Introduction
Insects are ectotherms and, as such, their body temperature reflects the temperature of their environment. In nature, this has a range of profound effects, such that temperature is often considered a ‘biological master factor’ for ectotherms (Clarke, Reference Clarke2017). During insect mass-rearing, transport and release, the temperature is often tightly controlled to maximize productivity and efficiency or to facilitate handling, storage, or synchronization (Chambers, Reference Chambers1977; FAO/IAEA, 2017). These mass-reared insects are then transported to and released into the field, where they may encounter very different thermal conditions to those experienced during rearing, and where their performance can be acutely temperature-dependent (Chidawanyika and Terblanche, Reference Chidawanyika and Terblanche2011; Nyamukondiwa et al., Reference Nyamukondiwa, Weldon, Chown, Le Roux and Terblanche2013). However, although their body temperatures are likely constrained by the ambient temperatures they encounter, insects are not passive victims of temperature variation, but can behaviourally thermoregulate (Harrison et al., Reference Harrison, Woods and Roberts2012) and respond physiologically at several time scales (Chown and Nicolson, Reference Chown and Nicolson2004; Harrison et al., Reference Harrison, Woods and Roberts2012). These plastic responses are reasonably well-understood (e.g. Cossins and Bowler, Reference Cossins and Bowler1987; Huey et al., Reference Huey, Berrigan, Gilchrist and Herron1999; Hoffmann et al., Reference Hoffmann, Sørensen and Loeschcke2003; Denlinger and Lee, Reference Denlinger and Lee2010) and can modify thermal biology in a way that could be harnessed to improve the performance of field released insects.
Here, we briefly summarize insect thermal biology and its plasticity. We place this into the context of ‘typical’ thermal profiles during rearing, handling, and release, and speculate on how modifications of these thermal profiles could be used to improve outcomes. We also highlight how these different stages (rearing, handling, and release) focus on different aspects of the thermal performance curve and, consequently, are likely to be impacted by different physiological mechanisms and processes. Although our focus here is on insects that are mass-reared for use in the sterile insect technique (SIT), many of these principles also apply to mass-reared insect biocontrol agents or even insects mass-reared for conservation purposes.
Thermal biology: its plasticity and consequences in insects – a brief guide
Temperature determines the rates of molecular interactions (i.e. biochemistry within cells), the state and function of cellular components (e.g. the flexibility of membranes), the rate of signal transduction (e.g. reaction time), and therefore almost all physiological processes in an ectotherm (Somero et al., Reference Somero, Lockwood and Tomanek2017). In turn, this means that biomechanics, behaviour, and digestion (among other things) are temperature-dependent, and that the ultimate success of ectotherms in the field will be determined by their responses to the temperatures they encounter (Clarke, Reference Clarke2017). The relationship between temperature and a measure of performance (usually a rate process such as flight speed, mating success, or feeding rate) can often be described by a thermal performance curve (Angilletta, Reference Angilletta2006). The complexities of the estimation and application of thermal performance curves are many (see Sinclair et al., Reference Sinclair, Marshall, Sewell, Levesque, Willett, Slotsbo, Dong, Harley, Marshall, Helmuth and Huey2016 for discussion), but the salient points here are: (1) there are both upper and lower limits to performance (often described by the critical thermal maximum, CT max, and critical thermal minimum, CT min); (2) in the lower portion of the curve, performance increases exponentially with temperature; and (3) after a peak in performance (misleadingly named the thermal optimum, T opt, in the literature), performance declines rapidly with increasing temperature. Importantly, the shape (e.g. asymmetry, concavity, inflection points) of the thermal performance curve can differ significantly among traits, so a temperature that favours high performance in one trait (such as development rate) may not necessarily favour high performance in another (such as mating) (Sgro et al., Reference Sgrò, Terblanche and Hoffmann2016; Kellermann et al., Reference Kellermann, Chown, Schou, Aitkenhead, Janion-Scheepers, Clemson, Scott and Sgrò2019).
Insect thermal biology is plastic. In general pre-conditioning at (e.g.) a high temperature will lead to better high-temperature performance, while low-temperature pre-conditioning will improve low-temperature performance (Chown and Terblanche, Reference Chown and Terblanche2007). This plasticity can occur over a range of time scales, from hardening responses induced after 30 min or less, to responses cued by development at different temperatures, or even the thermal experience of the mother days or weeks previously (table 1). Not all kinds of thermal plasticity have the same underlying mechanisms, which means that tolerance at one thermal extreme does not necessarily trade off with tolerance at the other end of the thermal range. For example, cold tolerance can be enhanced (e.g. at low temperatures via rapid cold-hardening; Teets and Denlinger, Reference Teets and Denlinger2013) without significantly compromising high-temperature tolerance (e.g. Thomson et al., Reference Thomson, Robinson and Hoffmann2001; Sørensen et al., Reference Sørensen, Kristensen, Loeschcke and Schou2015). Nevertheless, cold-acclimated codling moths (Cydia pomonella) are more likely to be recaptured under cold field conditions than under warm field conditions (Chidawanyika and Terblanche, Reference Chidawanyika and Terblanche2011), which implies improved performance (possibly survival) at low temperatures. Thus, improved low-temperature performance can be at the expense of high-temperature field performance and represent a classic trade-off between performing well under some conditions, at the expense of poorer performance under other conditions. Induced plasticity can persist across life stages; for example, exposing Drosophila to thermal perturbations as larvae affects their thermal biology as adults (MacLean et al., Reference MacLean, Kristensen, Overgaard, Sørensen and Bahrndorff2017). It is unclear how thermal tolerance mechanisms compare across life stages, making it difficult to predict these across-stage effects (Freda et al., Reference Freda, Ali, Heter, Ragland and Morgan2019).
Table 1. Examples of the cues and treatments that induce thermal plasticity in insects.
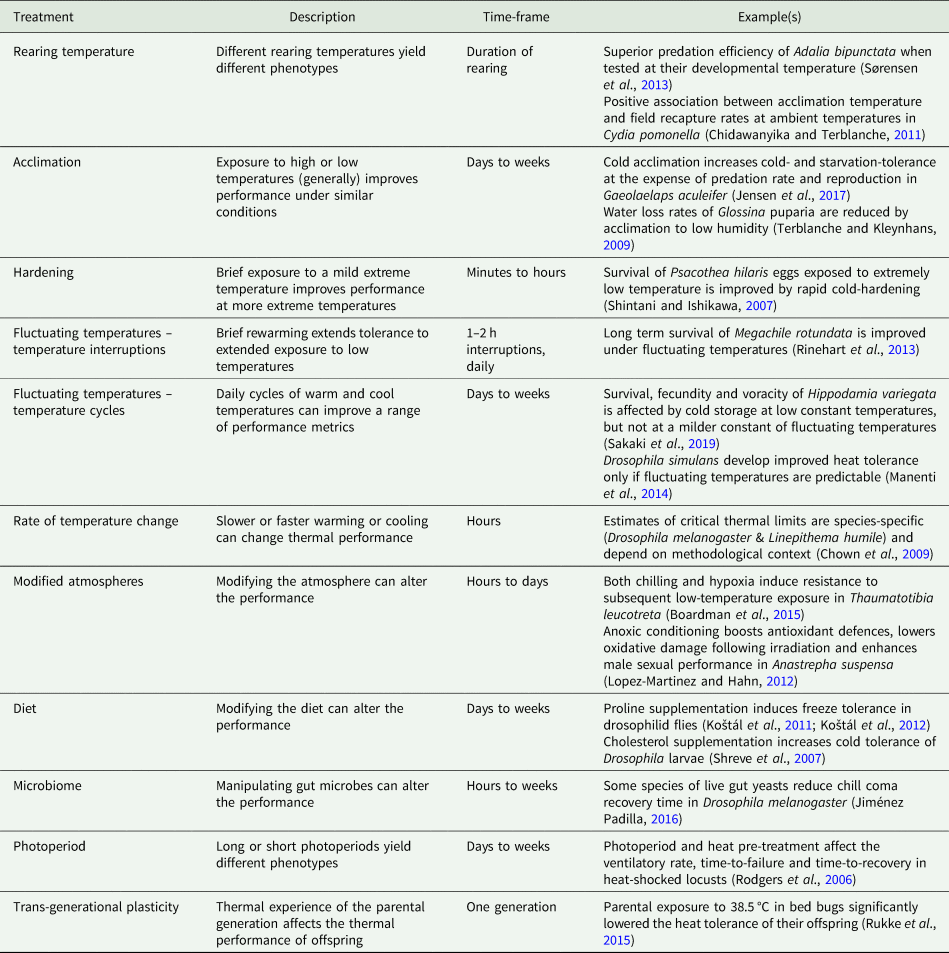
Within life stages, plasticity in insect thermal biology is sometimes irreversible (e.g. Sobek-Swant et al., Reference Sobek-Swant, Crosthwaite, Lyons and Sinclair2012), while in other cases, the plasticity (i.e. any induced response) is transient and reversible. The scale of reversibility is trait-dependent. For example, in Drosophila melanogaster, cold tolerance acquired during development is lost more readily than developmentally-induced heat tolerance (Slotsbo et al., Reference Slotsbo, Schou, Kristensen, Loeschcke and Sørensen2016). Furthermore, the timescale of reversibility of acquired thermal tolerance can range from minutes to days (summarized by Weldon et al., Reference Weldon, Terblanche and Chown2011), and depend on the magnitude of extreme temperature events, their timing during the day, and how recently they were applied (Xing et al., Reference Xing, Hoffmann, Zhao and Ma2019; Zhao et al., Reference Zhao, Xing, Hoffmann and Ma2019).
Both thermal tolerance and the plasticity of that tolerance can evolve rapidly, leading to variation among populations (summarized by Sinclair et al., Reference Sinclair, Williams and Terblanche2012). For example, populations of the sugarcane stalk borer Eldana saccharina (Lepidoptera: Pyralidae) from cool locations remain active at lower temperatures than moths from populations in nearby warmer locations. These populations also varied in their ability to acclimate to low temperatures (Kleynhans et al., Reference Kleynhans, Conlong and Terblanche2014). However, E. saccharina in this study lost their thermal plasticity after just two generations of laboratory rearing, suggesting that selection in the field maintains this trait's plasticity (Kleynhans et al., Reference Kleynhans, Conlong and Terblanche2014). This means that long-established laboratory populations may have lost some of the capacity to respond to treatments that may otherwise induce physiological plasticity (see also Terblanche and Chown, Reference Terblanche and Chown2007; Hoffmann and Ross, Reference Hoffmann and Ross2018). Thermal biology can evolve rapidly upon the release of insects that can reproduce (e.g. in weed biocontrol applications). For example, the water hyacinth control agent Eccritotarsus catarinensis (Hemiptera: Miridae) readily established in sites initially thought to be too cold to sustain viable populations likely as a result of a combination of plasticity and genetic adaptation (Griffith et al., Reference Griffith, Paterson, Owen and Coetzee2019).
In addition to thermal tolerances, other aspects of environmental physiology are plastic, for example water loss rates (e.g. Bazinet et al., Reference Bazinet, Marshall, MacMillan, Williams and Sinclair2010), metabolic rate (e.g. Kivelä et al., Reference Kivelä, Gotthard and Lehmann2019), anoxia resistance (e.g. Visser et al., Reference Visser, Williams, Hahn, Short and Lopez-Martinez2018), and accumulation of energy stores (e.g. Hahn and Denlinger, Reference Hahn, Denlinger, Berenbaum, Carde and Robinson2011). In some cases, this plasticity has overarching effects on physiology, such that improved tolerance to one stress changes tolerance to others (e.g. Weldon et al., Reference Weldon, Terblanche and Chown2011). This is generally encompassed by the concept of hormesis (e.g. Lopez-Martinez and Hahn, Reference Lopez-Martinez and Hahn2012), which can include interactions between thermal tolerance and irradiation effectiveness. The mechanisms underlying hormesis are not well-understood, but may arise from underlying similarities in the mechanisms underpinning tolerance to both stressors (‘cross-tolerance’) or because similar response pathways activate a suite of protective mechanisms (‘cross-talk’; see Sinclair et al., Reference Sinclair, Ferguson, Salehipour-shirazi and MacMillan2013). Thus, non-thermal conditions can affect thermal biology, and, conversely, temperature treatments have the potential to modify responses to other process-critical tolerances (Rodrigues and Beldade, Reference Rodrigues and Beldade2020). Unfortunately, there is not currently a strong understanding of how these interactions work, such that there is no general predictive framework available (see Kaunisto et al., Reference Kaunisto, Ferguson and Sinclair2016, for how this may be developed).
Currently, the mechanisms underlying plasticity (and return to the pre-acclimation physiological state) are not well-understood. Although there is a robust theory about how cells and whole insects modify performance at a given temperature (e.g. by changing membrane composition; Somero et al., Reference Somero, Lockwood and Tomanek2017), there is clearly considerable variation among species in the extent, cues, and underlying mechanisms for plasticity, such that there are no definitive rules. In some cases, the induction of a plastic response is restricted to a very precise set of conditions. For example, rapid cold-hardening is induced in Afrinus beetles immediately after a 2 h exposure to −2 °C, but rapid cold-hardening is only induced by a 0 °C exposure if it is followed by 30 min recovery at room temperature (Sinclair and Chown, Reference Sinclair and Chown2006). Indeed, even for well-known species, it is important to recognize that although the relative effects of treatments may be consistent among facilities, the magnitude of those effects can vary (Hoffmann and Sgrò, Reference Hoffmann and Sgrò2018). Most mass-rearing programmes focus on one (or a few) species, so gathering data on thermal biology and plasticity to understand the possibilities and constraints within a given system is achievable. Thus, we envisage that it may be possible to adjust rearing or holding conditions within existing mass-rearing programmes to achieve specific thermal biology goals in the targeted manipulation of performance for released insects.
Fluctuating temperatures
Most insect rearing – both at research and industrial scales – is done under constant temperatures. If there are fluctuations in temperature, they are within engineered limits (often less than 1 °C), or because of process constraints, which we discuss below. However, insects in nature may be exposed to temperatures that fluctuate considerably, and there is mounting evidence that these fluctuations can have profound biological effects. For example, insects can have very different thermal tolerances at different times of day (Kelty and Lee, Reference Kelty and Lee2001; Worland and Convey, Reference Worland and Convey2001; Sinclair et al., Reference Sinclair, Klok, Scott, Terblanche and Chown2003; Overgaard and Sørensen, Reference Overgaard and Sørensen2008), indicating a nimble repositioning of thermal biology in response to diel fluctuations in temperature (but see Nyamukondiwa et al., Reference Nyamukondiwa, Weldon, Chown, Le Roux and Terblanche2013 for an example where there is no temporal variation in thermal tolerance). Because the ambient temperature that an insect experiences is determined by both solar radiation and ambient air temperature, even insects living in apparently stable thermal environments can experience large fluctuations in body temperature (Potter et al., Reference Potter, Woods and Pincebourde2013).
Temperatures that fluctuate within a non-harmful temperature range can have profound, and usually positive, effects on insects (Fischer et al., Reference Fischer, Kolzow, Holtje and Karl2011; Colinet et al., Reference Colinet, Sinclair, Vernon and Renault2015). These can include faster development (e.g. Carrington et al., Reference Carrington, Armijos, Lambrechts, Barker and Scott2013), increased longevity (e.g. Economos and Lints, Reference Economos and Lints1986), increased reproductive output (e.g. Nyamukondiwa et al., Reference Nyamukondiwa, Kleynhans and Terblanche2010), and improved thermal tolerances (e.g. Fischer et al., Reference Fischer, Kolzow, Holtje and Karl2011). However, these positive effects are not universal, although most negative consequences appear to be associated with fluctuations that expose the insect to potentially deleterious conditions (e.g. Colinet et al., Reference Colinet, Renault, Hance and Vernon2006). Because growth and development rates increase exponentially with increasing temperature, high temperatures disproportionately increase these rates (a mathematical property called Jensen's Inequality; Ruel and Ayres, Reference Ruel and Ayres1999; Denny, Reference Denny2017). However, prolonged exposure to high temperatures can be detrimental (González-Tokman et al., Reference González-Tokman, Córdoba-Aguilar, Dáttilo, Lira-Noriega, Sánchez-Guillén and Villalobos2020). Thus, fluctuating temperatures can allow access to the benefits of high temperatures, without the penalties of prolonged exposure. In the context of insect mass-rearing and release, we might expect that these positive effects could yield faster development and adults that are better equipped to perform under the fluctuating temperatures in the field upon release.
At the other end of the temperature scale, fluctuating thermal regimes can significantly improve survival during prolonged cold storage. In these regimes, exposure to constant low temperatures is punctuated with brief (often only 1–2 h per day) exposures to warmer temperatures. These regimes can considerably increase the storage time for viable dormant insects. For example, the storage time of viable Megachile rotundata increases from 15 to 23 months using this strategy (Rinehart et al., Reference Rinehart, Yocum, Kemp and Greenlee2013). Thus, fluctuating temperatures are not only ecologically relevant, but also potentially valuable for increasing insect production, storing mass-reared insects, and improving the quality of released animals.
Finally, aside from the advantages we discuss here, fluctuating environments may be generally advantageous in mass-rearing by reducing lab adaptation or domestication. Long-term rearing in near-constant, predictable environments can homogenize genotypes and (in some cases) reduce quality (Ochieng’-Odero, Reference Ochieng’-Odero1994; Hoffmann and Ross, Reference Hoffmann and Ross2018). Although cryopreservation is the most-commonly used means to maintain genetic diversity by continuously re-introducing genetic variation (Leopold and Rinehart, Reference Leopold, Rinehart, Denlinger and Lee2010), variation in rearing conditions (primarily diet) has also been mooted as a method to prevent unidirectional selection (Leppla et al., Reference Leppla, Huettel, Chambers, Ashley, Miyashita, Wong and Harris1983; Hoffmann and Ross, Reference Hoffmann and Ross2018). We speculate that fluctuating thermal conditions during rearing could not only improve performance upon release through physiological conditioning, but that these fluctuating conditions could also help to maintain the genetic diversity that allows the success of individuals in variable field environments.
Effects of temperature on release performance of insects
In the context of insect mass-rearing for release, our take-home message is that the thermal biology of the insect at the point of release will be a product of the thermal environment throughout its rearing, handling, and transport. The impact of that thermal biology will depend on whether the thermal performance is a meaningful match with release conditions (fig. 1). In the field, there is an approximately linear increase in the number of released moths caught as a function of air temperature (Chidawanyika and Terblanche, Reference Chidawanyika and Terblanche2011; Terblanche, Reference Terblanche2014; Boersma et al., Reference Boersma, Boardman, Gilbert and Terblanche2019). However, field dispersal could be mediated by other factors, for example, dispersal behaviour itself can be thermally plastic (Fasolo and Krebs, Reference Fasolo and Krebs2004). However, the activity patterns of many insects can be temperature-dependent in more complicated ways (Dell et al., Reference Dell, Pawar and Savage2011).
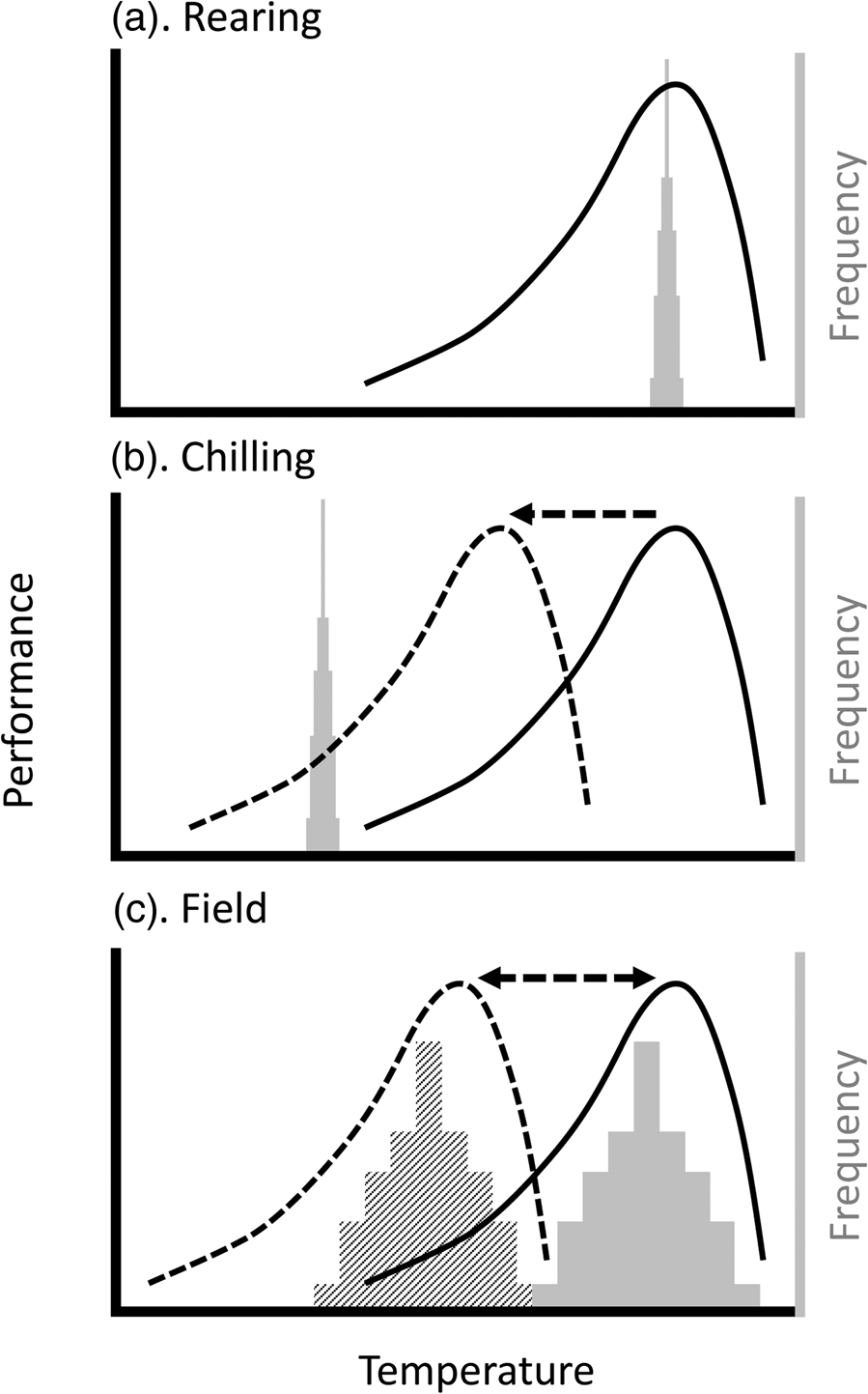
Figure 1. Plasticity can reduce mismatches between insect thermal performance and temperatures experienced during (a) mass rearing, (b) handling, and (c) release. Lines represent hypothetical thermal performance curves (TPCs) of insects being reared; histograms indicate the frequency distribution of ambient temperatures likely experienced during this process. (a) During rearing, temperatures are generally constant (i.e. temperature distribution is very narrow) and (because growth rate has been optimized) near the temperature where performance is maximized (often referred to as the T OPT). (b) During chilling for handling and transport, the temperatures are well below the normal range of the TPC (solid line), but the process of chilling may lead to plasticity (i.e. an acclimation response; dashed line), leading to a better match between TPC and ambient temperatures. (c) Upon or after release in the field, the temperature distributions will be significantly broader. They may also vary, for example by season or between day and night, such that different TPCs will maximize performance under different circumstances. Thus, a preconditioning treatment that matches the TPC to the expected ambient temperatures (e.g. dashed line to hatched histogram) will maximize performance, whereas release of animals that have, for example, been cold-acclimated (dashed TPC), will result in low performance if the ambient temperatures are more similar to the solid histogram.
Thermal preconditioning can mitigate low recapture rates under adverse environmental conditions. For example, warm-acclimated codling moths are twice as likely to be recaptured as their cold-acclimated counterparts under warm conditions and vice versa (i.e. cold-acclimated moths do well in cold but not heat) (Chidawanyika and Terblanche, Reference Chidawanyika and Terblanche2011). This effect is even greater in Drosophila (Kristensen et al., Reference Kristensen, Hoffmann, Overgaard, Sørensen, Hallas and Loeschcke2008), but does not appear to apply to C. capitata (Steyn et al., Reference Steyn, Mitchell, Nyamukondiwa and Terblanche2022). Thus, inducing physiological adjustments in key thermal traits has the potential to increase the performance of (some) insects when released in the field. We note, however, that released control agents act in interaction with the species or population it is intended to control. Differences in plasticity may affect the interaction between species or populations, and the net outcome of a release cannot be evaluated on the performance of the mass-reared insect alone. For example, laboratory thermal acclimation suggests that the sugarcane stemborer Chilo partellus expresses higher degrees of thermal plasticity than its larval endoparasitoid Cotesia flavipes, which could compromise control efficiency under certain thermal conditions (Mutamiswa et al., Reference Mutamiswa, Chidawanyika and Nyamukondiwa2018).
Temperatures experienced by mass-reared insects during rearing, transport, and release
Mass-reared insects are usually held at constant temperatures, which are often fairly warm (typically 25–27 °C). This maximizes the growth rate and therefore the number of animals produced (Sørensen et al., Reference Sørensen, Addison and Terblanche2012; Mamai et al., Reference Mamai, Lobb, Somda, Maiga, Yamada, Lees, Bouyer and Gilles2018). We do note that these temperatures are almost always set at the room level – high densities of larvae or eggs in an insulative rearing medium can elevate colony temperatures considerably above the set room temperature (e.g. Paz et al., Reference Paz, Carrejo and Rodriguez2015). In several SIT protocols, temperature-sensitive mutants are used to sex-select the animals due for release; this usually takes the form of a brief pulse of high temperature applied to the embryos: >28 °C in the case of C. capitata (Franz, Reference Franz, Dyck, Hendrichs and Robinson2005). Although insects are exposed to intense energy during irradiation, the actual increase in temperature during irradiation is on the order of a few degrees (R. Pereira, pers. comm; see also Sinclair et al., Reference Sinclair, Gibbs, Lee, Rajamohan, Roberts and Socha2009), and unlikely to elicit a heat shock response. Insects are usually chilled prior to handling and irradiation, as well as for packing and shipping (Dyck et al., Reference Dyck, Hendrichs and Robinson2005), and sometimes chilled specifically to aid dispersal (e.g. Mirieri et al., Reference Mirieri, Mutika, Bruno, Seck, Sall, Parker, van Oers, Vreysen, Bouyer and Abd-Alla2020). In most cases, a departure from chilling temperatures is because of inaccuracy in temperature maintenance rather than because of deliberately variable temperature conditions, which we discuss below.
We contend that mass-rearing insects provide both opportunities to control the thermal experience to maximize performance, and practical challenges because temperature control is usually optimized for the equipment and the process rather than to maximize insect performance. To begin addressing this, we examine the thermal experiences of mass-reared insects from two different SIT programmes focused on Lepidoptera in South Africa and Canada. We view these case studies through the lens of the kinds of temperatures experienced, the order in which they are experienced, and the contrasts between temperatures experienced during production with those experienced upon release. We follow-up with an examination of a dipteran release programme in Australia which benefits from an extensive body of thermal biology research.
False codling moth (Western Cape, South Africa)
False codling moth (Thaumatotibia leucotreta, Tortricidae) is a polyphagous pest of crops including macadamia nuts, citrus and cotton (Prinsloo and Uys, Reference Prinsloo and Uys2015). It is suppressed in the Western and Eastern Cape regions of South Africa by an area-wide SIT programme mainly focused on citrus-producing areas. Insects are reared in Citrusdal, South Africa, and transported in a cooled vehicle to the target area for release either by hand or from a helicopter. In both cases, they are re-warmed to ambient conditions for c. 1 h before release. Moths are reared through their larval and pupal stages at 25–27 °C, before 1–2 day-old adults are transferred to 6–8 °C for handling and irradiation, and shipped between 6 and 8 °C (Nevill Boersma, XSIT, pers. comm.). If the temperature exceeds 15 °C during the process, moths are not re-cooled to avoid decreased performance associated with repeated cold exposure, and are instead kept at 8–10 °C before release. They are released only if the orchard air temperature exceeds 12 °C, because moths typically are not capable of dispersing or being recaptured at cooler temperatures (Boersma et al., Reference Boersma, Boardman, Gilbert and Terblanche2019). False codling moths are typically released during the peak citrus growing season that coincides with low-temperature field conditions (in the morning, temperatures are often <15 °C) but field temperatures can exceed 30 °C.
Thus, in this system, rearing is at 27 °C, which is considerably warmer than field release conditions, and moths are typically handled for 12–24 h below 12 °C prior to release. The cold chain tolerances used in this system are based on a wealth of laboratory and field data specifically gathered for T. leucotreta (e.g. Blomefield and Giliomee, Reference Blomefield and Giliomee2011; Boardman et al., Reference Boardman, Grout and Terblanche2012; Boersma et al., Reference Boersma, Boardman, Gilbert and Terblanche2019; Karsten et al., Reference Karsten, Lebenzon, Sinclair and Terblanche2019). This is an excellent example of an outcome of a sustained research effort spanning several partnerships between researchers and the facility.
Codling moth (British Columbia, Canada)
Codling moth (Cydia pomonella Lepidoptera: Torticidae) is a pest of apples and pears. It is suppressed in the Okanagan Valley, British Columbia, Canada, via area-wide release of sterile adults. Insects are reared in a facility in Osoyoos, BC (Dyck et al., Reference Dyck, Graham and Bloem1993; Sterile Insect Release Program, 2016). Freshly-laid eggs are stored for 2–5 days at 2 °C, before hatching and rearing to adulthood at near-constant 27 °C. Adults are collected and held at 2 °C before irradiation 8–21 days after emergence. Post-irradiation, they are maintained at 2–4 °C during transport (2–4 h) and depot storage (an additional 0.5–4 h). Moths are transferred to all-terrain vehicles in coolers, and released in orchards at near ground-level via a blower mechanism. The goal for the release conditions is to maintain the moths at 2–4 °C, but this is anecdotally the most challenging part of the supply chain: release runs can last 0.5 to 6 h, and towards the end of a long run, moths are often at temperatures above 4 °C (Evan Esch pers comm.). Release temperatures are dependent on the date during the season and the time of day, and can range from 10–40 °C. Although there has been some concern that chilling might affect field performance and/or activity (Bloem et al., Reference Bloem, Carpenter and Dorn2006; Judd et al., Reference Judd, Arthur, Deglow and Gardiner2012), flight mill studies suggest that chilling doesn't affect overall flight performance (Matveev et al., Reference Matveev, Kwon, Judd and Evenden2017). Nevertheless, there is a documented trade-off in this species: the physiological response to cold reduces performance at high temperatures (Chidawanyika and Terblanche, Reference Chidawanyika and Terblanche2011) which means that there may be hidden compromises in the distribution process on hot days. Altogether, the adult moths may have been held anywhere between 11 and 35 h (at the extremes, although this upper bound is usually avoided) in the cold, prior to release.
Recently, OKSIR (the organization that runs the Okanagan Valley Sterile Insect Release Program) has been trialling shipment of sterile moths to New Zealand, which entails a flight of >13 h at low temperatures, and a total time of c. 32 h from the factory to release. Notably, time from irradiation to release is within the bounds of the supply chain in BC (which includes extensive transport by road), and the release temperatures in New Zealand do not reach the upper extremes seen in BC (Evan Esch, pers. comm.).
Queensland fruit fly (Queensland, Australia)
The Queensland fruit fly, B. tryoni, is a tropical and subtropical pest of fruits and vegetables in Australia and some Pacific Islands (Clarke et al., Reference Clarke, Powell, Weldon and Taylor2011), with the potential to spread into more temperate climates (Merkel et al., Reference Merkel, Schwarzmueller, Hulthen, Schellhorn, Williams and Clarke2019; Popa-Báez et al., Reference Popa-Báez, Lee, Yeap, Westmore, Crisp, Li, Catullo, Cameron, Edwards, Taylor and Oakeshott2021). Bactrocera tryoni are mass-reared, sterilized with irradiation, and released as part of area-wide Sterile Insect Technique control programmes (Jessup et al., Reference Jessup, Dominiak, Woods, De Lima, Tomkins, Smallridge, Vreysen, Robinson and Heindrichs2008). Here we wish to highlight a body of knowledge that leaves this programme primed to leverage the plasticity of thermal biology. There is a long history of investigation into thermal biology and the plasticity thereof for B. tryoni, in Australia (e.g. Meats, Reference Meats1973, Reference Meats1976a, Reference Meats1976b, Reference Meats1976c; Meats and Fay, Reference Meats and Fay1976; Meats, Reference Meats1983; Meats and Kelly, Reference Meats and Kelly2008). Already in the 1970s, Meats and colleagues were exploring the viability of thermal preconditioning for field releases. The SIT programme had mixed success, perhaps partly due to the diversity of field conditions encountered post-release (e.g. Meats and Fay, Reference Meats and Fay1977; Fay and Meats, Reference Fay and Meats1987a). One tantalizing result suggests that cold-acclimated sterile flies survived better than warm-acclimated flies, and the former survived at similar levels to the wild flies over a few weeks in early spring (Fay and Meats, Reference Fay and Meats1987b). Recently, the use of stage-specific thermal treatment has been explored in B. tryoni production, largely to manage production schedules by delaying and synchronizing development (Benelli et al., Reference Benelli, Ponton, Lallu, Mitchell and Taylor2019a, Reference Benelli, Ponton and Taylor2019b). Thus, there is a large existing body of knowledge available for fine-tuning the thermal biology of this species during rearing and release.
Manipulating the thermal biology of released insects – opportunities and challenges
We make several observations from our case studies. First, post-hatch rearing is primarily at constant benign warm conditions, but handling, storage, and distribution largely under near-constant cold conditions. Second, most animals are released after several (or many) hours of chilling. In Canada, this could lead to a substantial mismatch between (cold) transport conditions and the temperatures they encounter in the field. By contrast, in South Africa it may serve to pre-condition the animals for cool release conditions, although this is a fortuitous consequence of the production and release chain, not an explicit attempt to pre-condition the moths. Third, there is within-programme variation in the timing of transport and release which is driven by logistics and likely unavoidable. However, the relationship between increasing exposure duration and deleterious effects on insects is well-described (Overgaard and Macmillan, Reference Overgaard and Macmillan2017). Thus, supply-chain management to reduce chilling time or adoption of fluctuating thermal regimes to mitigate accumulated chilling injury could make the performance of released insects more consistent. Finally, to our knowledge, none of these programmes is deliberately harnessing phenotypic plasticity to improve performance.
In table 2, we summarize what we perceive as the opportunities to modify release performance or to mitigate mismatches between rearing and release conditions. Many of these lie within a general framework of hormesis (Costantini et al., Reference Costantini, Metcalfe and Monaghan2010), whereby mild stress exposure increases tolerance to other stresses, for example, brief stressful anoxia improves tolerance to radiation in Anastrepha suspensa (Lopez-Martinez and Hahn, Reference Lopez-Martinez and Hahn2012). One might argue that the simplest of these conditioning treatments is a non-stressful fluctuating temperature regime, which has the potential to improve many relevant performance metrics after chilling and release (even if applied to earlier life stages, see MacLean et al., Reference MacLean, Kristensen, Overgaard, Sørensen and Bahrndorff2017). However, we acknowledge that there are challenges to implementing any kind of temperature-based plasticity in a mass-rearing situation.
Table 2. A summary of the potential effects of temperature at different stages in the production and distribution of mass-reared insects, evidence-based modifications that address those effects, and the potential biological consequences and trade-offs of those modifications
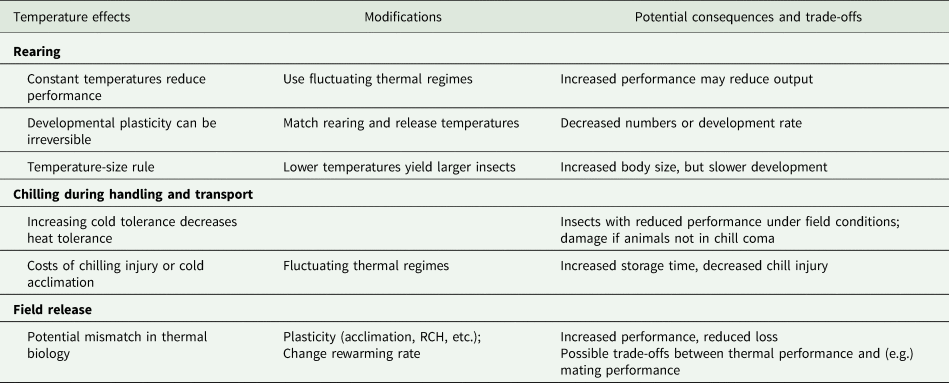
Performance metrics vary among programmes and among components within programmes. In particular, the number of insects produced is often used as a primary performance characteristic for a production facility (Sørensen et al., Reference Sørensen, Addison and Terblanche2012), with performance of the animals often assessed at the time they leave the factory or arrive at the release site, rather than when they are released. Modifying the temperature, especially during development, could lead to higher-quality individuals, but potentially in lower numbers or at a slower rate. Currently, most facilities produce ‘one size fits all’ insects, so the relationship between measurements of insect quality and release performance may vary seasonally. In situations where there is high variance in the performance of released insects, there may be considerable merit to understanding which quality metrics are associated with release performance, and how these change with different release conditions. Thus, identifying meaningful quality and performance metrics that allow the selection of individuals that will perform well in specific release conditions is a research priority.
The challenges to implementing these approaches may be simple engineering problems. For example, it can be difficult to control temperature precisely during aspects of transport (for example in the transport of moths during a long day of releases in the BC codling moth releases). Similarly, although fluctuating temperatures may be desirable, fluctuating temperatures are anecdotally much more challenging for temperature control systems and can reduce the lifespan of expensive refrigeration equipment (but see Greenspan et al., Reference Greenspan, Morris, Warburton, Edwards, Duffy, Pike, Schwarzkopf and Alford2016). The simplest form of fluctuating temperatures (brief interruptions of constant cold) can be effected by simply moving animals from one space to another for a brief period. In a research context, this involves removing them from the fridge and placing them on the bench, but we recognize that this may require retooling infrastructure in an industrial facility. Alternatively, one could move insects between climate-controlled rooms set to different temperatures, or make use of existing spatial variability in conditions within a facility (e.g. cool on the ground, warm at the ceiling). However, it is also important to recognize that chilling is an important component in mass-rearing, to prevent mating in mixed-sex releases, prevent damage, and reduce aging, so a thermal interruption that allows animals to recover from chill coma and mate could be undesirable. Thus, instituting controlled fluctuating temperatures during transport may be impractical, but we propose that fluctuating temperatures could significantly increase storage time at a facility.
Conclusions
In conclusion, the temperature is probably the most easily manipulated environmental variable that has large, and in many cases well-documented, phenotypic effects. Because of the possibility of generating mismatches between rearing and release conditions, these effects may have a negative impact on the performance of released insects. However, there are a variety of ways to manipulate thermal biology to better match animals to their release conditions, and/or reverse the negative impacts of transport or handling conditions. We believe that the challenges to harnessing plasticity to reduce negative impacts are practical, not intellectual. Determining how to optimize thermal performance is straightforward, and many exemplar studies that optimize thermal performance for a specific species already exist. However, we do caution that the possibility of performance-reducing trade-offs needs to be carefully explored. Regardless, the nature of infrastructure and the constraints of the distribution and release supply chain mean that there are nevertheless significant practical/engineering constraints to overcome.
Acknowledgements
Thanks to Nevill Boersma (XSIT) and Evan Esch (OKSIR) for helpful discussion and explanation of the production and shipping temperatures for their species, Rui Cardoso Pereira for discussion, and four anonymous reviewers and Dan Hahn for comments on earlier versions of this manuscript. This work arose from the IAEA/FAO Coordinated Research Project D41205 ‘Dormancy management to enable mass-rearing and increase the efficacy of sterile insects and natural enemies’, which also funded JST's contributions. We particularly acknowledge the leadership of Dan Hahn and David Denlinger in the CRP, and all of the participants for constructive and critical discussion. BJS is grateful to the Natural Sciences and Engineering Research Council (NSERC) of Canada for continued support for work on insect thermal biology via a Discovery Grant.
Conflict of interest
B. J. S. and J. G. S. declare none. J. S. T. has received funding from the False Codling Moth SIT programme.





