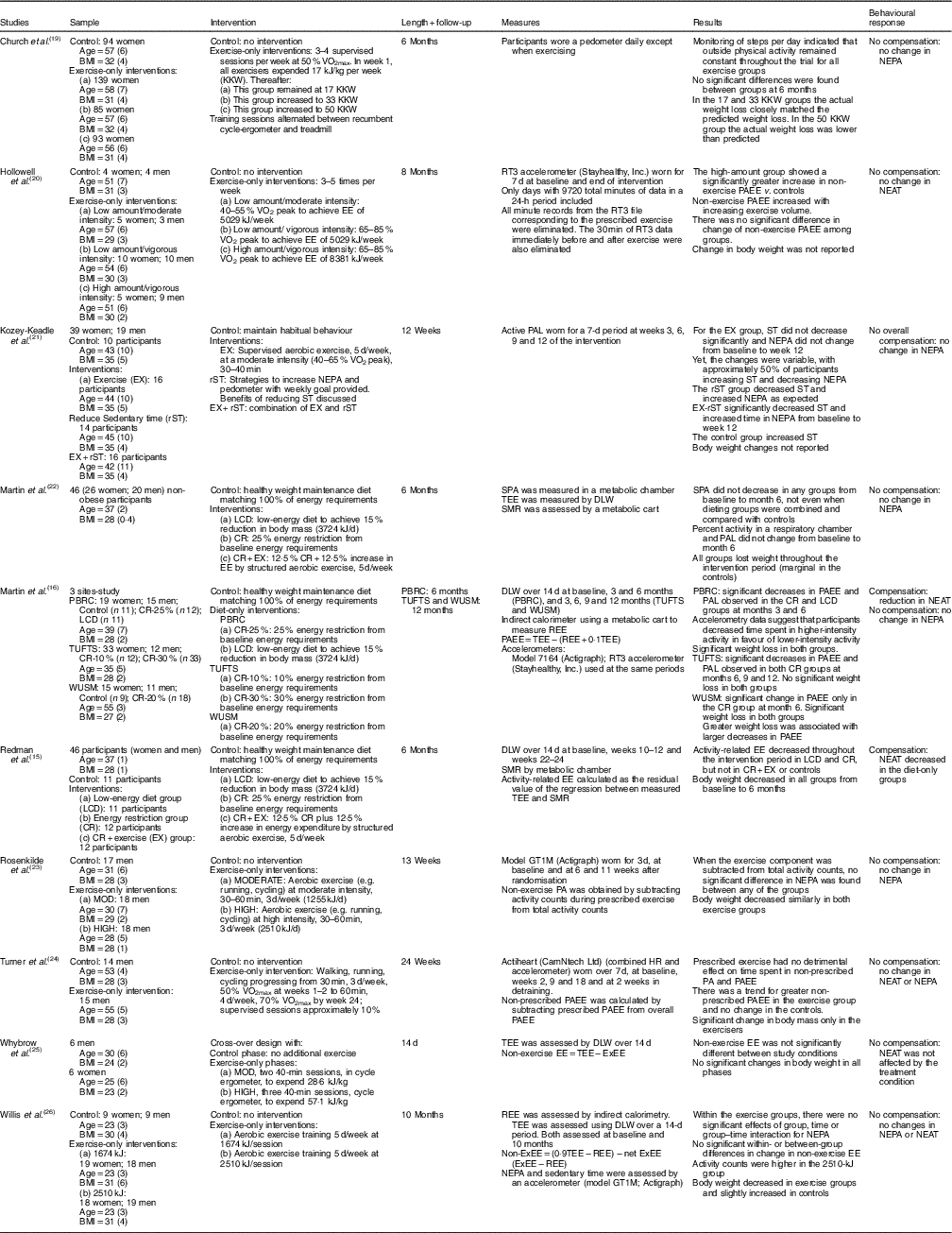
NEPA, non-exercise physical activity; PAEE, physical activity energy expenditure; NEAT, non-exercise activity thermogenesis; Ex, exercise; PAL, physical activity level; LCD, low-energy diet; CR, energy restriction; SPA, spontaneous physical activity; SRI, self-regulatory intervention; TEE, total energy expenditure; DLW, doubly labelled water; SMR, sleep metabolic rate; PA, physical activity; HRR, heart rate reserve; EE, energy expenditure; ExEE, exercise energy expenditure; HRmax, maximal heart rate; RM, repetition maximum; REE, resting energy expenditure; DO, diet-only; D-PA, diet plus physical activity; TEF, thermic effect of food; NR, non-reported; AT, aerobic training; RT, resistance training; PBRC, Pennington Biomedical Research Center; TUFTS, Tufts University; WUSM, Washington University School of Medicine.
Table 2 Randomised trials with no control group (nine studies)
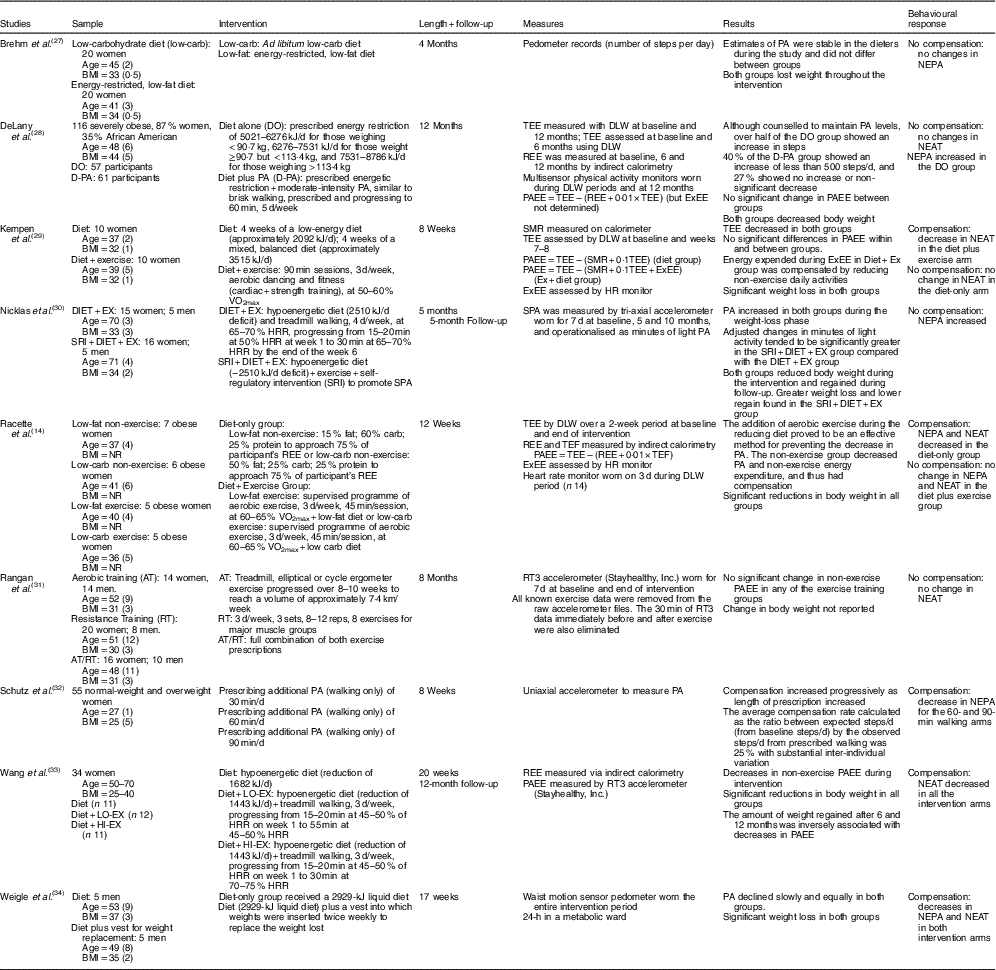
PA, physical activity; NEPA, non-exercise physical activity; DO, diet-only; D-PA, diet plus physical activity; TEE, total energy expenditure; DLW, doubly labelled water; REE, resting energy expenditure; PAEE, physical activity energy expenditure; ExEE, Exercise energy expenditure; NEAT, non-exercise activity thermogenesis; SMR, sleep metabolic rate; Ex, exercise; SRI, self-regulatory intervention; SPA, spontaneous physical activity; HRR, heart rate reserve; NR, non-reported; TEF, thermic effect of food; AT, aerobic training; RT, resistance training; LO-Ex, low exercise; HI-Ex, high exercise.
Table 3 Non-randomised trials (seventeen studies)
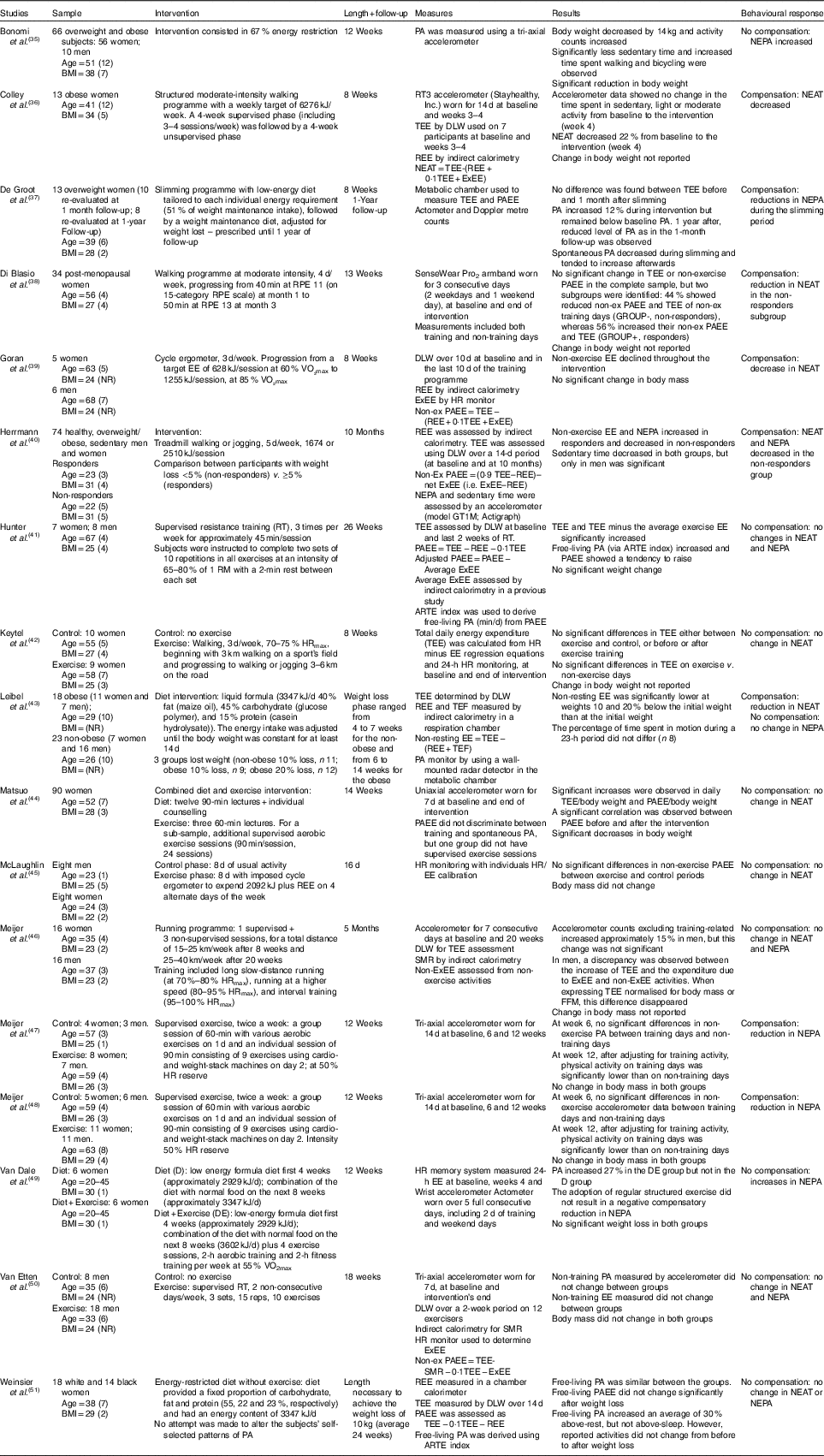
PA, physical activity; NEPA, non-exercise physical activity; TEE, total energy expenditure; DLW, doubly labelled water; REE, resting energy expenditure; NEAT, non-exercise activity thermogenesis; Ex, exercise; ExEE, Exercise energy expenditure; PAEE, physical activity energy expenditure; NR, non-reported; TEF, thermic effect of food; RM, repetition maximum; ARTE, activity-related time equivalent; FFM, fat-free mass; HR, heart rate; HRmax, maximal heart rate; RT, resistance training; SMR, sleep metabolic rate; RPE, rated perceived exertion.
Frequencies, medians, range and proportions were assessed using SPSS (version 24; IBM SPSS Statistics for Windows).
Quality assessment
Study quality was assessed with the Quality Assessment Tool for Quantitative Studies( Reference Armijo-Olivo, Stiles and Hagen 52 ) (online Supplementary material SII), evaluating six key methodological domains: study design, blinding, representativeness (selection bias), representativeness (withdrawals/dropouts), confounders and data collection. Each domain was classified as strong, moderate or weak methodological quality. A global rating was determined based on the scores of each component. Two authors independently rated the six domains and overall quality (P. B. J., E. V. C.). Discrepancies were discussed until a consensus was reached. Inter-rater agreement was good (Cohen’s κ=0·68). Quality assessment of all studies included in the review is provided as the online Supplementary material SIII.
Results
The initial search identified 1412 (1389 citations identified by database search and twenty-three through other sources) unique records, of which 314 were removed owing to duplication. From the remaining 1098 records, 1023 citations were excluded based on the screening of titles and abstracts. Full-text articles for the remaining seventy citations were retrieved and reviewed. A total of thirty-nine articles did not satisfy the inclusion criteria and were excluded; thus, thirty-six articles were considered (Fig. 1).
A total of thirty-six articles (10 (28 %) RCT, 9 (25 %) RT and 17 (47 %) NRT) with a total of seventy intervention arms (diet, exercise, diet plus exercise), comprising 1561 participants, met the inclusion criteria.
Behavioural compensation in NEPA or related decreases in NEAT were observed in twenty-six out of seventy intervention arms (fifteen out of thirty-six studies), whereas the remaining forty-four showed no compensation. From those who compensated (fifteen studies), fifteen intervention arms were diet-only interventions, eight were exercise-only interventions and three were diet plus exercise interventions.
Detailed information about the included studies is presented in Tables 1–3, divided by design type – that is NRT, RT and RCT.
Studies will be further detailed by intervention type (diet-only, exercise-only and combined diet and exercise), as follows.
Intervention arms that decreased NEAT (i.e. twenty-one arms) presented a higher median value of weight loss (available in eighteen intervention arms: average of −10 kg) compared with those who showed no changes (available in thirty-eight intervention arms; average of −5 kg). Similar medians were observed for trial length, BMI and age. A similar trend was found when observing weight-loss medians for diet-only, exercise-only and combined diet and exercise, with higher weight loss found in the groups that reduced NEAT. In studies that showed reductions in NEPA, similar median weight loss, BMI, trials length and age were observed compared with those studies that reported no changes in NEPA. However, in diet-only interventions, weight loss observed in participants who decreased NEPA was double the weight loss found in those who did not compensate. The median study length of exercise-only studies that showed decreases in NEAT was half the median length of trials that present no changes in NEAT. Compared with exercise-only studies without changes in NEAT or NEPA, the median exercise frequency was half in studies that showed reductions in NEAT, whereas the median exercise duration was double in trials that decreased NEPA. Studies with or without behavioural intervention had similar proportion of cases between compensators and non-compensator groups. In exercise-only and combined diet and exercise, studies with prescribed strength exercise are absent of cases with behavioural compensation (Table 4).
Table 4 Diet-only, exercise-only, combined diet and exercise and all studies combined, according to the presence or absence of changes in non-exercise activity thermogenesis (NEAT) and non-exercise physical activity (NEPA)(Medians and ranges; numbers and percentages)
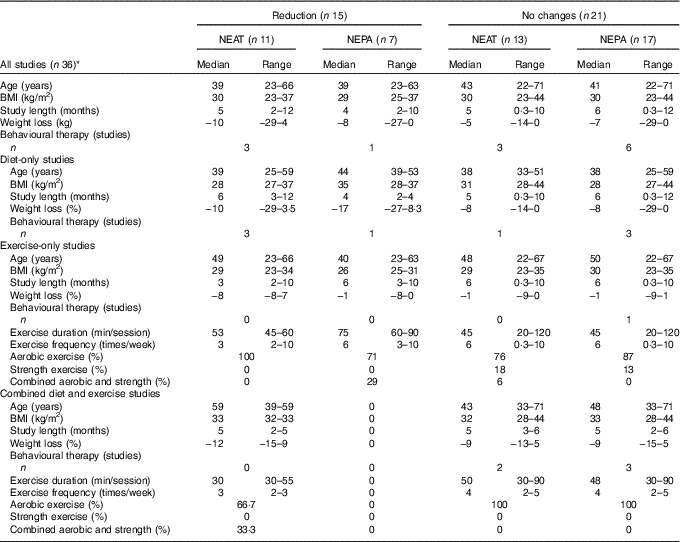
* Discrepancy between the number of overall studies that showed reductions (15) or no changes (21) in NEAT and NEPA and the number of studies displayed in the row below, according–the presence or absence of changes in NEAT (11 v. 13, respectively) and NEPA (7 v. 17, correspondingly), is due to studies that determined both NEAT and NEPA.
Diet-only interventions
The twenty-four diet-only interventions arms (i.e. fourteen diet-only trials) comprised approximately 39 % of the total number of studies included in this review, with a total of five NRT (36 %), six RT (43 %) and three RCT (21 %).
Study characteristics
Sample size
Diet-only interventions comprised a total of 400 participants with a median sample size of 18 (range 5–66). NRT included a median sample size of 23 (range 6–66), RT of 17 (range 5–57) and RCT of 15 (range 15–33).
Completion rate
Compliance to prescribed diet was only reported by DeLany et al.( Reference DeLany, Kelley and Hames 28 ) as 55 % and by Wang et al.( Reference Wang, Lyles and You 33 ) as 100 %. Since Leibel et al.( Reference Leibel, Rosenbaum and Hirsch 43 ) performed a laboratory-based study, full compliance with protocol was achieved. The remaining trials did not report compliance.
Trial length
The median length of the studies was 5·6 (range 2–12) months, varying from 3·5 (range 2–6) for NRT, 4 (range 2–6) for RT and 8 (range 6–12) for RCT.
Behavioural intervention
A total of five studies included behavioural therapy( Reference Racette, Schoeller and Kushner 14 – Reference Martin, Das and Lindblad 16 , Reference Martin, Heilbronn and de Jonge 22 , Reference Bonomi, Soenen and Goris 35 ) comprising 36 % of the diet-only studies included in this review.
Energy restriction
EI was restricted by 25( Reference Redman, Heilbronn and Martin 15 , Reference Martin, Heilbronn and de Jonge 22 ), 10, 20, 25 and 30 %( Reference Martin, Das and Lindblad 16 ), 33 %( Reference Bonomi, Soenen and Goris 35 ), 51 % of weight maintenance( Reference De Groot, Van Es and Van Raaij 37 ) and 75 % of resting EE (REE)( Reference Racette, Schoeller and Kushner 14 ). EI was prescribed as 3724 kJ/d( Reference Redman, Heilbronn and Martin 15 , Reference Martin, Heilbronn and de Jonge 22 ), approximately 2092 kJ/d in the first 4 weeks followed by 4 weeks at approximately 3515 kJ/d( Reference Kempen, Saris and Westerterp 29 ), 3347 kJ/d( Reference Weinsier, Hunter and Zuckerman 51 ), approximately 2929 kJ/d in the first 4 weeks and approximately 3347 kJ/d in the next 8 weeks( Reference Van Dale, Schoffelen and Ten Hoor 49 ), and according to body weight (<90·7 kg, 5021–6276 kJ/d; >90·7 kg and <113·4 kg, 6276–7531 kJ/d; and >113·4 kg, 7531–8368 kJ/d( Reference DeLany, Kelley and Hames 28 ). EI was also prescribed as a reduction of 2929 kJ/d( Reference Weigle 34 ), 3347 kJ/d( Reference Leibel, Rosenbaum and Hirsch 43 ) and 1682 kJ/d( Reference Wang, Lyles and You 33 ). EI was not reported in one study( Reference Brehm, Spang and Lattin 27 ).
Participant characteristics
Age
The median age across the fourteen studies was 40·5 years (range 25·0–58·6), with values of 35·5 years (range 25–51) for NRT, 46·3 years (range 36·6–58·6) for RT and 39·2 years (range 34·7–55·2) for RCT.
Sex
Seven studies included women only( Reference Racette, Schoeller and Kushner 14 , Reference Brehm, Spang and Lattin 27 , Reference Kempen, Saris and Westerterp 29 , Reference Wang, Lyles and You 33 , Reference De Groot, Van Es and Van Raaij 37 , Reference Van Dale, Schoffelen and Ten Hoor 49 , Reference Weinsier, Hunter and Zuckerman 51 ), one study included men only( Reference Weigle 34 ) and six studies included a combined sample of women and men( Reference Redman, Heilbronn and Martin 15 , Reference Martin, Das and Lindblad 16 , Reference Martin, Heilbronn and de Jonge 22 , Reference DeLany, Kelley and Hames 28 , Reference Bonomi, Soenen and Goris 35 , Reference Leibel, Rosenbaum and Hirsch 43 ).
BMI
Four studies included overweight/obese individuals( Reference Wang, Lyles and You 33 , Reference Bonomi, Soenen and Goris 35 , Reference Leibel, Rosenbaum and Hirsch 43 , Reference Van Dale, Schoffelen and Ten Hoor 49 ). Non-obese individuals were included in four studies( Reference Redman, Heilbronn and Martin 15 , Reference Martin, Das and Lindblad 16 , Reference Martin, Heilbronn and de Jonge 22 , Reference De Groot, Van Es and Van Raaij 37 , Reference Weinsier, Hunter and Zuckerman 51 ). Obese-only individuals were included in five studies( Reference Racette, Schoeller and Kushner 14 , Reference Brehm, Spang and Lattin 27 – Reference Kempen, Saris and Westerterp 29 , Reference Weigle 34 ). In the studies that provided data on this parameter, BMI was 31·2 kg/m2 (range 27·4–43·6), with a median of 31·4 kg/m2 (range 28·1–38·3) for NRT, 35·0 kg/m2 (range 31·7–43·6) for RT and 27·8 kg/m2 (range 27·4–27·9) for RCT.
Ethnicity
Two studies described ethnic groups as Caucasian, Black, Asian and Hispanics( Reference Redman, Heilbronn and Martin 15 , Reference Martin, Heilbronn and de Jonge 22 ), and four studies reported participants as Caucasian and Black( Reference Brehm, Spang and Lattin 27 , Reference DeLany, Kelley and Hames 28 , Reference Wang, Lyles and You 33 , Reference Weinsier, Hunter and Zuckerman 51 ). One study included Caucasian only( Reference De Groot, Van Es and Van Raaij 37 ) and seven studies did not report ethnic groups( Reference Racette, Schoeller and Kushner 14 , Reference Martin, Das and Lindblad 16 , Reference Kempen, Saris and Westerterp 29 , Reference Weigle 34 , Reference Bonomi, Soenen and Goris 35 , Reference Leibel, Rosenbaum and Hirsch 43 , Reference Van Dale, Schoffelen and Ten Hoor 49 ).
Physical activity level
Only six studies characterised the level of PA of the participants as sedentary( Reference Racette, Schoeller and Kushner 14 – Reference Martin, Das and Lindblad 16 , Reference Martin, Heilbronn and de Jonge 22 , Reference Wang, Lyles and You 33 , Reference Bonomi, Soenen and Goris 35 ).
Methods for assessing non-exercise activity thermogenesis
Among RCT, Martin et al.( Reference Martin, Das and Lindblad 16 ) assessed NEAT by subtracting the sum of REE from indirect calorimetry and thermic effect of food (TEF) (assumed as 0·1 TEE) from TEE by DLW. Redman et al.( Reference Redman, Heilbronn and Martin 15 ) assessed NEAT (referred to as activity-related EE) as the residual value of the regression between measured TEE obtained from DLW and measured sleeping metabolic rate (SMR) using indirect calorimetry. In RT, DeLany et al.( Reference DeLany, Kelley and Hames 28 ) assessed NEAT (referred to as PAEE, as exercise was not prescribed) as TEE from DLW minus the sum of REE by indirect calorimetry with TEF (assumed as 0·1TEE). Kempen et al.( Reference Kempen, Saris and Westerterp 29 ) assessed NEAT (referred to as PAEE, as exercise was not prescribed) by subtracting the sum of SMR from indirect calorimetry and the TEF (assumed as 0·1TEE) from TEE by DLW. Racette et al.( Reference Racette, Schoeller and Kushner 14 ) assessed NEAT (referred to as PAEE, as exercise was not prescribed) with DLW for TEE, indirect calorimetry for REE and TEF as TEE – (REE+TEF). Wang et al.( Reference Wang, Lyles and You 33 ) used ACC for non-exercise PAEE. Weigle( Reference Weigle 34 ) used a 24-h EE in a metabolic ward to assess NEAT (referred to as non-resting EE=24EE−REE). In NRT, Leibel et al.( Reference Leibel, Rosenbaum and Hirsch 43 ) assessed NEAT (referred to as non-resting EE) calculated as TEE from DLW minus the sum of REE and TEF obtained using a respiratory chamber. Weinsier et al.( Reference Weinsier, Hunter and Zuckerman 51 ) determine NEAT (referred to as PAEE, as exercise was not prescribed) by subtracting the sum of REE by indirect calorimetry with TEF (assumed as 0·1TEE) from TEE obtained by DLW.
Methods for assessing non-exercise physical activity
In RCT, Martin et al.( Reference Martin, Das and Lindblad 16 ) assessed NEPA with ACC (model 716 (Actigraph) and RT3 accelerometer (Stayhealthy, Inc.)). In another trial under the CALERIE study, Martin et al.( Reference Martin, Heilbronn and de Jonge 22 ) used a metabolic chamber to assess NEPA by determining the percent time participants were active. In RT, DeLany et al.( Reference DeLany, Kelley and Hames 28 ) assessed NEPA by counting steps/d using multisensor PA monitors (SenseWearPro3; BodyMedia Inc.). Racette et al.( Reference Racette, Schoeller and Kushner 14 ) assessed NEPA from HR monitors for PA assessment (excluding exercise data on exercise activity). Weigle( Reference Weigle 34 ) used a pedometer for assessing NEPA. In NRT, Bonomi et al.( Reference Bonomi, Soenen and Goris 35 ) assessed NEPA through a combined actometer and Doppler measures. Brehm et al.( Reference Brehm, Spang and Lattin 27 ) used pedometers for assessing NEPA.
De Groot et al.( Reference De Groot, Van Es and Van Raaij 37 ) assessed NEPA using an actometer and Doppler metre counts. Leibel et al.( Reference Leibel, Rosenbaum and Hirsch 43 ) assessed NEPA using a respiratory chamber equipped with a wall-mounted radar detector to monitor PA. Van Dale et al.( Reference Van Dale, Schoffelen and Ten Hoor 49 ) assessed NEPA using an actometer and HR monitor. Weinsier et al.( Reference Weinsier, Hunter and Zuckerman 51 ) determined NEPA by using the activity-related time equivalent (Arte) index for free-living PA (min/d).
Weight loss
Median weight loss was −11·0 kg (range −29·2 to 0·1) with a median of −12·0 kg (range −29·2 to 0·1) for NRT, −13·0 (range −26·9 to −6·1) for RT and −8·4 (range −11·2 to −3·5) for RCT.
Risk of bias
The quality of assessment tool rated one trial as weak( Reference Leibel, Rosenbaum and Hirsch 43 ), twelve as moderate( Reference Racette, Schoeller and Kushner 14 – Reference Martin, Das and Lindblad 16 , Reference Martin, Heilbronn and de Jonge 22 , Reference Brehm, Spang and Lattin 27 , Reference Kempen, Saris and Westerterp 29 , Reference Wang, Lyles and You 33 – Reference Bonomi, Soenen and Goris 35 , Reference De Groot, Van Es and Van Raaij 37 , Reference Van Dale, Schoffelen and Ten Hoor 49 , Reference Weinsier, Hunter and Zuckerman 51 ) and one trial as strong( Reference DeLany, Kelley and Hames 28 ) (online Supplementary material SIII).
Main outcome
A total of fifteen out of twenty-four intervention arms (seven out of fourteen diet-only interventions) reported a significant decrease in NEAT or NEPA resulting from the prescribed diet.
Among the nine diet-only trials assessing NEAT, decreases were observed in six studies (fourteen diet-only trial arms) – specifically three intervention arms of an NRT( Reference Leibel, Rosenbaum and Hirsch 43 ), four intervention arms of RT( Reference Racette, Schoeller and Kushner 14 , Reference Wang, Lyles and You 33 , Reference Weigle 34 ) and seven intervention arms of RCT( Reference Redman, Heilbronn and Martin 15 , Reference Martin, Das and Lindblad 16 ).
From the eleven studies assessing NEPA, behavioural compensation was observed in three diet-only interventions (four intervention arms), specifically one intervention arm of an NRT( Reference De Groot, Van Es and Van Raaij 37 ) and three intervention arms of RT( Reference Racette, Schoeller and Kushner 14 , Reference Weigle 34 ).
Exercise-only interventions
The thirty-five exercise-only intervention arms (twenty studies) comprised approximately 56 % of the total number of studies included in this review, with a total of eleven NRT (55 %), two RT (10 %) and seven RCT (35 %).
Study characteristics
Sample size
Exercise-only studies comprised 917 participants with a median sample size of 28 (range 8–139). NRT included a median sample size of 20 (range 9–40), RT included a median sample size of 27 (range 26–28) and RCT included a median sample size of 35 (range 8–139).
Completion rate
Fourteen trials reported the percentage of exercise sessions attended as >99( Reference Church, Martin and Thompson 19 , Reference Kozey-Keadle, Staudenmayer and Libertine 21 ), 62·5( Reference Willis, Herrmann and Honas 26 , Reference Herrmann, Willis and Honas 40 ), 94( Reference Turner, Markovitch and Betts 24 ), 99 % for moderate intensity and 96 % for high intensity( Reference Rosenkilde, Auerbach and Reichkendler 23 ), 90 % for aerobic training, 84 % for combined aerobic and resistance training, but % was not available for resistance training( Reference Rangan, Willis and Slentz 31 ), 100 % for 30-min and 60–80 % for 60- and 90-min groups( Reference Schutz, Nguyen and Byrne 32 ), 86( Reference Di Blasio, Ripari and Bucci 38 , Reference Meijer, Westerterp and Verstappen 48 ), >90( Reference Hunter, Byrne and Sirikul 41 ), 85( Reference Meijer, Westerterp and Verstappen 47 ), 95( Reference Van Etten, Westerterp and Verstappen 50 ), 100( Reference McLaughlin, Malkova and Nimmo 45 ) and 64 %( Reference Willis, Herrmann and Honas 26 , Reference Herrmann, Willis and Honas 40 ), whereas one trial( Reference Colley, Hills and King 36 ) reported the level of exercise EE (prescribed 6276 kJ/week; achieved 6000 kJ/week). Whybrow et al.( Reference Whybrow, Hughes and Ritz 25 ) and Meijer et al.( Reference Meijer, Janssen and Westerterp 46 ) reported good compliance, but no percent values were given. Compliance with the exercise protocol was not reported in three trials( Reference Hollowell, Willis and Slentz 20 , Reference Goran and Poehlman 39 , Reference Keytel, Lambert and Johnson 42 ).
Trial length
The median duration of the studies was 5·3 (range 0·3–10) months, varying from 4·2 (range 0·3–10) for NRT, 8 months for the two RT and 5·2 (range 0·5–10) for the RCT.
Behavioural intervention
Only the study of Kozey-Keadle et al.( Reference Kozey-Keadle, Staudenmayer and Libertine 21 ) included behavioural therapy.
Exercise mode
NRT included a variety of indoor or outdoor walking/running( Reference Colley, Hills and King 36 , Reference Di Blasio, Ripari and Bucci 38 , Reference Keytel, Lambert and Johnson 42 , Reference Meijer, Janssen and Westerterp 46 ), cycle ergometer exercise( Reference Goran and Poehlman 39 , Reference McLaughlin, Malkova and Nimmo 45 ), fitness classes and resistance training( Reference Meijer, Westerterp and Verstappen 47 , Reference Meijer, Westerterp and Verstappen 48 ), resistance training only( Reference Hunter, Byrne and Sirikul 41 , Reference Van Etten, Westerterp and Verstappen 50 ) and treadmill( Reference Herrmann, Willis and Honas 40 ). RT intervention arms involved a combination of resistance and aerobic training( Reference Rangan, Willis and Slentz 31 ) and daily walking( Reference Schutz, Nguyen and Byrne 32 ). The remaining RCT intervention arms used laboratory-based aerobic exercise conducted on cycle ergometers/rowers/steppers/Arc Trainer/treadmills( Reference Church, Martin and Thompson 19 – Reference Kozey-Keadle, Staudenmayer and Libertine 21 , Reference Rosenkilde, Auerbach and Reichkendler 23 , Reference Whybrow, Hughes and Ritz 25 , Reference Willis, Herrmann and Honas 26 ), or outdoor walking/running( Reference Turner, Markovitch and Betts 24 ).
Exercise supervision
All exercise sessions were supervised in thirteen NRT trials( Reference Goran and Poehlman 39 – Reference Hunter, Byrne and Sirikul 41 , Reference Meijer, Westerterp and Verstappen 47 , Reference Meijer, Westerterp and Verstappen 48 , Reference Van Etten, Westerterp and Verstappen 50 ) and RCT( Reference Church, Martin and Thompson 19 – Reference Kozey-Keadle, Staudenmayer and Libertine 21 , Reference Rosenkilde, Auerbach and Reichkendler 23 – Reference Willis, Herrmann and Honas 26 ), partially supervised in five NRT trials( Reference Colley, Hills and King 36 , Reference Di Blasio, Ripari and Bucci 38 , Reference Keytel, Lambert and Johnson 42 , Reference McLaughlin, Malkova and Nimmo 45 , Reference Meijer, Janssen and Westerterp 46 ) or not reported in RT( Reference Rangan, Willis and Slentz 31 , Reference Schutz, Nguyen and Byrne 32 ).
Exercise prescription (frequency)
The median exercise frequency was 4·1 (range 2·0–7·0) d/week with daily week values of 3·4 (range 2·0–5·0) for NRT, 3·9 (range 2·5–5·0) for RT and 4·8 (range 3·5–7·0) for RCT.
Exercise prescription (intensity)
Six NRT-prescribed intensity as a percentage of maximal VO2max (53 %( Reference McLaughlin, Malkova and Nimmo 45 ), 85 %( Reference Goran and Poehlman 39 )), percentage of maximal HR (70–80 %HRmax (40) Reference Herrmann, Willis and Honas , 70–75 %( Reference Keytel, Lambert and Johnson 42 )), by level of EE relative to body weight (moderate intensity: 28·6kJ/kg and high intensity: 57·1kJ/kg)( Reference Whybrow, Hughes and Ritz 25 ), one based on HR reserve (50 % HRR( Reference Meijer, Westerterp and Verstappen 48 )) and one based on ratings of perceived exertion (11–13 on a 15-point scale( Reference Di Blasio, Ripari and Bucci 38 )). Resistance training in the study by Hunter et al.( Reference Hunter, Byrne and Sirikul 41 ) was conducted at 65–85 % of one maximum repetition. Exercise intensity in RT/RCT studies that included aerobic exercise was prescribed as a percentage of maximal/peak VO2 in five trials (50 %( Reference Church, Martin and Thompson 19 ); three sessions >70 % and remaining self-selected( Reference Rosenkilde, Auerbach and Reichkendler 23 ), 70 %( Reference Turner, Markovitch and Betts 24 ); 65–80 % – vigorous and 40–55 – moderate( Reference Hollowell, Willis and Slentz 20 ), 75 %( Reference Rangan, Willis and Slentz 31 )), maximum HR (70–80 %( Reference Willis, Herrmann and Honas 26 )), between 40 and 65 % HR reserve( Reference Kozey-Keadle, Staudenmayer and Libertine 21 ). Prescribed exercise intensity was not reported in four trials( Reference Colley, Hills and King 36 , Reference Meijer, Janssen and Westerterp 46 , Reference Meijer, Westerterp and Verstappen 47 , Reference Van Etten, Westerterp and Verstappen 50 ) and self-selected in an RT( Reference Schutz, Nguyen and Byrne 32 ). Resistance training was performed at 8–12 repetition maximum (approximately 80 %)( Reference Rangan, Willis and Slentz 31 ), whereas Van Etten et al.( Reference Van Etten, Westerterp and Verstappen 50 ) did not report intensity.
Exercise prescription (duration)
Median session duration was approximately 55 min (20–120), with values of about 50 min (35–75) in NRT, 67 min (30–111) and 52 min (20–120). Four NRT-prescribed exercise duration was performed by time (50 min( Reference Di Blasio, Ripari and Bucci 38 ), 60 min aerobic plus 90 min aerobic/strength( Reference Meijer, Westerterp and Verstappen 47 ), 60 min aerobic plus 90 min aerobic/strength( Reference Meijer, Westerterp and Verstappen 48 ) and 39 and 55 min( Reference Herrmann, Willis and Honas 40 ), respectively, for the 1674 kJ/session and 2510 kJ/session), three based on level of exercise EE (1569–2092 kJ/session( Reference Colley, Hills and King 36 ), 1255 kJ/session( Reference Goran and Poehlman 39 ), 2092 kJ/session( Reference McLaughlin, Malkova and Nimmo 45 )) and two based on walking/running distance( Reference Keytel, Lambert and Johnson 42 , Reference Meijer, Janssen and Westerterp 46 ). The median duration of exercise for the five trials prescribing exercise by time was 150 (range 60–200) min/week. The median for trials prescribing exercise by EE was 4094 (range 1883–6276) kJ/week. Prescribed walking distance was 3–6 km/d( Reference Keytel, Lambert and Johnson 42 ), and running distance was 25–40 km/week( Reference Meijer, Janssen and Westerterp 46 ). One study dosed exercise by level of EE relative to body weight 28·6 or 57·1kJ/kg( Reference Whybrow, Hughes and Ritz 25 )) or absolute EE (2092 kJ/d above REE( Reference McLaughlin, Malkova and Nimmo 45 )). In three trials, prescribed exercise duration was calculated as EE per body weight ( Reference Church, Martin and Thompson 19 , Reference Hollowell, Willis and Slentz 20 , Reference Rangan, Willis and Slentz 31 ), four based on time( Reference Kozey-Keadle, Staudenmayer and Libertine 21 , Reference Turner, Markovitch and Betts 24 , Reference Whybrow, Hughes and Ritz 25 , Reference Schutz, Nguyen and Byrne 32 ) and two according to level of exercise EE (1255 and 2510 kJ/session( Reference Rosenkilde, Auerbach and Reichkendler 23 ); 1674 and 2510 kJ/session( Reference Willis, Herrmann and Honas 26 )). In three RCT, exercise time was prescribed for 60( Reference Turner, Markovitch and Betts 24 ), 40( Reference Kozey-Keadle, Staudenmayer and Libertine 21 ) and 40 min( Reference Whybrow, Hughes and Ritz 25 ). Schutz et al.( Reference Schutz, Nguyen and Byrne 32 ) involved three arms with different durations (30, 60 and 90 min). Willis et al.( Reference Willis, Herrmann and Honas 26 ) prescribed 39 and 55 min for the 1674 kJ/session and 2510 kJ/session; Rosenkilde et al.( Reference Rosenkilde, Auerbach and Reichkendler 23 ) prescribed 1255 and 2510 kJ/d; Church et al.( Reference Church, Martin and Thompson 19 ) prescribed 17, 33 and 50 kJ/kg per week; and Rangan et al.( Reference Rangan, Willis and Slentz 31 ) and Hollowell et al.( Reference Hollowell, Willis and Slentz 20 ) dosed duration as 58·6 kJ/kg per week and 59 and 96 kJ/kg per week. Resistance training in the study of Van Etten et al.( Reference Van Etten, Westerterp and Verstappen 50 ) consisted of three sets of fifteen reps over ten exercises, whereas for Hunter et al.( Reference Hunter, Byrne and Sirikul 41 ) duration of 45 min, ten repetitions with 2 min of rest by set was used.
Participant characteristics
Age
Studies were generally conducted in adults with a median age of 44·1 years (22·1–66·8), specifically 49·0 years (range 22·1–66·8) for NRT, 38·7 years (range 27·0–52) for RT and 42·2 years (range 23–57·5) for RCT.
Sex
Five studies included women only( Reference Church, Martin and Thompson 19 , Reference Schutz, Nguyen and Byrne 32 , Reference Colley, Hills and King 36 , Reference Di Blasio, Ripari and Bucci 38 , Reference Keytel, Lambert and Johnson 42 ), three studies included men only( Reference Rosenkilde, Auerbach and Reichkendler 23 , Reference Turner, Markovitch and Betts 24 , Reference Van Etten, Westerterp and Verstappen 50 ) and twelve studies included a combined sample of women and men( Reference Hollowell, Willis and Slentz 20 , Reference Kozey-Keadle, Staudenmayer and Libertine 21 , Reference Whybrow, Hughes and Ritz 25 , Reference Willis, Herrmann and Honas 26 , Reference Rangan, Willis and Slentz 31 , Reference Goran and Poehlman 39 – Reference Hunter, Byrne and Sirikul 41 , Reference McLaughlin, Malkova and Nimmo 45 – Reference Meijer, Westerterp and Verstappen 48 ).
BMI
Nine studies included overweight/obese individuals( Reference Church, Martin and Thompson 19 – Reference Kozey-Keadle, Staudenmayer and Libertine 21 , Reference Turner, Markovitch and Betts 24 , Reference Willis, Herrmann and Honas 26 , Reference Rangan, Willis and Slentz 31 , Reference Di Blasio, Ripari and Bucci 38 , Reference Herrmann, Willis and Honas 40 , Reference Meijer, Westerterp and Verstappen 48 ). Non-obese participants were included in ten studies( Reference Rosenkilde, Auerbach and Reichkendler 23 , Reference Whybrow, Hughes and Ritz 25 , Reference Schutz, Nguyen and Byrne 32 , Reference Goran and Poehlman 39 , Reference Hunter, Byrne and Sirikul 41 , Reference Keytel, Lambert and Johnson 42 , Reference McLaughlin, Malkova and Nimmo 45 – Reference Meijer, Westerterp and Verstappen 47 , Reference Van Etten, Westerterp and Verstappen 50 ). Obese-only individuals were included in one study( Reference Colley, Hills and King 36 ). Median BMI was 31·2 kg/m2 (range 27·4–43·6) in the studies that provided data on this parameter, with a median of 27·0 kg/m2 (range 22·8–33·9) for NRT, 27·8 kg/m2 (range 22·8–33·9) for RT and 42·2 kg/m2 (range 23·0–57·9) for RCT.
Ethnicity
Two studies described the ethnicity representation in the study sample as Caucasian( Reference Rosenkilde, Auerbach and Reichkendler 23 , Reference Hunter, Byrne and Sirikul 41 ), whereas three studies included Caucasian, Black, Hispanics and Asians( Reference Church, Martin and Thompson 19 , Reference Willis, Herrmann and Honas 26 , Reference Herrmann, Willis and Honas 40 ). The remaining studies did not report ethnicity.
Physical activity level/fitness
In all, seventeen studies characterised the level of PA of the participants as sedentary or exercising <2–3 d/week( Reference Church, Martin and Thompson 19 – Reference Kozey-Keadle, Staudenmayer and Libertine 21 , Reference Rosenkilde, Auerbach and Reichkendler 23 – Reference Willis, Herrmann and Honas 26 , Reference Rangan, Willis and Slentz 31 , Reference Colley, Hills and King 36 , Reference Herrmann, Willis and Honas 40 – Reference Keytel, Lambert and Johnson 42 , Reference McLaughlin, Malkova and Nimmo 45 – Reference Meijer, Westerterp and Verstappen 48 , Reference Van Etten, Westerterp and Verstappen 50 ). Di Blasio et al.( Reference Di Blasio, Ripari and Bucci 38 ) reported that PA level ranged from sedentary to highly active and fitness level from poor to good. Two studies did not report PA level or fitness( Reference Schutz, Nguyen and Byrne 32 , Reference Goran and Poehlman 39 ).
Methods for assessing non-exercise activity thermogenesis
Hollowell et al.( Reference Hollowell, Willis and Slentz 20 ) used an ACC (RT3 accelerometer; Stayhealthy, Inc.) for assessing NEAT (although authors referred ‘non-exercise PAEE’) by excluding exercise EE (ExEE) (including the 30 min before and after exercise). Turner et al.( Reference Turner, Markovitch and Betts 24 ) assessed NEAT (referred to as ‘non-prescribed PAEE’) through a combined HR monitor and ACC (Actiheart; CamNtech Ltd) by, respectively, subtracting the ExEE from the overall PAEE. Whybrow et al.( Reference Whybrow, Hughes and Ritz 25 ) calculated non-exercise EE as the difference between TEE from DLW and ExEE by an individual calibrated HR monitor. Willis et al.( Reference Willis, Herrmann and Honas 26 ) determined NEAT (referred as ‘non-exercise EE’) as (0·9 TEE–RMR)-net ExEE (ExEE–RMR), where TEE was assessed by DLW and REE and ExEE by indirect calorimetry. Rangan et al.( Reference Rangan, Willis and Slentz 31 ) assessed NEAT (referred to as non-exercise PAEE) with ACC (RT3 accelerometer; Stayhealthy, Inc.) by removing exercise data EE. Across NRT, Colley et al.( Reference Colley, Hills and King 36 ) assessed NEAT by subtracting the sum of exercise EE (using HR monitor), REE (indirect calorimetry) and TEF (assumed as 10 % of TEE) from TEE obtained by DLW. Di Blasio et al.( Reference Di Blasio, Ripari and Bucci 38 ) used a SenseWear Pro 2 armband on training and non-training days to assess whether PAEE between responders and non-responders differed in non-exercise days. Goran & Poehlman( Reference Goran and Poehlman 39 ) used DLW (TEE), indirect calorimetry (REE) and HR monitoring during exercise training to determine NEAT as TEE-(REE+0·1TEE+ExEE). Herrman et al.( Reference Herrmann, Willis and Honas 40 ) assessed NEAT (referred to as ‘non-exercise EE’) as (0·9 TEE–RMR)-net Exercise EE (exercise EE–RMR), where TEE was assessed by DLW and REE and ExEE by indirect calorimetry. Hunter et al.( Reference Hunter, Byrne and Sirikul 41 ) used DLW (TEE) and indirect calorimetry (REE) to determine PAEE (as TEE-01TEE-REE) and adjusted PAEE (adjusted for energy cost of average ExEE). HR monitoring with individual HR/EE calibration was used by Keytel et al.( Reference Keytel, Lambert and Johnson 42 ) to assess 24-h daily EE between training and non-training days and between exercise and control group. McLaughlin et al.( Reference McLaughlin, Malkova and Nimmo 45 ) calculated NEAT from HR monitor using HR/EE individual calibration between control and exercise periods. Meijer et al.( Reference Meijer, Janssen and Westerterp 46 ) assessed TEE with DLW, SMR by indirect calorimetry and NEAT (referred as EE from non-exercise activities). Van Etten et al.( Reference Van Etten, Westerterp and Verstappen 50 ) assessed NEAT (referred to as non-training EE) as TEE from DLW, minus SMR from indirect calorimetry minus ExEE from HR measurements.
Methods for assessing non-exercise physical activity
Church et al.( Reference Church, Martin and Thompson 19 ) used pedometers outside the training sessions for assessing NEPA. Kozey-Keadle et al.( Reference Kozey-Keadle, Staudenmayer and Libertine 21 ) assessed NEPA through ActivPAL (PAL Technologies). In RT, Schutz et al.( Reference Schutz, Nguyen and Byrne 32 ) determined NEPA through steps/d using an ACC (uniaxial), calculating the ratio between expected (from baseline steps/d) and observed steps/d from the prescribed walking. Rosenkilde et al.( Reference Rosenkilde, Auerbach and Reichkendler 23 ) assessed NEPA through ACC (model GT1M; Actigraph), by subtracting exercise counts from PA counts. Turner et al.( Reference Turner, Markovitch and Betts 24 ) assessed NEPA (as the time spent participating in PA above pre-determined thresholds) by a combined HR monitor and ACC (Actiheart; CamNtech Ltd) after subtracting exercise activity. Willis et al.( Reference Willis, Herrmann and Honas 26 ) determined NEPA was assessed through ACC (model GT1M; Actigraph) by subtracting ACC data from the exercise training sessions. In the studies of Meijer et al.( Reference Meijer, Janssen and Westerterp 46 – Reference Meijer, Westerterp and Verstappen 48 ), NEPA was assessed by ACC. Herrman et al.( Reference Herrmann, Willis and Honas 40 ) determined NEPA from ACC (model GT1M; Actigraph) after removing ACC data from the exercise sessions. Hunter et al.( Reference Hunter, Byrne and Sirikul 41 ) used an arte index for free-living PA (min/d) that reflects the amount of time a person spend in free-living PA. Van Etten et al.( Reference Van Etten, Westerterp and Verstappen 50 ) assessed NEPA through a triaxial ACC.
Weight loss
Median weight loss was −2·3 kg (range −9·1 to 0·1) with a median and range of −2·1 kg (range −9·1 to 0·1) for NRT and −2·4 kg (range −5·2 to −0·8) for RCT (data not available for RT).
Risk of bias
Using the quality assessment tool, eight trials were rated as weak( Reference Hollowell, Willis and Slentz 20 , Reference Turner, Markovitch and Betts 24 , Reference Whybrow, Hughes and Ritz 25 , Reference Rangan, Willis and Slentz 31 , Reference Schutz, Nguyen and Byrne 32 , Reference Colley, Hills and King 36 , Reference Keytel, Lambert and Johnson 42 , Reference McLaughlin, Malkova and Nimmo 45 ), eleven as moderate( Reference Kozey-Keadle, Staudenmayer and Libertine 21 , Reference Rosenkilde, Auerbach and Reichkendler 23 , Reference Willis, Herrmann and Honas 26 , Reference Di Blasio, Ripari and Bucci 38 – Reference Hunter, Byrne and Sirikul 41 , Reference Meijer, Janssen and Westerterp 46 – Reference Meijer, Westerterp and Verstappen 48 , Reference Van Etten, Westerterp and Verstappen 50 ) and one trial as strong( Reference Church, Martin and Thompson 19 ) (online Supplementary material SIII).
Main outcome
A total of seven out of twenty studies (eight in thirty-five intervention arms) reported a significant decrease in NEPA or NEAT, resulting from the prescribed exercise.
From the fourteen exercise-only interventions, reductions in NEAT were observed in four NRT (four intervention arms)( Reference Colley, Hills and King 36 , Reference Di Blasio, Ripari and Bucci 38 – Reference Herrmann, Willis and Honas 40 ).
In a total of twelve exercise-only trials, decreases in NEPA were observed in four studies, specifically three arms of NRT( Reference Herrmann, Willis and Honas 40 , Reference Meijer, Westerterp and Verstappen 47 , Reference Meijer, Westerterp and Verstappen 48 ) and two arms of a RT( Reference Schutz, Nguyen and Byrne 32 ).
Combined diet and exercise interventions
The 11 combined diet and exercise interventions arms (9 studies) comprised 25 % of the total number of studies included in this review with a total of 2 NRT (22 %), 5 RT (56 %) and 2 RCT (22 %).
Study characteristics
Sample size
Combined diet and exercise trials comprised a total of 244 participants with a median sample size of 23·8 (range 5–90). NRT included a median sample of 48 (range 6–90), RT of 20 (range 7–61) and two RCT, from the same large study (CALERIE), included twelve participants.
Completion rate
Two studies reported compliance to the exercise sessions (>90( Reference Wang, Lyles and You 33 ) and 22 %( Reference DeLany, Kelley and Hames 28 )). Two studies reported compliance to diet (100( Reference Wang, Lyles and You 33 ) and 55 %( Reference DeLany, Kelley and Hames 28 )). The remaining studies did not include data on compliance to either exercise or diet.
Trial length
The median duration of the studies was 4·4 months (range 2–0–6·0). Median duration was 2·8 months (range 2·5–3·0) for NRT, 4·4 months (range 2·0–6·0) for RT and 6 months for the two RCT.
Behavioural intervention
A total of five studies included behavioural therapy( Reference Racette, Schoeller and Kushner 14 , Reference Redman, Heilbronn and Martin 15 , Reference Martin, Heilbronn and de Jonge 22 , Reference Nicklas, Gaukstern and Beavers 30 , Reference Matsuo, Okura and Nakata 44 ) comprising 44 % of the combined diet and exercise studies included in this review.
Energy restriction
EI was reduced by 12·5 %( Reference Redman, Heilbronn and Martin 15 , Reference Martin, Heilbronn and de Jonge 22 ) and 75 % of RMR( Reference Racette, Schoeller and Kushner 14 ). EI was prescribed as 5021 kJ/dReference Matsuo, Okura and Nakata ( 44 ), approximately 2092 kJ/d in the first 4 weeks followed by 4 weeks at approximately 3515 kJ/d( Reference Kempen, Saris and Westerterp 29 ), about 2929 kJ/d in the first 4 weeks and about 3602 kJ/d in the next 8 weeks( Reference Van Dale, Schoffelen and Ten Hoor 49 ), and according to body weight (<90·7 kg, 5021–6276 kJ/d; >90·7 kg and <113·4 kg, 6276–7531 kJ/d; and >113·4 kg, 7531–8368 kJ/d( Reference DeLany, Kelley and Hames 28 )). EI was also prescribed as a reduction of 2510 kJ/d( Reference Nicklas, Gaukstern and Beavers 30 ) and 1443 kJ/d( Reference Wang, Lyles and You 33 ).
Exercise mode
One study used indoor aerobic and strength training( Reference Kempen, Saris and Westerterp 29 ), indoor aerobic/fitness training( Reference Matsuo, Okura and Nakata 44 , Reference Van Dale, Schoffelen and Ten Hoor 49 ), treadmill/walking( Reference Nicklas, Gaukstern and Beavers 30 , Reference Wang, Lyles and You 33 ), treadmill/stairstep/rowing/bicycling( Reference Racette, Schoeller and Kushner 14 ), walking/running/bicycling( Reference Redman, Heilbronn and Martin 15 , Reference Martin, Heilbronn and de Jonge 22 ) and outdoor brisk walking( Reference DeLany, Kelley and Hames 28 ).
Exercise supervision
Exercise sessions were supervised in NRT( Reference Matsuo, Okura and Nakata 44 , Reference Van Dale, Schoffelen and Ten Hoor 49 ) and RT( Reference Racette, Schoeller and Kushner 14 , Reference Nicklas, Gaukstern and Beavers 30 ), partially supervised in RT( Reference Kempen, Saris and Westerterp 29 ) and RCT( Reference Redman, Heilbronn and Martin 15 , Reference Martin, Heilbronn and de Jonge 22 ), or not reported in two RT( Reference DeLany, Kelley and Hames 28 , Reference Wang, Lyles and You 33 ).
Exercise prescription (frequency)
The median exercise frequency was 3·7 d/week (range 2·0–5·0) with 3·0 d/week (range 2·0–4·0) for NRT, 3·6 d/week (range 3·0–5·0) for RT and 5 d/week in the two RCT.
Exercise prescription (intensity)
Three trials prescribed intensity as a percentage of maximal VO2max (NRT: 55 %( Reference Van Dale, Schoffelen and Ten Hoor 49 ); RT: 50–60 %( Reference Kempen, Saris and Westerterp 29 ); 60–65 %( Reference Racette, Schoeller and Kushner 14 )), and two based on HR reserve (65–70 %( Reference Nicklas, Gaukstern and Beavers 30 ); 45–50 %; and 70–75 %( Reference Wang, Lyles and You 33 )). Prescribed exercise intensity was not reported in one trial( Reference Matsuo, Okura and Nakata 44 ) and was self-selected in three trials( Reference Redman, Heilbronn and Martin 15 , Reference Martin, Heilbronn and de Jonge 22 , Reference DeLany, Kelley and Hames 28 ).
Exercise prescription (duration)
Exercise duration was prescribed by time in most studies (NRT: 90 min/session( Reference Matsuo, Okura and Nakata 44 ) and 60 min/session( Reference Van Dale, Schoffelen and Ten Hoor 49 ); RT: 30 min/session( Reference Nicklas, Gaukstern and Beavers 30 ), 55 and 30 min/session( Reference Wang, Lyles and You 33 ); 60 min/session( Reference DeLany, Kelley and Hames 28 ); 45 min/session( Reference Racette, Schoeller and Kushner 14 ); 90 min/session( Reference Kempen, Saris and Westerterp 29 )). Prescribed exercise duration was self-selected in two RCT( Reference Redman, Heilbronn and Martin 15 , Reference Martin, Heilbronn and de Jonge 22 ).
Participant characteristics
Age
Studies were generally conducted in adults with a median age of 44·1 years (range 22·1–66·8), specifically 49·0 years (range 22·1–66·8) for NRT, 38·7 years (range 27·0–52) for RT and 42·2 years (range 23–57·5) for the two RCT.
Sex
Five studies included women only( Reference Racette, Schoeller and Kushner 14 , Reference Kempen, Saris and Westerterp 29 , Reference Wang, Lyles and You 33 , Reference Matsuo, Okura and Nakata 44 , Reference Van Dale, Schoffelen and Ten Hoor 49 ), and four studies included a combined sample of women and men( Reference Redman, Heilbronn and Martin 15 , Reference Martin, Heilbronn and de Jonge 22 , Reference DeLany, Kelley and Hames 28 , Reference Nicklas, Gaukstern and Beavers 30 ).
BMI
Three studies included overweight/obese individuals( Reference Wang, Lyles and You 33 , Reference Matsuo, Okura and Nakata 44 , Reference Van Dale, Schoffelen and Ten Hoor 49 ), four studies included obese participants( Reference Racette, Schoeller and Kushner 14 , Reference DeLany, Kelley and Hames 28 – Reference Nicklas, Gaukstern and Beavers 30 ) and two trials included non-obese individuals( Reference Redman, Heilbronn and Martin 15 , Reference Martin, Heilbronn and de Jonge 22 ). In the studies that provided data on this parameter, BMI was 32·3 kg/m2 (range 27·5–43·6), with a median and range of 29·1 kg/m2 (range 27·8–30·3) for NRT, 34·6 kg/m2 (range 32·4–43·6) for RT and 27·7 kg/m2 (range 27·5–27·8) for the two RCT.
Ethnicity
Two studies include all ethnic groups – that is, Caucasian, Black, Asian and Hispanics( Reference Redman, Heilbronn and Martin 15 , Reference Martin, Heilbronn and de Jonge 22 ); one study included Asians only( Reference Matsuo, Okura and Nakata 44 ); three studies included Caucasian and Black participants( Reference DeLany, Kelley and Hames 28 , Reference Nicklas, Gaukstern and Beavers 30 , Reference Wang, Lyles and You 33 ); and three studies did not report ethnic groups( Reference Racette, Schoeller and Kushner 14 , Reference Kempen, Saris and Westerterp 29 , Reference Van Dale, Schoffelen and Ten Hoor 49 ).
Participant activity level/fitness
Three studies characterised the level of PA of the participants as sedentary( Reference Racette, Schoeller and Kushner 14 , Reference Nicklas, Gaukstern and Beavers 30 , Reference Wang, Lyles and You 33 ).
Methods for assessing non-exercise activity thermogenesis
Redman et al.( Reference Redman, Heilbronn and Martin 15 ) assessed NEAT (referred as activity-related EE) as the residual value of the regression between measured TEE obtained from DLW and measured SMR using indirect calorimetry, but ExEE was not assessed. DeLany et al.( Reference DeLany, Kelley and Hames 28 ) assessed NEAT (referred to as PAEE) as TEE from DLW minus the sum of REE by indirect calorimetry with TEF (assumed as 0·1TEE) but ExEE was not calculated, restricting an accurate assessment of NEAT. Kempen et al.( Reference Kempen, Saris and Westerterp 29 ) assessed NEAT (referred to as non-exercised PA) by subtracting the sum of SMR from indirect calorimetry plus TEF (assumed as 0·1TEE) plus ExEE (from HR monitor) from TEE by DLW. Racette et al.( Reference Racette, Schoeller and Kushner 14 ) assessed NEAT (referred to as non-exercise PAEE) with DLW (for TEE), TEF and REE by indirect calorimetry and ExEE from HR monitors as TEE – (REE+TEF+ExEE). Wang et al.( Reference Wang, Lyles and You 33 ) used ACC (RT3 accelerometer; Stayhealthy, Inc.) for non-exercise PAEE (excluding exercise data on EE). Matsuo et al.( Reference Matsuo, Okura and Nakata 44 ) assessed NEAT using an ACC (Lifecorder; Suzuken Co. Ltd).
Methods for assessing non-exercise physical activity
Martin et al.( Reference Martin, Heilbronn and de Jonge 22 ) used a metabolic chamber to assess NEPA by determining the percent time participants were active. DeLany et al.( Reference DeLany, Kelley and Hames 28 ) assessed NEPA through steps/d using a multisensor PA monitor. Nicklas et al.( Reference Nicklas, Gaukstern and Beavers 30 ) used an ACC for assessing NEPA. Racette et al.( Reference Racette, Schoeller and Kushner 14 ) assessed NEPA from HR monitors for PA assessment (excluding exercise data on exercise activity). Van Dale et al.( Reference Van Dale, Schoffelen and Ten Hoor 49 ) assessed NEPA using an actometer and HR monitor.
Weight loss
Median weight loss was −9·8 kg (range −14·8 to −5·2) – specifically −9·2 kg (range −13·2 to −5·2) for NRT, −10·4 kg (range −14·8 to −6·6) for RT and −8·8 kg for the two RCT.
Risk of bias
On the basis of the quality assessment tool, one trial was rated as weak( Reference Matsuo, Okura and Nakata 44 ), seven as moderate( Reference Racette, Schoeller and Kushner 14 , Reference Redman, Heilbronn and Martin 15 , Reference Martin, Heilbronn and de Jonge 22 , Reference Kempen, Saris and Westerterp 29 , Reference Nicklas, Gaukstern and Beavers 30 , Reference Wang, Lyles and You 33 , Reference Van Dale, Schoffelen and Ten Hoor 49 ) and one trial as strong( Reference DeLany, Kelley and Hames 28 ) (online Supplementary material SIII).
Main outcome
A total of two out of nine combined diet and exercise interventions (three in eleven intervention arms) reported a significant decrease in NEPA or NEAT resulting from the prescribed diet plus exercise.
From the six combined diet and exercise trials that assessed NEAT, reductions were observed in three intervention arms of two RT( Reference Kempen, Saris and Westerterp 29 , Reference Wang, Lyles and You 33 ), whereas no behavioural compensation was observed in the five interventions that assessed NEPA.
Discussion
We systematically reviewed thirty-six studies with a variety of designs including NRT and RT to address whether the prescribed diet and/or exercise led to reductions in NEPA/NEAT in healthy adults. A reduction in NEAT has been hypothesised as a way to compensate for the increased EE of prescribed exercise and/or energy deficit from energetic restriction diets, resulting in less-than-expected negative energy balance and related weight loss( Reference Dhurandhar, Kaiser and Dawson 17 ).
Overall, our review found decreases in NEPA or NEAT in fifteen out of thirty-six studies conducted in healthy adults using diet-only intervention, combined diet and exercise intervention and exercise-only intervention (twenty-six out of a total of seventy intervention arms). Decreases in NEPA and/or NEAT were observed in seven out of fourteen diet-only interventions, two out of nine combined diet and exercise trials and seven out twenty exercise-only trials. In addition, it is important to highlight some other relevant findings. This review reported that the intervention arms that decreased NEAT were the ones presenting higher median values of weight loss (approximately 10 kg) compared with those who reported no changes in NEAT (approximately 5 kg). This observation suggests that reductions in NEAT may play a protective role when substantial body weight is lost.
Only seven of twenty exercise-only studies (eight out thirty-five intervention arms – 23 %) included in this review reported a significant decrease in NEAT assessed by DLW/HR/metabolic chambers/cart( Reference Colley, Hills and King 36 , Reference Di Blasio, Ripari and Bucci 38 – Reference Herrmann, Willis and Honas 40 ) or NEPA assessed by pedometer/ACC/actometer/doppler( Reference Schutz, Nguyen and Byrne 32 , Reference Herrmann, Willis and Honas 40 , Reference Meijer, Westerterp and Verstappen 47 , Reference Meijer, Westerterp and Verstappen 48 ). Studies that reported decreased NEPA/NEAT used a non-randomised design and were mainly conducted in sedentary overweight or obese adults. Age varied from young( Reference Schutz, Nguyen and Byrne 32 , Reference Herrmann, Willis and Honas 40 ), middle-aged( Reference Colley, Hills and King 36 , Reference Di Blasio, Ripari and Bucci 38 , Reference Meijer, Westerterp and Verstappen 47 , Reference Meijer, Westerterp and Verstappen 48 ) to older adults( Reference Goran and Poehlman 39 ). We observed that median age was similar between those who compensated compared with those who did not compensate, although Washburn et al.( Reference Washburn, Lambourne and Szabo 10 ) suggest that NEPA/NEAT may decrease in response to exercise training in older individuals.
In exercise-only studies that showed reductions in NEAT, the median duration of the studies was half the median duration of trials that did not present behavioural compensation. Apparently, compensation seems to occur in exercise studies of reduced duration. These results do not extend the findings observed by Riou et al.( Reference Riou, Jomphe-Tremblay and Lamothe 53 ), reporting that the degree of energy compensation is near 84 % for exercise interventions of a longer duration.
In contrast, seven out fourteen studies (fifteen out twenty-four intervention arms – 63 %) testing the effects of diet-only interventions reported a significant decrease in NEAT assessed by DLW/HR/ ACC/metabolic chambers/cart( Reference Racette, Schoeller and Kushner 14 – Reference Martin, Das and Lindblad 16 , Reference Wang, Lyles and You 33 , Reference Weigle 34 , Reference Leibel, Rosenbaum and Hirsch 43 ) or NEPA assessed by pedometer/ACC/actometer/doppler( Reference Racette, Schoeller and Kushner 14 , Reference Weigle 34 , Reference De Groot, Van Es and Van Raaij 37 ). Studies that reported decreased NEPA/NEAT were conducted in sedentary overweight or obese adults and used a randomised design. Median age was below 40 years( Reference Racette, Schoeller and Kushner 14 – Reference Martin, Das and Lindblad 16 , Reference De Groot, Van Es and Van Raaij 37 , Reference Leibel, Rosenbaum and Hirsch 43 ) in the majority of the trials, but middle-aged to older adults were studied( Reference Wang, Lyles and You 33 , Reference Weigle 34 ).
Considering the combined effects of diet and exercise, only two out of nine studies (three out eleven intervention arms – 27 %) testing the effects of diet plus exercise interventions showed a reduction in NEAT( Reference Kempen, Saris and Westerterp 29 , Reference Wang, Lyles and You 33 ) but not in NEPA by means of DLW/ACC/metabolic carts( Reference Kempen, Saris and Westerterp 29 , Reference Wang, Lyles and You 33 ). Studies that reported reductions in NEAT were conducted in sedentary overweight or obese adults and used a randomised design with a median age of 37 years( Reference Kempen, Saris and Westerterp 29 ) and approximately 60 years( Reference Wang, Lyles and You 33 ).
Reductions in NEAT/NEPA were observed in more than half of the diet-only intervention arms (approximately 63 %), followed by diet plus exercise (27 %) and exercise-only (23 %) intervention arms. It is possible that diet-only interventions are more prone to cause reductions in NEAT/NEPA compared with exercise-only or diet plus exercise, but this hypothesis has not been evaluated in a trial comparing changes in NEPA/NEAT in response to diet, exercise training protocols and combined diet and exercise training protocols. Moreover, in studies that involved exercise-only and diet plus exercise studies, the decrease in NEAT was absent in trials that prescribed resistance training. These observations suggest that exercise prescription may indeed have benefits for weight management interventions, although well-designed trials are required to definitively clarify the role of exercise dose on NEAT and NEPA.
Further, considering all the intervention arms that presented behavioural compensation in free-living PA, approximately 81 % reduced NEAT and only 19 % presented decrements in NEPA (twenty-one and nine intervention arms, respectively, out of twenty-six). Indeed, studies using methods to assess both NEAT and NEPA found reductions in the former but not in the latter( Reference Martin, Das and Lindblad 16 ). These observations may be owing to methodological limitations in assessing NEPA in free-living conditions, although we only included those studies that used objective measures of PA. Indeed, obtaining accurate measures of NEPA and NEAT in free-living conditions is challenging, specifically during an energy balance intervention given the variable nature of human adaptive response. DLW method and activity monitors are the most common approaches( Reference Hollowell, Willis and Slentz 20 , Reference Di Blasio, Ripari and Bucci 38 , Reference Goran and Poehlman 39 , Reference Westerterp 54 ). DLW is the state-of-the-art method for measuring TEE( Reference Westerterp 54 ). When DLW is used in exercise training trials, NEAT is typically estimated using the measured or estimated REE and ExEE. Most of the studies assume that the TEF represents 10 % of TEE without changes over the intervention. Therefore, NEAT is calculated as the difference between the TEE and the sum of REE, TEF (or the assumption of 0·1 TEE) and ExEE prescribed in exercise or combined diet and exercise interventions. In diet-only studies, authors refer to NEAT as PAEE, assuming that participants did not engage in exercise activities outside the energy-restricted intervention. A major drawback of determining NEAT is the involved cost, specifically owing to the use of DLW measurements, limiting the number of participants included in the studies. Nevertheless, DLW provides one value of TEE over a period of days, which means that for assessing NEAT when exercise is prescribed the related EE needs to be accounted for. In addition, DLW does not provide the type of non-Ex PA performed (i.e. sitting and ambulatory movement) or PA patterns. These limitations can partly be overcome using activity monitors, but estimates of EE from accelerometry are less accurate than those from DLW( Reference Westerterp 54 ). Indeed, PAEE might be somewhat independent of measurements of body movement for wide ranges of PA amounts( Reference Pontzer, Durazo-Arvizu and Dugas 55 ). Pontzer et al.( Reference Pontzer, Durazo-Arvizu and Dugas 55 ) observed that after controlling for body composition and size TEE was positively related with PA, but the association was stronger over the lower range of PA, whereas TEE plateaued in individuals whose PA was considered in the upper range, supporting a constrained TEE model.
Limitations of the studies
There are important short-comings in the studies included in this systematic review. The methodological issues in assessing PA and EE, described previously, may limit the accuracy in evaluating the impact of diet and/or exercise training on NEAT/NEPA. Considering the relevance of energy balance interventions for weight management, it is important to assess the effect of diet and/or exercise training on compensatory responses using accurate techniques. Only fourteen out of thirty-six studies( Reference Racette, Schoeller and Kushner 14 – Reference Martin, Das and Lindblad 16 , Reference Martin, Heilbronn and de Jonge 22 , Reference Whybrow, Hughes and Ritz 25 , Reference DeLany, Kelley and Hames 28 , Reference Kempen, Saris and Westerterp 29 , Reference Colley, Hills and King 36 , Reference Goran and Poehlman 39 – Reference Hunter, Byrne and Sirikul 41 , Reference Meijer, Janssen and Westerterp 46 , Reference Van Etten, Westerterp and Verstappen 50 , Reference Weinsier, Hunter and Zuckerman 51 ) assessed NEAT using DLW, the state-of-the-art method for TEE measurements in free-living individuals( Reference Schoeller 56 ). In addition, for determining NEAT, REE measures through indirect calorimetry and accurate methods for assessing ExEE are required. Only two studies included in this review provided measures of exercise EE by indirect calorimetry( Reference Willis, Herrmann and Honas 26 , Reference Herrmann, Willis and Honas 40 ), although REE was assessed with this technique in eleven studies along with DLW( Reference Racette, Schoeller and Kushner 14 , Reference Redman, Heilbronn and Martin 15 , Reference Martin, Heilbronn and de Jonge 22 , Reference DeLany, Kelley and Hames 28 , Reference Kempen, Saris and Westerterp 29 , Reference Colley, Hills and King 36 , Reference Goran and Poehlman 39 , Reference Hunter, Byrne and Sirikul 41 , Reference Meijer, Janssen and Westerterp 46 , Reference Van Etten, Westerterp and Verstappen 50 , Reference Weinsier, Hunter and Zuckerman 51 ).
Studies were not specifically designed and appropriately powered to detect differences in NEAT/NEPA between- or within-group with statistical significance in response to diet and/or exercise. The majority of these trials were conducted in small samples of <20 participants( Reference Racette, Schoeller and Kushner 14 , Reference Turner, Markovitch and Betts 24 , Reference Kempen, Saris and Westerterp 29 , Reference Weigle 34 , Reference Colley, Hills and King 36 , Reference De Groot, Van Es and Van Raaij 37 , Reference Goran and Poehlman 39 , Reference Hunter, Byrne and Sirikul 41 , Reference Keytel, Lambert and Johnson 42 , Reference McLaughlin, Malkova and Nimmo 45 , Reference Meijer, Westerterp and Verstappen 47 , Reference Van Dale, Schoffelen and Ten Hoor 49 , Reference Van Etten, Westerterp and Verstappen 50 ). In addition, in those studies that were specifically designed to address the effect of diet and/or exercise training on NEAT/NEPA, small samples were used( Reference Redman, Heilbronn and Martin 15 , Reference Martin, Heilbronn and de Jonge 22 , Reference Turner, Markovitch and Betts 24 , Reference Van Etten, Westerterp and Verstappen 50 ).
Other limitations include the lack of studies that tested the impact of the degree of energy restriction, weight-loss magnitude and exercise dose.
Limitations of this review
The findings of our review are based on data coming essentially from weak to moderate study designs (NRT and RT with an elevated risk of bias).
Conclusions
Although the present systematic review did not find evidence to suggest that diet and/or exercise training has a significant effect, decreases in NEPA (four studies), NEAT (eight studies) or both (three studies) were observed in 63 % of the total diet-only intervention arms, with only 23 and 27 % of the declines observed in exercise-only or combined diet and exercise trial arms. We also reported that participants who decreased NEAT presented a median amount of weight loss that was almost double the amount of those participants who did not compensate, suggesting that behavioural compensation leading to reductions in NEAT may depend on the degree of energy stores used when substantial body weight is lost, thus conserving energy.
Nevertheless, additional RT designed to specifically evaluate the impact of diet and/or exercise on NEPA/NEAT should be conducted in overweight/obese adults. In particular, studies should be powered to detect clinically significant differences. In addition, measures of daily and exercise EE should be included for an accurate assessment of NEAT. Studies must also analyse the impact of the degree of energy restriction, weight-loss magnitude, exercise dose and participant characteristics in more detail.
Acknowledgements
The data sets generated for this analysis are available from the corresponding author on reasonable request.
The current work was supported by National funding from the Portuguese Foundation for Science and Technology within the R&D units 472 (UID/DTP/00447/2013). P. B. J. is supported by the Portuguese Foundation for Science and Technology with a post-doctoral scholarship (SFRH/BPD/115977/2016).
A. M. S., P. J. T., N. K. and L. B. S. designed the study. A. M. S. performed the literature searches. P. B. J. and E. V. C. conducted the title, abstract and full text screening and data. All authors contributed to the interpretation of the results and critically reviewed the manuscript. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S000711451800096X







