Amongst the micronutrients, deficiencies of Zn and vitamin A are thought to have the largest impact on disease burden in preschool children, and together have been estimated to account for 9 % of the global burden in this age group( Reference Black, Allen and Bhutta 1 ). A recent meta-analysis of randomised controlled trials of Zn supplementation showed that supplementation reduced significantly the frequency and severity of childhood respiratory illnesses, but the authors also noted significant heterogeneity in trial outcomes( Reference Aggarwal, Sentz and Miller 2 ). Vitamin A supplementation has resulted in significant reductions in mortality in several community-based trials in apparently healthy children( Reference Villamor and Fawzi 3 ), prompting many governments to introduce routine supplementation programmes. However, the benefits of vitamin A supplementation have not been consistent across trials, particularly for respiratory morbidity, where evidence suggests that supplementation may increase the risk of adverse outcomes in some conditions( Reference Villamor and Fawzi 3 ).
Some aspects of a functional interaction between Zn and vitamin A are well described. For example, Zn is essential for the synthesis of retinol-binding protein, is needed for the conversion of retinol to retinal for dark adaptation and for the lymphatic absorption of retinol( Reference Christian and West 4 , Reference Solomons and Russell 5 ). Zn supplementation has also been shown to improve vitamin A biochemical indices in children with low or marginal vitamin A status( Reference Rahman, Wahed and Fuchs 6 , Reference Munoz, Rosado and Lopez 7 ). However, few supplementation trials have examined the practical importance of the interaction for childhood respiratory morbidity. In a trial amongst children aged 12–35 months in the urban slums of Dhaka, Bangladesh, Rahman et al. ( Reference Rahman, Vermund and Wahed 8 ) showed a significant increase in acute lower respiratory infection with Zn alone, but an interaction and net positive effect from combined vitamin A and Zn supplementation. In contrast, Long et al. ( Reference Long, Montoya and Hertzmark 9 ) in children aged 6–15 months from a peri-urban area of Mexico City showed that vitamin A supplementation was associated with an increase in cough with fever, Zn supplementation had no independent effect on respiratory morbidity, and there was no significant interaction. While the weight of evidence is toward Zn and vitamin A supplementation having generally beneficial effects on childhood morbidity, these studies showed potentially adverse effects on respiratory morbidity from supplementing with Zn or vitamin A alone, and the need to understand better the conditions under which Zn alone or combined with vitamin A supplementation will be beneficial.
Vitamin A deficiency has been a significant public health nutrition problem in Indonesia and Zn deficiency also appears to be widespread( Reference Dijkhuizen, Wieringa and West 10 , Reference Soekatri 11 ). Further, upper respiratory tract infection (URTI) is a frequent cause of childhood morbidity, which could have an impact on growth( Reference Hadi, Dibley and West 12 ) and risk of other diseases such as otitis media and sinusitis or exacerbate asthma attacks( Reference Greenberg 13 ). Therefore, we carried out a placebo-controlled trial of Zn supplementation in the presence and absence of vitamin A supplementation to examine the effects on URTI morbidity in preschool children in an urban area of Indonesia.
Subjects and methods
Mothers of all children aged 2–5 years attending thirty-six posyandu (health centres) in Semarang, Indonesia were invited to join the study. The age group was selected due to their relatively high risk of both malnutrition – including vitamin A deficiency – and URTI, and the feasibility of selecting a community-based sample of this age through the posyandu. Apparently healthy children aged 2–5 years were included. Moderately and severely malnourished children, defined by a weight-for-height ≤ − 2 sd of the WHO/National Center for Health Statistics reference( 14 ) were excluded, as malnutrition may modify a treatment effect from supplementation. Of the 1047 children who were present at recruitment, 826 apparently healthy children were included in the study.
The present study was randomised and double-blinded for Zn or placebo supplementation. Children were recruited during June 2003 and given a Zn supplement or placebo daily for 4 months. At 2 months after recruitment (i.e. August), children received a single-dose vitamin A supplement (200 000 IU; 60 mg retinol) as part of the routine bi-annual national vitamin A supplementation programme. This scheduling meant that the vitamin A status of the children was likely to be at its lowest in June and July – before the vitamin A supplementation – while the opposite would be true in September and October. Thus the two treatment groups (Zn or placebo) could be compared during two time periods, before and after vitamin A supplementation.
The ‘before vitamin A’ supplementation period was defined as starting at 1 d after recruitment, which was 4 months after the previous routine vitamin A supplementation, and ending on the day of vitamin A supplementation for each child. The ‘after vitamin A’ supplementation period started 1 d after vitamin A supplementation and ended on the last day of supplement consumption and observation, that is, 2 months after routine vitamin A supplementation.
The effect of Zn supplementation was measured by comparing the URTI morbidity in the Zn (415 children) and placebo (411 children) groups. The effect of vitamin A supplementation was measured by comparing URTI morbidity in the same 826 children during the two different time periods. Thus, four comparison groups were defined: placebo (no Zn and no vitamin A) (A); Zn only (B); vitamin A only (C); and Zn plus vitamin A (D). Fig. 1 shows the study design, participant selection process and reasons for loss to follow-up.

Fig. 1 Study design and included subjects.
Micronutrient supplementation
In each posyandu, one of the physicians (not investigators) used random numbers to allocate each child to receive daily either Zn (10 mg elemental Zn) or a placebo syrup for the 4 months. Supplements were prepared and labelled with alphabetic codes by the Pharmacy Department of Diponegoro University. There was no difference between the syrups in taste or appearance. Trained health workers were recruited from the thirty-six posyandu and made home visits to each subject every third day. During these visits they supervised consumption of syrup, recorded compliance data and observed the amount of syrup left in the bottles. They supplied new bottles of syrup to mothers each fortnight, rotating strawberry and lemon flavours to maximise compliance. Both health workers and mothers were blinded to the syrup content. Non-compliance was defined as syrup consumption on less than 75 % of the total supplement days. Of the children, four stopped taking the supplement during the first 2 months of the study as they moved out of the areas and did not take part for the remainder of the study, and twenty-four stopped during the last 2 months.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Medical Research Ethics Committee of The University of Queensland and Ethical Committee of Medical Research of Medical Faculty, Diponegoro University. Written informed consent was obtained from the mothers of all subjects.
Sample size
The underlying incidence rate for URTI was assumed to be 8·11 (sd 2·43) per child year( Reference Dibley, Sadjimin and Kjolhede 15 ). A sample of 764 children, 382 in each group, was required to detect a 20 % difference in URTI incidence rate between comparison groups with a power of 80 %, a 95 % significance level, and 20 % loss to follow-up.
Morbidity data collection
The health workers also collected morbidity data during their visits every third day by observing the child's health status on that day and interviewing mothers regarding their child's health status for the two previous days. If the child was found to have a cough, respiratory rate was measured. If they had at least one URTI symptoms (see below), axillary temperature was measured using a digital thermometer. The results of the observation and interview were recorded on structured questionnaires and reported to supervising physicians fortnightly. The latter diagnosed URTI based on symptoms reported, and randomly checked children's health status and visited the subjects if necessary.
An URTI episode was defined as the presence of at least two of the following clinical symptoms experienced for at least 1 d: runny nose (ongoing discharge from the nose), cough, sore throat and raised body temperature, or one of the symptoms for at least 2 d, without the signs of difficult or rapid breathing. A total of three aspects of URTI morbidity, namely the number of episodes, percentage of days with URTI and duration per episode, were used as dependent variables in the present study. A period of three illness-free days between the episodes was required for a new episode to be defined. This definition of URTI episode is adapted from definitions used in previous randomised controlled Zn supplementation studies( Reference Ruel, Rivera and Santizo 16 – Reference Osendarp, Van Raaij and Darmstadt 19 ) modified to specifically measure URTI (instead of respiratory infections more generally, or irritation problem) in preschool-age children. The percentage of days with URTI was defined as the number of days with URTI divided by the number of observation days, expressed as a percentage. The duration of URTI episodes was defined as the number of days with URTI for each episode.
Other measurements
Child and family characteristics and socio-economic data were collected at baseline by interview using structured questionnaires. Anthropometric assessment was conducted at baseline by trained undergraduate nutrition students using standard protocols( Reference Gibson 20 ) – weight measured on a Seca portable digital scale to the nearest 100 g, and height measured standing using a measuring board to the nearest 0·1 cm. Z scores for anthropometric indicators were calculated based on WHO/National Center for Health Statistics reference distributions using Nutrisoft software (Nutrition Research and Development Centre, Health Research and Development Institute of Indonesian Ministry of Health).
Non-fasting venous blood was drawn from the children at baseline, between 09.00 and 12.00 hours. Serum retinol was determined using HPLC at the Institute of Nutrition, Mahidol University, Thailand. Intra-assay CV was 3·5 % and inter-assay CV 6·1 %. Vitamin A deficiency was defined as serum retinol < 0·7 μmol/l and low vitamin A status defined as serum retinol < 1·05 μmol/l( Reference Baeten, Richardson and Bankson 21 ). C-reactive protein was measured using an ELISA kit (K9710s; Immundiagnostik) with optical density values read at 450 nm. Intra- and inter-assay CV were 8·5 and 10·0 %, respectively. Hair samples (1–2 cm; about 50 mg) were cut from close to the scalp in the occipital area using stainless steel scissors that had been washed in deionised water. The samples were stored in labelled polyethylene zip-locked bags at room temperature. Analysis was conducted at Queensland Health Scientific Services, Australia. Hair samples were washed three times using the hexane–ethanol method( Reference Assarian and Oberleas 22 ), acid digested using concentrated nitric acid and assayed using a Varian inductively coupled plasma atomic emission spectrometer (ICP-AES). The intra- and inter-assay CV were 4·7 and 8·6 %, respectively. Hair Zn is known to vary by day-length or season and different cut-offs are recommended for spring and summer ( < 1·07 μmol/g) v. winter ( < 1·68 μmol/g) seasons( 23 ). Prevalence using both criteria is reported.
Data analysis
SAS software (version 9.1; SAS Institute, Inc.) was used for analysing the number of episodes and percentage of days with URTI, and SPSS (version 13.0; SPSS, Inc.) for URTI duration. The analysis was initially conducted on an intention-to-treat basis, with the code of Zn or placebo syrup broken only after the initial analyses were completed. Compliance and subgroup effects were considered in subsequent analyses.
Potential confounders considered were socio-demographic status, sex, compliance (days of supplement consumption), initial anthropometric status, Zn and vitamin A status at baseline and posyandu code. The latter was included to control for cluster effects. Although in a randomised controlled study these factors could be regarded as similar across the groups, their significance was assessed by inclusion in the models. The final models included only the significant variables.
The effects of supplementation on the number of episodes of URTI and percentage of days ill were measured in relative risks. The GENMOD procedure of SAS 9.1 was used with a negative binomial distribution. In the models for URTI episodes the number of observation days was included as a covariate to control for observation period. The effect of supplementation on URTI duration per episode was assessed using the repeated measures option in the general linear model (GLM) procedure of SPSS 13.0. URTI duration was analysed in log form, as this variable was not normally distributed in the original value but normalised after log transformation. The results presented in the tables have been back-transformed to the original values. Log URTI duration per episode before and log URTI duration per episode after vitamin A supplementation were set as the dependent variables, time (before and after vitamin A supplementation period) as two levels of within-subject factor, treatment as the between-subjects factor and potential confounding variables as the covariates. Only important covariates as judged by P value less than 0·05 were included in the models.
The effect of the interaction between Zn and vitamin A supplementation was assessed by comparing morbidity rates in the Zn plus vitamin A and the placebo groups (D+A) with the rates in the Zn and the vitamin A groups (B+C). P values of less than 0·05 were regarded as significant and P values between 0·05 and 0·1 as marginally significant.
Results
Characteristics of the 826 study children at commencement of the study are presented in Table 1. There were no differences in age, anthropometric status and socio-economic conditions between the subjects who received Zn v. placebo. Baseline vitamin A and Zn status were also similar across groups, as reflected in the mean serum retinol and hair Zn levels. The overall prevalence of vitamin A deficiency was very low (2·3 %); however, a significant proportion (32·4 %) was at risk of low vitamin A status. The proportion with hair Zn levels < 1·07 μmol/g (spring and summer cut-off) was low at 5·3 %, with 20·3 % below < 1·68 μmol/g (winter cut-off).
Table 1 Subject characteristics at the start of the study and compliance with zinc and placebo supplementation by treatment group (total n 826) (Number of subjects, mean values with their standard errors, or percentages)

* One-way ANOVA for continuous variables; χ2 tests for categorical variables.
† Poverty line level used: 175 000 rupiahs/month (Indonesian Central Bureau of Statistics, 2006( 33 )).
Compliance with supplement consumption was high; 96 % consumed the supplement on >75 % of days, with amounts consumed slightly higher in the last 2 months of the study. The mean number of days with syrup consumption in each group was: A, 56·4 (se 0·28); B, 56·1 (se 0·32); C, 61·9 (se 0·44); D, 60·9 (se 0·47), respectively. The median number of days with syrup consumption in each group was: A, 58 (range 29–67); B, 58 (range 21–73); C, 64 (range 0–80); D, 64 (range 0–79), respectively.
Overall, 84 % of the study children had an episode of URTI at some point during the study period, the number of episodes ranging from zero to twelve per child, with a mean of 2·84 (se 0·08) episodes. About 70 % of the subjects had fever during these episodes. The percentage of days with URTI during the study period was 13·4 (se 0·5) %, and the mean duration per episode was 5·7 (se 0·2) d. By contrast, the next most common illness was diarrhoea, with 14 % of children affected, and only two children were diagnosed with an episode of lower respiratory tract infection (LRTI) during the 4 months.
Table 2 shows URTI morbidity by treatment group and Table 3 shows the treatment effects. The main effect of Zn across the 4 months of supplementation was a 12 % reduction in the percentage of days with URTI (P = 0·09). The effect was different between the before and after vitamin A supplementation periods, with a reduction of 20 % following the distribution of vitamin A. Vitamin A alone had no effect on the percentage of days with URTI (no difference in the placebo before and after vitamin A supplementation); however, the effect was very strong on children who received Zn supplementation, with a reduction of 9 % in the percentage of days with URTI associated with the ‘interaction effect’, and an overall significant main effect for vitamin A.
Table 2 Levels of upper respiratory tract infections (URTI) morbidity in a trial of zinc supplementation with and without concurrent vitamin A supplementation in Indonesian preschool children (Mean values with their standard errors)
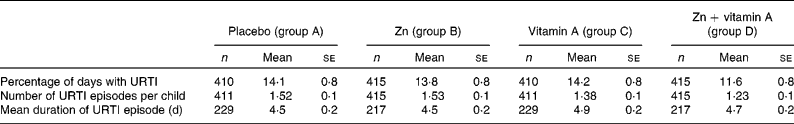
Table 3 Treatment effects in a trial of zinc supplementation with and without concurrent vitamin A supplementation in Indonesian preschool children (Relative risks (RR) and 95 % confidence intervals)

Group A, placebo; group B, Zn supplementation; group C, vitamin A supplementation; group D, Zn + vitamin A supplementation; URTI, upper respiratory tract infection.
* Used GENMOD procedure in SAS, with negative binomial distribution; covariates in final model = posyandu (health centre), observation days.
† Used GENMOD procedure in SAS, with negative binomial distribution; covariate in final model = posyandu.
‡ Used repeated measures option in the general linear model (GLM) procedure of SPSS; covariate in final model = posyandu.
The main effect of Zn was not significant for URTI episodes; however, vitamin A supplementation was associated with a significant (P = 0·00) reduction (23 %) in the number of URTI episodes. The children who received both Zn and vitamin A had the lowest number of episodes, with a 6 % reduction in the number of episodes associated with the ‘interaction effect’ compared with the combined effects of Zn and vitamin A individually (P = 0·07).
For URTI duration per episode, Zn had no significant main effect and there was no significant interaction between Zn and vitamin A supplementation. The vitamin A main effect was a significant increase in the duration of URTI by 0·6 d.
Figs. 2 and 3 present results of stratified analysis with effects estimated for subgroups of sex, age and baseline nutritional status. In the final model the number of observation days was a significant covariate for the analysis of number of URTI episodes but not other models; posyandu was retained in the models to control for possible cluster effects. The vitamin A main effect on URTI episodes was statistically significant for all subgroups. The effect magnitude was the greatest in the younger children ( < 3·5 years old) and girls, though the CI for all subgroups overlapped. The Zn main effect on episodes was variable and not significant for any subgroup. The interaction tended to show a protective effect across all subgroups, and was statistically significant only amongst girls.

Fig. 2 Effect of supplementation on number of upper respiratory tract infection episodes by subgroups of weight-for-age, baseline vitamin A, age and sex. (a) Zn main effect; (b) vitamin A main effect; (c) interaction effect. Values are relative risks, with 95 % confidence intervals represented by horizontal bars.

Fig. 3 Effect of supplementation on the percentage of days with upper respiratory tract infection by subgroups of weight-for-age, baseline vitamin A, age and sex. (a) Zn main effect; (b) vitamin A main effect; (c) interaction effect. Values are relative risks, with 95 % confidence intervals represented by horizontal bars.
The results for the percentage of days with URTI suggest greater heterogeneity. The vitamin A main effect was greater in those < 3·5 years old than older children, with other subgroup results showing a general pattern of modest protection across subgroups. The Zn main effect was variable, and significantly protective amongst girls, those underweight and older children. The interaction effect was more consistent, suggesting a moderate additional protection by combining the treatments, with the most marked difference between boys and girls that suggests no effect amongst boys.
Discussion
The subjects were from a generally healthy but relatively poor suburban area of Semarang, in the province of Central Java. Assessment of Zn status is hampered by the lack of a single, specific and sensitive index, but hair Zn is frequently used as an indicator of chronic Zn status( 23 ). Serum retinol is most useful as an indicator of vitamin A status when liver stores are depleted( Reference Gibson 24 ). Despite these limitations, the results, together with relatively low anthropometric measures, suggest a poor nutritional history, including marginal micronutrient status for vitamin A and Zn. In terms of factors that might influence estimation of treatment effects, there were no important differences in characteristics between the treatment groups, compliance was very high, with 96 % of the children consuming the supplement on more than 75 % of days and similar compliance across groups.
The children in the present study had a relatively high incidence of URTI, with 84 % of the children affected, and a mean 2·84 episodes during 4 months of follow-up or 8·52 episodes per child-year. This was similar to the incidence of 8·11/child-year reported elsewhere in Indonesia( Reference Dibley, Sadjimin and Kjolhede 15 ).
The Zn supplementation, which was conducted as a double-blind randomised controlled trial over the full 4-month study showed a reduction of 12 % in the percentage of days with URTI. Furthermore, the effect was modified following vitamin A supplementation, with a greater reduction (20 %) seen after vitamin A supplementation. The results show a potentially important interaction between the vitamin A and Zn supplementation, with those receiving both supplements having 9 % fewer days with URTI and 6 % fewer URTI episodes than expected from the independent effects of vitamin A and Zn.
Because we could not randomly allocate the timing for vitamin A supplementation it is possible that the interaction effect was caused by other differences beside the treatments that might alter URTI risk between the before and after vitamin A supplementation periods. However, available evidence suggests that this is not the case. First, both periods (June–July and August–September) were in the dry season period in Indonesia (Indonesia has only two seasons: dry from April to October and wet from October to April). Data from the local primary health care centre showed no important differences between the numbers of visits for respiratory infection between June–July and August–September in 2002, 2003 and 2004 (Puskesmas Bangetayu Report, unpublished results). Data from the City Health Office (unpublished results) showed that the number of LRTI reported was also not different during that time period. Second, the percentage of days with URTI in the placebo group before and after vitamin A supplementation was essentially identical, suggesting a relatively constant level of risk over the 4 months.
There is growing evidence of an interaction between Zn and vitamin A that is consistent with vitamin A status modifying the effect of Zn supplementation on URTI morbidity. The combined effects in the present study are estimated to be a reduction of 34 % in episodes and 30 % in the percentage of days with URTI. This may slightly overestimate the interaction because children starting Zn supplementation at baseline (analysis group B) would be expected to have poorer Zn status than at the start of the second period following vitamin A supplementation (group D), when they will already have had 2 months of Zn supplement and would be expected to be Zn replete.
As well as a marked effect on episodes and percentage of days with URTI, the combined treatment appears to have changed the nature of the morbidity. While the overall effect on the percentage of days ill with URTI was a 9 % reduction (across both treatment groups), there was a 23 % reduction in the number of episodes and a 0·6 d increase in the average duration of each episode. For the reasons above, it appears unlikely that this effect can be explained by time difference.
The present results differ from other studies in several ways. In terms of the Zn effect, a recent meta-analysis of Zn supplementation trials showed a greater effect of Zn supplementation on episodes with LRTI or pneumonia than we observed (20 % reduction), but not days with respiratory illness (non-significant 5 % reduction)( 25 ). The authors noted significant heterogeneity across studies. An important difference between the present study and most of the others is the type of morbidity, with most previous trials focusing on acute LRTI or broad definitions that would probably encompass both URTI and LRTI. To the extent that the differences in morbidity definitions distinguish between URTI and LRTI, the divergence in findings may be due to differences in the pathogens generally associated with upper v. lower respiratory infections, often viral v. bacterial, respectively( Reference Peltola and Ruuskanen 26 ), and a differential effect across these pathogens of immune changes induced by vitamin A and by Zn.
Reviews of vitamin A supplementation trials and recently reported studies have not shown a general effect of vitamin A on respiratory infections in young children( Reference Villamor and Fawzi 3 , Reference Dibley, Sadjimin and Kjolhede 15 , Reference Fawzi, Mbise and Spiegelman 27 , Reference Barreto, Santos and Assis 28 ). Furthermore, most studies showing benefit suggest that it is in reducing the severity rather than incidence of infections( Reference Villamor and Fawzi 3 ). The present study showed a reduction in the incidence of URTI, but increase in URTI duration, which is one measure of severity. However, Zn supplementation suggested a modification of the deleterious effect in duration to lessen this effect.
Few trials have reported on the effects of combined vitamin A and Zn supplementation on respiratory morbidity. Rahman et al. ( Reference Rahman, Vermund and Wahed 8 ) conducted a trial in children aged 12–35 months living in urban slums of Dhaka, Bangladesh. They found no effect of vitamin A alone on acute LRTI over 6 months of follow-up, a significant increase in both episodes and days ill associated with Zn supplementation alone, but a reduction in both aspects of morbidity with combined vitamin A and Zn (significant for days ill). They also reported that one-third of the children had low vitamin A status, one-third had low serum Zn at baseline, and vitamin A alone failed to correct vitamin A deficiency, whereas combined supplementation with vitamin A and Zn effectively corrected the deficiency( Reference Rahman, Wahed and Fuchs 6 ). Long et al. ( Reference Long, Montoya and Hertzmark 9 ) also reported on the individual and combined effects of vitamin A and Zn on respiratory infections in children aged 6–15 months living in a peri-urban area of Mexico City. They found a 23 % increase in episodes of ‘cough with fever’ associated with vitamin A alone, no effect of Zn alone, and the interaction to be non-significant. Importantly, the authors noted that the prevalence of low serum vitamin A was likely to be low ( < 5 %) with Zn deficiency likely to be lower than the national prevalence (one-third of the children).
The heterogeneity across study findings suggests that the vitamin A and Zn effects are modified by other factors. The subgroup analysis in the present study provides some evidence that the younger children ( < 3·5 years old) and girls responded most overall, with no consistent patterns for any influence of vitamin A status and underweight on treatment effects. However, the present study was not primarily powered for subgroup analyses and so these results are only indicative. Furthermore, the supplementation period (2 months plus 2 months) was short relative to most other Zn supplementation trials. Longer supplementation periods would have strengthened the capacity to examine both the overall and subgroup effects (increased person-time exposure; longer periods Zn replete). Importantly, the present study showed no evidence for adverse outcomes for any subgroups.
A key finding in each of the studies testing the combined effects of vitamin A and Zn on respiratory infection is that the combined effect has been synergistic, with significant positive effects from the combination even when an individual main effect has been negative. We noted earlier the evidence for an interaction between vitamin A and Zn in storage, mobilisation and nutrient conversion( Reference Christian and West 4 , Reference Solomons and Russell 5 ). This could explain part of the synergy, but it is probably also due to complementarity in their effects on the immune response. Vitamin A is important for maintaining the integrity of epithelial barriers. It also plays an important role in other innate immune functions such as phagocytic and oxidative burst activity of macrophage and natural killer cell activities( Reference Villamor and Fawzi 3 ). Vitamin A also has an important role in the development and differentiation of Th1 and Th2 lymphocytes. This role in Th2 lymphocytes is essential for extracellular pathogen defence( Reference Maggini, Wintergerst and Beveridge 29 ). Zn has also been known as an essential cofactor for thymulin, which modulates cytokine release and proliferation, supporting a Th1 response, maintaining skin and mucosal membrane integrity as well as having a direct antiviral effect( Reference Maggini, Wintergerst and Beveridge 29 ). The combination of these effects could lead to only the more virulent pathogens surviving to cause URTI, producing the effect of fewer episodes but the longer duration or higher severity of the URTI we observed. In contrast, studies of Zn supplementation on diarrhoeal morbidity show that it reduces the severity of the illness( Reference Strand, Chandyo and Bahl 30 – Reference Hambidge 32 ). Trends in the present results and the Bangladesh study provide support for this also occurring for respiratory infections, but it needs to be further examined.
Conclusion
Zn supplementation reduced the percentage of days with URTI, a very common illness in this population of preschool Indonesian children with marginal nutritional status, including marginal Zn and vitamin A status. The greatest impact of Zn supplementation was following routine vitamin A supplementation when the combined effects were estimated to be a reduction of 34 % in episodes and 30 % in the percentage of days with URTI.
The present study indicates an important public health impact of combined Zn and vitamin A supplementation on URTI in young children, especially in developing countries, where the burden associated with URTI is high, as is the associated morbidity due to otitis media, sinusitis and asthma attacks. Moreover, the effect was sufficiently large to protect the children against the aggravating effect of URTI on growth rates, which are already low as a result of low energy and micronutrient intakes in Indonesia.
Acknowledgements
The research was conducted with funding support from a Nestlé Foundation Research Grant. We would like to give special thanks to Professor Gail Williams for statistical advice. The authors' responsibility were as follows; M. I. K., G. C. M. and F. A. conceived of and designed the study; M. I. K., H. W. S. and M. Z. R. designed and conducted field-work and collected data; M. I. K. interpreted data and wrote the draft of the manuscript; F. A. and G. C. M. interpreted data and critically reviewed the manuscript. There are no conflicts of interest in the conduct or reporting of the present study.








