Infection or inflammation causes marked responses in amino acid (AA) and protein metabolism. These include alterations in plasma AA concentrations, with many being decreased( Reference Bruins, Soeters and Lamers 1 – Reference Hoskin, Bremner and Holtrop 3 ), plus accompanying changes in whole-body AA irreversible loss rates (ILR)( Reference Bruins, Soeters and Lamers 1 , Reference Hoskin, Bremner and Holtrop 3 , Reference Bruins, Soeters and Deutz 4 ). These responses probably reflect complex interactions between the immune system and key regulatory organs. For example, lowered AA plasma concentrations may result from reduced net absorption, either from the inhibition of intake that accompanies inflammation and sepsis( Reference Faure, Moennoz and Montigon 5 ) or increased oxidation by the portal-drained viscera (PDV), as observed with certain gastrointestinal infections( Reference Yu, Bruce and Calder 6 ). Alternatively, demands within specific tissues can increase the removal of AA from the blood circulation to support the synthesis of either additional proteins or specific metabolites. For example, manufacture of positive acute-phase proteins increases liver utilisation of essential AA( Reference Bruins, Soeters and Deutz 4 ), especially phenylalanine( Reference Reeds, Fjeld and Jahoor 7 ), whereas elevated hepatic glucose synthesis during major stress( Reference Shaw and Wolfe 8 ) elevates metabolism of glucogenic AA, although this is not always observed( Reference Bruins, Soeters and Deutz 4 ). Similarly, production of additional glutathione to provide antioxidant protection adjacent to sites of inflammation and pro-oxidant activity can alter the demand for cysteine( Reference Malmezat, Breuille and Pouyet 9 ). Furthermore, activation of the immune system elevates the net use of AA to support proliferative responses associated with infection or surgery( Reference Essen, McNurlan and Thorell 10 ), and it also increases the hepatic use of glutamine( Reference Bruins, Soeters and Deutz 4 ), a known regulator of intermediary metabolism( Reference Stoll, Gerok and Lang 11 ). Although some of these needs are general and require most AA, as in the case of cellular proliferation, other reactions will be restricted to one, or just a few, AA and this will leave the remainder in disproportionate excess and lead to their disposal as urea and lead to the net N losses characteristic of inflammation and sepsis( Reference Biolo and Tessari 12 ).
Reduced plasma AA during infection or inflammation can be offset by either additional protein or AA supply( Reference Laurichesse, Tauveron and Gourdon 13 ), but the quantities required differ between AA( Reference Hoskin, Bremner and Holtrop 3 ) and between type and magnitude of the challenge( Reference Le Floc’h, Deblanc and Cariolet 2 ). The effectiveness of such approaches has been demonstrated in septic rodents, in which a cocktail of AA reduces the severity of the challenge and enhances the rate of recovery( Reference Breuille, Bechereau and Buffiere 14 ). Future nutritional strategies to help combat the deleterious effects of infection and inflammation and to aid recovery require knowledge of both the absolute demands for specific AA( Reference Hoskin, Bremner and Holtrop 3 ) and where in the body these requirements arise. The effectiveness of targeted intervention in sheep, on the basis of previous kinetic quantification of AA demands during the acute phase of an inflammatory challenge( Reference Hoskin, Bremner and Holtrop 3 ), is addressed in the current study. The focus is on splanchnic tissue metabolism, with consequences on absorption, liver-related protein metabolism and net AA supply to peripheral tissues. This was tested with a cocktail of six AA, based on information gained from an earlier study( Reference Hoskin, Bremner and Holtrop 3 ). Three (methionine, serine and threonine) were chosen because the ILR through plasma decreased markedly in response to lipopolysaccharide (LPS)( Reference Hoskin, Bremner and Holtrop 3 ), suggestive of a deficient supply during endotoxaemia. Cysteine has been reported as beneficial for septic rats( Reference Breuille, Bechereau and Buffiere 14 ) and LPS-challenged pigs( Reference Hou, Wang and Zhang 15 ) but showed no change in ILR in the previous sheep study( Reference Hoskin, Bremner and Holtrop 3 ). Also included was proline, which showed similar responses to cysteine, with no effects on ILR but marked decreases in plasma concentration under an LPS challenge( Reference Hoskin, Bremner and Holtrop 3 ). The final AA in the cocktail was glutamine, the supplementation of which is often used in clinical situations( Reference Wernerman 16 ) and exhibits increased turnover during cancer( Reference de Blaauw, Heeneman and Deutz 17 ) and endotoxin challenge( Reference Hoskin, Bremner and Holtrop 3 ). For all these AA, the amounts infused were based on the product of their ILR and the fractional reduction in plasma concentration, as observed under similar experimental conditions previously( Reference Hoskin, Bremner and Holtrop 3 ).
Methods
Sheep and diets
All procedures were approved by the Ethical Review Committee of the Rowett Institute of Nutrition and Health and conformed to UK legislation under the Animals (Scientific Procedures) Act 1986. Suffolk cross lambs (eight lambs, two female, six castrate male; 12–16 months old, 37–54 kg live weight) were prepared with silicone rubber catheters in the aorta, mesenteric vein, portal vein and hepatic vein( Reference Lobley, Connell and Lomax 18 ). During the 2-week recovery period from surgery, the sheep were offered a mixed roughage–concentrate diet (daily 2×500 g as fed) with the following composition (g/kg as fed): hay 500, barley 300, molasses 100, fishmeal 90, vitamin and mineral mix 10 (2×500 g/d as fed); 830 g DM/kg; 21·3 g N/kg DM, 11·0 MJ metabolisable energy/kg DM, at an estimated 1·0–1·3×energy maintenance based on metabolic body weight (kg0·75). Subsequently, they were acclimatised over 1 week to metabolism crates, with the daily feed provided as 24-h portions, and then allocated to treatments when a temporary polyvinyl catheter was inserted into the jugular vein( Reference Lobley, Connell and Lomax 18 ).
At treatment allocation, the sheep (n 8) were divided between two groups, balanced for sex and weight. Within each group, the sheep were measured on 2 experimental days, 7 d apart. For group A (n 4), the 2 infusion days involved either sterile 0·15 m-sodium chloride (control) infused into both the jugular vein (15 g/h) and mesenteric vein (40 g/h) for 20 h, whereas for the other experimental day the jugular vein infusion (15 g/d) involved LPS (from Escherichia coli 155, serotype 055:B5, 2 ng/min per kg body weight (BW)), as described previously( Reference Hoskin, Bremner and Holtrop 3 ). For group B (n 4), 1 experimental day was identical to the control procedure used for group A, whereas the other experimental day involved a jugular vein infusion of LPS, identical to group A, plus a sterile mixture of AA into the mesenteric vein (40 g/h). The order of infusions (saline v. LPS, with or without AA) was randomised between sheep. Therefore, all eight sheep received a control (saline) jugular infusion, whereas four received LPS as a treatment and four received LPS plus AA. The amounts for each of the AA infused were calculated from the product of their ILR and the fractional decrease in arterial concentration from control value in response to a similar dose of LPS, both as reported previously( Reference Hoskin, Bremner and Holtrop 3 ). For sheep with a BW of 50 kg, the concentrations of AA-N in the supplement were cysteine (68 mm), glutamine (320 mm), methionine (38 mm), proline (64 mm), serine (124 mm) and threonine (138 mm) dissolved in 0·15 m-sodium chloride. For sheep of other weight, the concentration of the infusate was adjusted based on BW0·75.
Just before the start of each 20-h infusion period, a background blood sample was taken for evaluation of clinical parameters (including blood Hb, plasma albumin, leucocytes count and cell type distribution) and determination of blood and plasma DM (by freeze-drying, each in triplicate). In order to measure plasma flow across the splanchnic tissues, sterile sodium p-aminohippurate (0·1 m prepared in 0·05 m-sodium phosphate buffer pH 7·4) containing 250 IU/g heparin (Leo Laboratories) was also infused at a rate of 40 g/h into the mesenteric vein over a period of 15–20 h. This was mixed with the appropriate AA or 0·15 m-sodium chloride infusate via a t-piece connector. From 12 to 20 h, a solution of 15 mm-[2H5]phenylalanine (99 at%; Cambridge Isotope Laboratories) in 0·15 m sterile saline was infused at 10 g/h into the jugular vein, again via a t-piece connector.
Between 16 and 20 h of LPS infusion, blood was withdrawn continuously over iced water as 4×1 h samples from the arterial, portal and hepatic venous catheters( Reference Lobley, Connell and Lomax 18 ), with 12 ml taken for each collection. At 12 and 20 h, 20 ml of arterial blood was withdrawn and maintained at room temperature for immediate processing of lymphocytes. At 20 h, the various infusions were stopped and 6 g of a sterile saline containing 24 mg of Evan’s Blue was injected via the jugular catheter. Then, 2·5 ml of blood was withdrawn at 3, 6, 9 and 12 min after injection to allow the estimation of plasma volume( Reference Mackie 19 ).
Chemical analyses
Clinical blood parameters were determined as described previously( Reference Hoskin, Bremner and Holtrop 3 ). Immediately following each hourly collection, blood analysis (pH, pO2, pCO2 and Hb) was performed with an ABL650 Blood Gas Analyser (Radiometer) and packed cell volume was determined using a microcentrifuge. Blood samples were then centrifuged at 1000 g for 15 min, and the plasma was used for various analyses using gravimetric procedures( Reference Lobley, Connell and Lomax 18 ). All blood samples taken were analysed individually, parameters were then calculated separately for each hour of collection and then the mean of these was used for statistical analysis.
The p-aminohippurate concentration was quantified on 0·7 g of plasma( Reference Lobley, Connell and Lomax 18 ), whereas 1 g of plasma from each sample site was retained for enrichment analysis of [2H5]phenylalanine. To another 1·4 g of plasma was added 0·6 g of a mixture of [U-13C] algal hydrolysate containing [5-15N]glutamine, [indole-15N]tryptophan, [1-13C]cysteine and [15N2]urea to allow the determination of AA concentrations by isotope dilution( Reference Calder, Garden and Anderson 20 , Reference Lobley, Holtrop and Bremner 21 ). This sample was divided into two equal portions, with one kept in reserve. A further portion of fresh plasma (0·4 g) was analysed with commercial kits for total protein, albumin, glucose (kits 981-387, 981-767, 981-304, respectively; Thermo Scientific), ammonia (kit 17660; Sentinel Diagnostic) and lactate (kit 735-10; Trinity Biochemicals) on a clinical analyser (Kone Limited). Plasma albumin was isolated from arterial plasma( Reference Connell, Calder and Anderson 22 ). Concentrations of Evans Blue bound to plasma protein were determined spectrophotometrically( Reference Mackie 19 ) and values were extrapolated to zero time injection to allow the estimation of plasma volume. Determination of the enrichments of plasma free [2H5]phenylalanine and the extraction, hydrolysis and analysis of both albumin and plasma total protein labelled with [2H5]phenylalanine were performed as described previously( Reference Connell, Calder and Anderson 22 ). Lymphocytes were isolated from the 20-ml blood samples taken at the start and end of the isotope infusion. The blood was first gently diluted 1:1 with 0·15 m-NaCl, and 5-ml portions were slowly layered onto 5 ml of Histopaque 1077 lymphocyte separation medium (Sigma Bioscience), in eight separate tubes, with care taken to avoid mixing of the two layers. The tubes were then centrifuged at 700 g for 20 min at 20°C with no brake applied. The lymphocyte layer at the interface was then carefully removed from each tube using a Pasteur pipette and was transferred to a 10-ml glass hydrolysis tube and diluted to 10 ml with ice-cold 0·15 m-NaCl saline. This solution was centrifuged at 1500 g for 15 min at 4°C, and the pellet was resuspended in 4 ml of ice-cold lysis buffer (9:1 (v/v) ammonium chloride (8·3 g/l): 0·17 m-Tris-HCl buffer pH 7·65). This was re-centrifuged at 1500 g for 15 min at 4°C, and the supernatant containing the contents from erythrocyte lysis was decanted. The pellet was then washed with ice-cold saline and re-centrifuged on three more occasions. After the final wash, the pellet was resuspended in 5 ml of distilled water and stored frozen at −20°C until further analysis. Lymphocyte protein was prepared in a manner similar to the general procedure for albumin and total plasma protein and involved thawing the stored suspension and deproteinisation with the addition of 0·8 ml of 48 % (w/v) sulphosalicylic acid (SSA). After allowing the sample to stand on ice for 10 min, it was centrifuged at 1500 g for 20 min at 4°C. The sample was then washed twice with 8 ml of 8 % ice-cold SSA and centrifuged on each occasion. The supernatant was removed and to the pellet a phenol crystal was added (to protect aromatic AA against oxidation) followed by 4 ml of 4 m HCl. This was then hydrolysed for 18 h at 105°C in a heating block. Subsequent steps were then performed as described previously( Reference Connell, Calder and Anderson 22 ).
Calculations
Plasma flows (kg/min) were determined by a gravimetric approach( Reference Lobley, Connell and Lomax 18 ) with blood flow calculated from plasma flow/(1–packed cell volume). Hepatic artery (FA) flow (blood or plasma) was determined as the difference between the flows in the hepatic vein (FH) and hepatic portal vein (FP). The plasma and blood water flows were calculated from the DM in order to quantify urea transfers( Reference Lobley, Bremner and Nieto 23 ). In general, net mass transfers (µmol/min) of individual AA or metabolites across the PDV were calculated as follows:
where C a , C p and C h are metabolite concentrations (µmol/kg) in arterial, hepatic portal vein and hepatic vein fluids (blood for O2, ammonia; blood water for urea; plasma for all other measurements).
For protein synthesis (µmol/min), estimates based on isotope transfers across the gastrointestinal tract and liver the appropriate calculations were as follows:
across the PDV:
and across the liver:
where E h , E p and E a are the respective enrichments (molar % excess) of free [2H5]phenylalanine in plasma from the hepatic vein, portal vein and artery, and where E x is the enrichment value selected as representative of the precursor pool. For comparison with whole-body ILR measurements, E x was based on the arterial value, but for other comparisons E x was assumed to be similar to hepatic venous enrichments, as this has been shown to reflect well values for export proteins( Reference Connell, Calder and Anderson 22 ). Both estimates of precursors, however, are less than values observed in the intracellular pools of either the liver( Reference Connell, Calder and Anderson 22 ) or the gastrointestinal tract( Reference Lobley, Shen and Le 24 ). For both net transfer data and isotope kinetics, the concentrations, enrichments and flows were calculated for each individual hour of collection and then averaged.
Whole-body ILR (mmol/h) of the tracee was estimated by the standard procedure – that is
where 99 is the enrichment (molar % excess) of the [2H5]phenylalanine infusate.
Enrichments for both total protein and albumin from plasma were altered in a linear manner over the times of collection, and their respective gradients (change molar % excess/h) were divided by E h (representative of the precursor for export proteins( Reference Connell, Calder and Anderson 22 )) and adjusted to give daily fractional synthesis rates (FSR). These FSR were then converted to absolute synthesis rates (g/d) by
where total plasma volume was determined by dilution of Evans Blue( Reference Mackie 19 ). For lymphocytes, a value of 28·2 pg protein/cell was adopted( Reference Schroder, Schafer and Schauder 25 ).
Power calculations and statistical analyses
The number of sheep per treatment group (control combined with LPS or control combined with LPS+AA) was based on comparing the effect of LPS+AA with the effect of LPS alone. The power calculations were performed at a power of 80 % for the 5 % significance level, with the between-sheep variance obtained from previous studies( Reference Hoskin, Bremner and Holtrop 3 , Reference Connell, Calder and Anderson 22 ). A considerable number of parameters were to be assessed in this study, but the decision on the number of sheep per treatment group was based on the following two outcomes that were deemed most indicative of the restorative effect of AA supplementation on an LPS challenge. The first parameter involved restoration of the arterial concentrations for the six AA infused in combination with LPS to at least 90 % of the control (saline) values. Expected decreases for these six AA were expected to be 23–73 % by the action of LPS alone( Reference Hoskin, Bremner and Holtrop 3 ) with between-sheep sd 13–43 % of control values( Reference Hoskin, Bremner and Holtrop 3 ) yielding n 4 per treatment group. The second parameter involved changes in FSR for plasma albumin, a negative acute-phase protein, and based on an sd of 0·49 %/d( Reference Connell, Calder and Anderson 22 ) a change of 1·4 %/d, equivalent to the difference between fed and fasted sheep( Reference Connell, Calder and Anderson 22 ), would be observed with n 4 per treatment group. Additional power was gained by initial selection of six sheep per treatment group. Unfortunately, one sheep required to be euthanised during the surgical procedure, whereas three others developed non-patent catheters in either the portal or hepatic veins before the end of the study. Thus, eleven sheep had measurements reliant on arterial samples (this included the two criteria used for the power calculations above), but only four sheep per treatment group were used for the full splanchnic transfers. In practice, the changes based on arterial values only were similar between the eleven and eight sheep comparisons. Therefore, for the main text, data are presented for the eight sheep that had the full working splanchnic-bed catheters. Of these, four received a saline infusion or LPS infusion on 2 separate days. The other four sheep received a saline infusion or LPS+AA infusion on 2 separate days. The order of infusions was randomised within each group. Although this is a small study, the use of a frequent feeding regimen, continuous administration of a low dose of LPS over the experimental duration and collection of integrated blood samples all helped reduce the associated variance and meant that fewer animals were needed to detect statistical differences. In addition, where appropriate, results from the eleven sheep are presented as the online Supplementary Material.
Data were analysed as a mixed model using the residual maximum likelihood (REML) estimation procedure in Genstat 13th edition, release 13.2 (VSN International). The influence of LPS (independent of whether AA were infused or not) was assessed with sheep and period (experimental day) within sheep as random effects, whereas period and LPS status (present or absent) and their interaction were regarded as fixed effects. To assess the influence of AA infusion, sheep and period within sheep were considered as random effects, whereas period and treatment and their interaction were considered as fixed effects, where treatment involved saline, LPS and LPS+AA infusions.
All data are presented as predicted means from the REML analysis, with the maximum standard error of the difference between means value also given for the various comparisons. P<0·05 was taken as evidence of a significant response and 0·05<P<0·10 as weak evidence.
Results
Whole-body responses and arterial concentration changes
Within the first 4 h of LPS infusion, there were increases in body temperature (1·0–2·0°C) and respiration rate, as observed previously( Reference Hoskin, Bremner and Holtrop 3 ). During the same period, occasionally there were mild reluctances to eat, but this never exceeded more than two-hourly meals; the meals were consumed soon thereafter, and thus for the total 20 h there were no refusals. Over the period of blood collection (16–20 h after the start of LPS or vehicle infusion), endotoxaemia did not affect either arterial blood pH or Hb status (data not shown). Hb was lower during the second period (10·7 v. 9·1, sed 0·61, P=0·027), as was plasma albumin (27·1 v. 26·5 g/l, sed 0·22, P=0·013), both possibly related to the amount of blood withdrawn over the various procedures. Blood leucocyte numbers were slightly increased just before LPS infusion for the second period (10·24 v. 12·25×109 cells/l blood, sed 0·870, P=0·046). Most of this could be attributed to whether the sheep had received LPS or saline during the first period (14·63 v. 11·35×109 cells/l blood, sed 1·487, P=0·055, respectively). There were no differences between allocated groups (saline or LPS) in leucocyte count before period 1. There were no period or previous treatment effects on the proportion of neutrophils (34 %) and lymphocytes (64 %) in the monocyte population before each infusion period. At the end of the 20-h infusion, numbers of lymphocyte cells had more than doubled in response to LPS challenge compared with saline infusion (Table 1).
Table 1 Impact of a 20-h infusion of saline or lipopolysaccharide (LPS; 2 ng/kg live weight per min), either with or without six supplemental amino acids (AA), on arterial concentrations of albumin, total protein, glucose, lactate and lymphocytes in eight sheep (Predicted means with their standard errors of the difference (sed) between means for the effect of treatment)
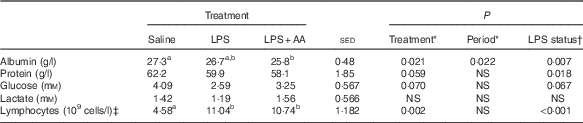
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05); post hoc t test was performed to compare the treatment means.
* Analysed by random-effects model, with sheep and period within sheep as random effects and period and treatment plus their interaction as fixed effects, where treatment was either a saline, LPS or LPS+AA infusion. There were no period×treatment effects (P>0·05).
† Analysed by random-effects model as described above, where treatment reflects LPS status – that is either saline or LPS (both alone and in combination with AA).
‡ One missing value (for LPS treatment).
Endotoxaemia tended to cause a 30 % decrease in arterial glucose (P=0·067), whereas lactate concentrations were unaffected (Table 1). Arterial plasma concentrations of both total protein (−6 %, P=0·018) and albumin (−4 %, P=0·007; Table 1) were reduced by LPS. In contrast, although the FSR of albumin was also decreased (−48 %, P<0·001), that of total protein was increased (+37 %, P=0·012; Table 2). As total plasma volume (average 2·18 litres) was unaffected by either period or treatment, similar directions of change were also observed for the ASR of both albumin and total protein (both P<0·01; Table 2) in response to LPS. The substantial increase in lymphocyte numbers was accompanied by a 63 % increase in FSR (P=0·043; Table 2), whereas the ASR was elevated by 386 % (P<0·001). For all these variables, the responses to LPS were independent of whether the supplemental AA were provided or not. Responses reported in both Tables 1 and 2 for the eight sheep with complete functional catheters across splanchnic tissues at the end of the study were similar to data obtained from the original eleven sheep (online Supplementary Tables S1 and S2).
Table 2 Effect of a 20-h infusion of saline or lipopolysaccharide (LPS; 2 ng/kg live weight per min), either with or without six supplemental amino acids (AA), on synthesis rates of plasma albumin, total plasma protein and lymphocytes in eight sheep (Predicted means with their standard errors of the difference between means for the effect of treatment)
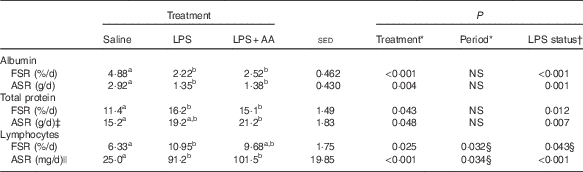
FSR, fractional synthesis rate; ASR, absolute synthesis rate.
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05); post hoc t test was performed to compare the treatment means.
* Analysed by random-effects model, with sheep and period within sheep as random effects and period and treatment plus their interaction as fixed effects, where treatment was either a saline, LPS or LPS+AA infusion.
† Analysed by random-effects model as described above, where treatment reflects LPS status – that is either saline or LPS (both alone and in combination with AA).
‡ Period×treatment effect (P=0·029) values lower during period 2 for LPS treatment (similar between periods for saline and LPS+AA infusions).
§ Values lower during period 2.
|| One missing value (LPS).
Arterial plasma concentrations of amino acids
For most non-infused AA, the arterial plasma concentrations were reduced substantially with both LPS and LPS+AA treatments (by 20–50 %, P<0·01; Table 3). The exceptions were tryptophan, where only a tendency was observed (−15 %, P=0·079), whereas for phenylalanine the plasma concentration increased (+30 %, P<0·001). The reductions for the non-infused AA were not influenced by the infusion of the six AA. LPS infusion also increased plasma urea (22 %, P=0·014).
Table 3 Effect of a 20-h infusion of saline or lipopolysaccharide (LPS; 2 ng/kg live weight per min), either with or without six supplemental amino acids (AA), on plasma arterial concentrations (µmol/kg) of AA in eight sheep (Predicted means with their standard errors of the difference between means for the effect of treatment)

a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05); post hoc t test was performed to compare the treatment means.
* Analysed by random-effects model, with sheep and period within sheep as random effects and period and treatment plus their interaction as fixed effects, where treatment was either a saline, LPS or LPS+AA infusion.
† Analysed by random-effects model as described above, where treatment reflects LPS status – that is either saline or LPS (both alone and in combination with AA).
‡ Values greater in period 2.
§ Period×treatment effect (P=0·009), with lower values for LPS+AA during period 2.
|| Period×treatment effect (P=0·003 for Gln; P=0·04 for Thr), with greater values for LPS+AA during period 2.
For the six AA that formed the supplement, these also showed decreased plasma concentrations when the sheep were challenged with LPS but infused with saline (reduced by 14–74 %, all P<0·05). For the four sheep in which the supplement was infused, this restored plasma concentrations to saline-infused values for cysteine, glutamine, methionine, proline and serine (Table 3). The infusion of threonine over-compensated (P=0·007).
Splanchnic bed metabolism
Plasma flows in the portal vein, hepatic vein and hepatic artery (means 1·347, 1·428 and 0·080 kg/min, respectively) were not altered by the endotoxin challenge (data not shown), nor were there any effects of LPS (with or without supplemental AA) on splanchnic tissue O2 uptake (−112 and –113 μmol/min for PDV and liver, respectively). Although glucose concentrations were higher in the hepatic vein than in either the portal vein or hepatic artery (3·81, 3·56, 3·51 mm, respectively, sed 0·041, P<0·001) with, in consequence, more glucose appearance across the liver than the PDV (+360 v. +89 μmol/min, sed 79·1, P<0·001), there was no effect of LPS infusion, with or without infused AA present. Similarly, lactate transfers were also unaffected by endotoxaemia (−130 and +64 μmol/min for PDV and liver, respectively).
Portal-drained viscera amino acid transfers
All AA, except glutamine, showed net positive appearances (uptake) across the PDV under the various experimental conditions (Table 4). Over the 4 h of sampling, infusion of LPS did not alter net PDV appearance, compatible with the lack of effect of the endotoxin on food intake. In contrast, the net PDV appearance of glutamine across the PDV was negative during saline infusions (indicative of metabolism of endogenous glutamine by the gut tissues), but this was reduced by 67 % (P=0·014) with LPS infusion (without supplemental AA). Neither removal of urea nor appearance of ammonia across the PDV was affected by LPS infusion. As expected, mesenteric vein infusion of the six AA increased their portal vein appearance (all P<0·001). For the six infused AA, apparent recovery within sheep across the PDV was not significantly different from 100 % for methionine, proline, serine, threonine and glutamine, but only 71 % for cysteine (P=0·004). The amount of glutamine infused was sufficient to provide a net positive supply to the liver (Table 4). PDV appearances of alanine and lysine (both P<0·05) were also increased by infusion of the AA supplement. For alanine, this restored values to those of the saline-infused period, whereas for arginine net supply increased above that for the control (saline) period (P=0·012).
Table 4 Net portal-drained viscera supply (absorbed from diet+any infused amino acid (AA)) of AA-N (µmol N/min) in eight sheep in response to 20-h infusions of saline or lipopolysaccharide (LPS; 2 ng/kg live weight per min), either with or without six supplemental AA (Predicted means with their standard errors of the difference between means for the effect of treatment)

a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05); post hoc t test was performed to compare the treatment means.
* Analysed by random effects model, with sheep and period within sheep as random effects and period and treatment plus their interaction as fixed effects, where treatment was either a saline, LPS or LPS+AA infusion. There were no period×treatment effects (P>0·05).
† Analysed by random-effects model as described above, where treatment reflects LPS status – that is either saline or LPS (both alone and in combination with AA).
‡ Values lower for period 1 than period 2, except for Arg where period 1 was greater.
§ Only three sheep for the LPS+AA treatment.
|| Infusion rates (µmol-N/min) into mesenteric vein, 17·8 (Pro), 10·8 (Met), 34·7 (Ser), 38·4 (Thr), 19·2 (Cys), 89·2 (Gln) for LPS+AA treatment.
Hepatic amino acid transfers
Except for glutamine and glutamate, during saline infusion there was net removal of each AA across the liver and these were different from zero (P<0·05), except for aspartate and cysteine. During saline infusion, there was net hepatic export of glutamate (P<0·001). LPS infusion (without supplemental AA) increased net hepatic removal (P<0·05) of six AA (Table 5), and these contributed to the 75 % extra extraction of total AA-N (P=0·012). For the non-infused AA, hepatic removal was not affected by the supplemental AA in the presence of LPS (Table 5). In contrast, extraction by the liver of all the infused AA was markedly increased (P<0·01), and this resulted in an additional 40 % removal of total AA-N (P=0·030).
Table 5 Net hepatic removals (µmol N/min) of amino acid (AA)-N in eight sheep in response to 20-h infusions of saline or lipopolysaccharide (LPS; 2 ng/kg live weight per min), either with or without six supplemental AA (Predicted means with their standard errors of the difference between means for the effect of treatment)

a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05); post hoc t test was performed to compare the treatment means.
* Analysed by random-effects model, with sheep and period within sheep as random effects and period and treatment plus their interaction as fixed effects, where treatment was either a saline, LPS or LPS+AA infusion.
† Analysed by random-effects model as described above, where treatment reflects LPS status – that is either saline or LPS (both alone and in combination with AA).
‡ Only three sheep for the LPS+AA treatment.
§ Period×treatment interaction (P<0·05) with less uptake during period 2 for LPS and LPS+AA.
|| More hepatic removal during period 1.
Net splanchnic amino acid transfers
The difference between net absorption (plus any infused AA) and hepatic removal represents net splanchnic flow to peripheral tissues (Table 6). With saline infusion, only glycine and glutamine had significant (P<0·05) negative net transfers – that is hepatic uptake exceeded PDV absorption, and thus additional amounts were removed from the peripheral circulation. In terms of positive net post-splanchnic supply, most AA had values that were significantly different from 0 (P<0·05); the exceptions were alanine, cysteine, histidine, phenylalanine, tryptophan and tyrosine. In response to LPS alone, net splanchnic supply decreased for alanine and arginine (P<0·05). Provision of supplemental AA increased net splanchnic supply for cysteine, methionine, proline, threonine (all P<0·010) and serine (P=0·037), but not glutamine.
Table 6 Net total splanchnic appearances (µmol N/min) of amino acid (AA)-N in response to a 20-h infusion of saline or lipopolysaccharide (LPS; 2 ng/kg live weight per min), either with or without six supplemental AA, LPS, in eight sheep (Predicted means with their standard errors of the difference between means for the effect of treatment)

TSP total splanchnic release.
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05); post hoc t test was performed to compare the treatment means.
* Analysed by random effects model, with sheep and period within sheep as random effects and period and treatment plus their interaction as fixed effects, where treatment was either a saline, LPS or LPS+AA infusion.
† Analysed by random-effects model as described above, where treatment reflects LPS status – that is either saline or LPS (both alone and in combination with AA).
‡ Values lower for period 1 than period 2.
§ Only three sheep for the LPS+AA treatment.
|| Period×treatment interaction for Met with lower TSP (P=0·014) during period 2 for saline infusion, whereas values for LPS and LPS+AA infusions were greater.
Isotope transfers
Phenylalanine whole-body ILR was increased in response to LPS infusion, both with and without the AA supplement (mean 17 %, P<0·001; Table 7). Endotoxaemia did not alter ILR across the PDV, regardless of whether the arterial or venous plasma enrichment was chosen as precursor (latter data not shown). In contrast, with either method of calculation, both liver (P=0·023) and total splanchnic (P=0·034) metabolism was increased by LPS. In combination, LPS plus supplemental AA increased hepatic protein synthesis by 12 % (P=0·036) compared with LPS alone. Together, the hepatic and PDV response accounted for approximately 40 % of whole-body ILR under both saline and LPS infusions, but with the contribution from the liver more than 2-fold that from the PDV.
Table 7 Impact of saline or lipopolysaccharide (LPS; 2 ng/kg live weight per min), either with or without six supplemental amino acids (AA), on ILR (mmol/h) of plasma phenylalanine for the whole body (WB) and across the tissues of the splanchnic bed (Predicted means with their standard errors of the difference between means for the effect of treatment)
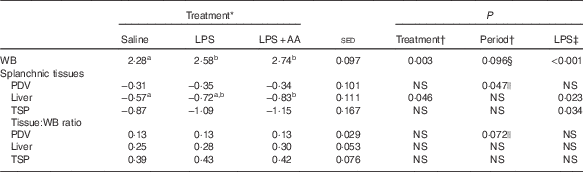
PDV, portal-drained viscera (total gut); TSP, total splanchnic preparation (liver+PDV).
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05); post hoc t test was performed to compare the treatment means.
* All values based on arterial enrichments.
† Analysed by random-effects model, with sheep and period within sheep as random effects and period and treatment plus their interaction as fixed effects, where treatment was either a saline, LPS or LPS+AA infusion. There were no period×treatment effects (P>0·05).
‡ Analysed by random-effects model as described above, where treatment reflects LPS status – that is either saline or LPS (both alone and in combination with AA).
§ Values greater for period 1 than period 2.
|| Values greater for period 2 than period 1.
Discussion
Although experimentally induced endotoxaemia has been proven to be a popular research tool to study the metabolic events and beneficial nutrition interventions related to inflammation, the responses observed can vary because of many factors, including species, severity of dose and period of measurement. For example, in both pigs( Reference Bruins, Soeters and Deutz 4 ) and sheep( Reference Hoskin, Bremner and Holtrop 3 ), LPS causes a decrease in plasma concentration for most AA. In contrast, in rodents, concentrations can increase( Reference Soeters, Hallemeesch and Bruins 26 ), whereas in humans both null( Reference Fong, Matthews and He 27 ) or decreased( Reference Vesali, Klaude and Rooyackers 28 ) responses have been reported. The dose used in the current study is below that usually adopted – for example only 4 % of the hourly dose used in pigs( Reference Bruins, Soeters and Deutz 4 ) – but it does give the advantage that pyrexia and anorexia responses are mild and of limited duration yet with similar responses in arterial AA concentrations and metabolic flows( Reference Hoskin, Bremner and Holtrop 3 ).
Whole-body and splanchnic tissue response to endotoxin challenge
The LPS dose increased whole-body ILR of plasma phenylalanine in the sheep, as observed previously( Reference Hoskin, Bremner and Holtrop 3 ), in support of observations in pigs( Reference Bruins, Soeters and Lamers 1 ) and humans( Reference Fong, Matthews and He 27 ). Similarly, the increase in lymphocyte cell numbers is in agreement with other findings during infection and inflammation challenges( Reference Hoskin, Bremner and Holtrop 3 , Reference Papet, Ruot and Breuille 29 ), although in shorter-term endotoxaemia studies (<2·5 h) in human such changes are not observed( Reference Januszkiewicz, Lore and Essen 30 ). Increased cell numbers may involve mobilisation of lymphocytes from pre-existing stores and/or a higher fractional rate of synthesis, and the current data confirm that the latter occurred. Nonetheless, the 4-fold increase in total protein synthesis of lymphocytes represented <0·1 % of whole-body protein synthesis and would only require <0·2 % of the phenylalanine absorbed from the diet. In contrast, the net increase in total plasma protein synthesis (4 g/d, from Table 2) would require an additional 5 % of the absorbed phenylalanine. The latter value compares with estimates for the total immune system of approximately 8 % in humans( Reference Hellerstein and Munro 31 ) and with nutritional costs increased from 1·2 to 6·7 % between saline-infused and LPS-challenged chicks( Reference Klasing and Calvert 32 ). Other components of the immune system, which are not monitored in the current study and including the thymus, spleen, bone marrow and immune cells, may also be activated( Reference Breuille, Arnal and Rambourdin 33 ) with a possible greater contribution from secondary lymphoid organs compared with primary tissues plus blood lymphocytes( Reference Papet, Ruot and Breuille 29 ).
Although endotoxaemia is associated with changes in NO status( Reference Soeters, Hallemeesch and Bruins 26 ), blood (and plasma) flows across the splanchnic tissues remained unaltered. Again, this is in line with observations in both pigs( Reference Bruins, Soeters and Deutz 4 , Reference Soeters, Hallemeesch and Bruins 26 ) and rodents( Reference Soeters, Hallemeesch and Bruins 26 ). In humans, a near doubling of splanchnic blood flow was observed( Reference Fong, Matthews and He 27 ), but this had disappeared 6 h after the bolus injection of endotoxin. LPS also exerts direct effects on the gut, with consequent damage( Reference Effenberger-Neidnicht, Jagers and Verhaegh 34 ) and altered permeability( Reference Hou, Wang and Zhang 15 ). It is clear, however, that there was no impact on net absorption of most AA in the current experiment, but this may relate to the parenteral route of LPS infusion and the low dose used. Therefore, the altered arterial AA concentrations must be a consequence of altered post-intestinal tract metabolism. This contrasts with pigs, in which, following a more severe endotoxin challenge supplied enterally, increases in net portal vein appearance for several AA were approximately double compared with that supplied from the diet, suggestive of mobilisation of intestinal tissue( Reference Bruins, Soeters and Deutz 4 ).
In the current study, glutamine showed lowered net removal in response to LPS. This has similarities to observations both in pigs post surgery( Reference Deutz, Reijven and Athanasas 35 ) and in tumour-bearing rats( Reference de Blaauw, Heeneman and Deutz 17 ), in which the gut consumed less glutamine, possibly in response to lowered arterial concentrations. Indeed, arterial glutamine, and thus systemic supply, was reduced by 38 % in the current study.
Under control conditions, hepatic AA removal followed patterns previously reported( Reference Lobley, Connell and Revell 36 , Reference Lobley, Bremner and Brown 37 ), with most of the net absorbed histidine and phenylalanine extracted, but with only limited uptake of the branched-chain AA (17–27 %). There was net output of glutamate, but, contrary to earlier observations, this was not balanced by similar net hepatic glutamine removal( Reference Lobley, Bremner and Nieto 23 , Reference Nieto, Obitsu and Fernandez-Quintela 38 ). More N was extracted from plasma by the liver as combined AA-N and ammonia-N than was released as urea-N, compatible with hepatic needs to support other processes( Reference Lobley, Bremner and Nieto 23 ), including the synthesis of constitutive and export proteins. Part of the difference (4·5 mmol/h) would be used to support the measured albumin synthesis (1·4 mmol-N/h), but it would be insufficient to account for estimated total plasma protein synthesis (7·2 mmol-N/h), although not all plasma proteins are synthesised by the liver (e.g. globulins).
LPS infusion increased hepatic removal for several AA, including glutamine, leucine, lysine, proline and threonine, and these contributed to the additional 6·5 mmol AA-N/h net removal by the liver, similar to the 8·3 mmol-N/h estimated from the change in total liver ILR (from Table 7). How much of this additional uptake of AA-N is catabolised is unclear, because the numerical change in arterial urea was not supported by increased hepatic ureagenesis. Studies in humans have reported increased AA catabolism due to LPS, including hepatic oxidation of leucine( Reference Fong, Matthews and He 27 ), greater fractional extraction of leucine across the splanchnic bed during sepsis( Reference Kao, Hsu and Bandi 39 ) and elevated whole-body conversion of phenylalanine to tyrosine( Reference Lichter-Konecki, Hipke and Konecki 40 ), primarily a liver event. In addition, infection or inflammation stimulate the synthesis of constitutive and/or export proteins( Reference Bruins, Soeters and Lamers 1 , Reference Bruins, Soeters and Deutz 4 , Reference Breuille, Arnal and Rambourdin 33 , Reference Ruot, Breuille and Rambourdin 41 ).
Phenylalanine is unusual in that the arterial concentration increases during endotoxaemia in pigs( Reference Bruins, Soeters and Deutz 4 ) and sheep( Reference Hoskin, Bremner and Holtrop 3 ), although not in humans( Reference Fong, Matthews and He 27 , Reference Vesali, Klaude and Rooyackers 28 ). Hepatic removal of phenylalanine remained unchanged by LPS treatment even though it has been suggested that liver demands for phenylalanine (and tryptophan) would increase during infection and inflammation because of their relatively high abundance in positive acute-phase proteins( Reference Reeds, Fjeld and Jahoor 7 ), the synthesis of which is increased by infection( Reference Jahoor, Gazzard and Phillips 42 ). In the current study, such demands would be offset by simultaneous decreased synthesis (−50 %) of albumin, a negative acute phase protein that contains 6 % (w/w) phenylalanine. Therefore, the increased arterial plasma phenylalanine probably relates to mobilisation of protein from non-splanchnic tissues, particularly skeletal muscle( Reference Bruins, Soeters and Lamers 1 , Reference Orellana, Suryawan and Wilson 43 ).
Effect of amino acid supplementation
Targeted supply of nutrients in response to specific physiological, developmental or environmental events is a key nutritional aim. In various clinical situations, much attention has been focused on demands for specific AA. For example, supplementation with large amounts of glutamine has been proposed for a variety of surgical and clinical states( Reference Wernerman 16 , Reference de Oliveira, Silva and de Araujo 44 – Reference Fan, Wu and Li 46 ) associated with specific needs for the immune system( Reference Newsholme 47 ). In addition, claims as effective therapies have been made for a number of other AA supplied alone( Reference Poeze, Bruins and Kessels 48 – Reference Xu, You and Li 50 ). Other benefits have involved AA in combination( Reference Laurichesse, Tauveron and Gourdon 13 , Reference Breuille, Bechereau and Buffiere 14 ), although these have not always been successful( Reference Hoskin, Lobley and Coop 51 ).
A recent approach involved dynamic measurements in sheep subjected to an LPS challenge in order to quantify the demands for specific AA( Reference Hoskin, Bremner and Holtrop 3 ), and these findings were applied within the current protocol. The success of such an approach can be assessed at several levels. The simplest involves the effect of supplementation on plasma AA concentrations, and this produced statistical restoration to the saline-infused values for five of the AA, although further numerical improvement would be preferred for cysteine and serine. In contrast, threonine was probably over-supplied based on previous sheep data( Reference Hoskin, Bremner and Holtrop 3 ), and there may be differences between studies in the sensitivity of the animals to the LPS dose. Notably, LPS caused an 80 % reduction in plasma threonine in the earlier study( Reference Hoskin, Bremner and Holtrop 3 ), whereas for the current animals the decrease was only 56 %; therefore, an amount less than that actually given might have been needed to restore to control values. Recovery of AA infused into the post-absorptive venous drainage was not different from unity, except in the case of cysteine, possibly because of the requirement for synthesis of taurine and glutathione – processes that occur within the intestinal cells( Reference Malmezat, Breuille and Capitan 52 , Reference Bauchart-Thevret, Stoll and Chacko 53 ).
On the basis of the hypothesis that AA are mobilised from tissue protein – particularly muscle( Reference Orellana, Suryawan and Wilson 43 , Reference Wolfe and Miller 54 ) – to combat inflammation, if one (or more) of these AA are needed in considerable amounts then this will leave the remainder that are released in excess to requirements for protein synthesis. These will then be removed from the body via ureagenesis and lead to depletion of plasma concentrations. If the supplement contains those AA that are needed to support the anti-inflammatory responses, then this should reduce the need for peripheral tissue mobilisation and lower the overall hepatic removal for catabolic purposes. Despite the reasonable success in restoring the plasma concentrations of the supplemented AA to normal, there was no improvement in the plasma concentrations of the non-supplemented AA and neither was there a reduction in their removal by the liver between the LPS and LPS+AA treatments. For the supplemented AA, only for glutamine was there complete removal of the extra provided. This suggests that the increased arterial glutamine concentrations are probably because of mobilisation from other tissues; muscle is an obvious candidate in which millimolar quantities of free glutamine are present( Reference Mittendorfer, Volpi and Wolfe 55 ) and which is released during severe illness, even in the presence of supplemental glutamine( Reference Gore and Wolfe 56 ). For the other supplemented AA, hepatic removal ranged from 55 % (proline) to 84 % (serine), so the fates were partitioned between increased post-hepatic delivery, which is necessary to restore arterial concentrations to, or above, normal and potential support of liver-based mechanisms. The latter did not, however, involve restoration of the synthesis of the negative acute-phase protein, albumin. This non-response was despite the fact that the increased hepatic uptake of the supplemented AA would, in theory, have the potential to support 30–130 g/d of albumin synthesis, in considerable excess of the 1 g/d decrease observed. Similarly, the additional AA supplement did not alter rates of synthesis of lymphocytes. These observations might suggest that either other AA are needed in the supplement or that the immediate events during inflammation are less responsive to supplementation, for example there is a metabolic ‘over-ride’, and perhaps the focus should be on longer-term responses and enhanced recovery, as has been shown to occur in rats treated with exotoxin and supplemented with AA( Reference Breuille, Bechereau and Buffiere 14 ).
In summary, infusion of six AA predicted as key requirements during the response to an LPS challenge restored or exceed arterial concentrations of these to control values but not any of the other non-infused AA. Similarly, there was no restoration of the synthesis of the negative acute-phase protein, albumin, and no change in the elevated protein synthesis of lymphocytes. The parenteral infusion of the low dose of LPS did not affect gut metabolism or net AA absorption except for glutamine, where net removal was reduced. Hepatic uptake of leucine, lysine, glutamine and serine was increased by LPS, with liver removal of the latter two plus the other four infused AA increased during the AA supplementation. The data suggest that AA supplementation does not mitigate certain metabolic demands during the acute phase of an endotoxin challenge, but whether supplementation provides benefits on later responses and the period of recovery requires further investigation.
Acknowledgements
The expertise of A. Graham Calder and Susan Anderson for the various stable isotope analyses is gratefully recognised. Ngaire Dennison is also thanked for her surgical expertise with the trans-splanchnic tissue catheter preparations.
This study was supported by funds provided to the Rowett Institute of Nutrition and Health, University of Aberdeen and Biomathematics and Statistics Scotland by the Rural and Environment Science and Analytical Services Division of the Scottish Government. S. O. H. was a recipient of a FoRST (NZ) award to study abroad.
The contributions of the authors were as follows: C. J. M. N., S. O. H., G. H. and G. E. L. were responsible for the study concept and design. C. J. M. N., S. O. H., G. E. L. and D. M. B. were responsible for data collection and collation. G. E. L. and G. H. were responsible for data analysis and statistical matters. C. J. M. N., S. O.H., G. E. L. and G. H. were responsible for the first draft and critical revision of the manuscript for important intellectual content.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114516001860










