Introduction
Agriculture in Madagascar is one of the major farming activities owing to the relatively heavy economic value of the products and improving food security (FAO, 2018). The diverse agro - climatic conditions and altitude (from 0 to 2, 876 m asl) enable the year-round production of a wide range of horticultural crops. In Madagascar, fruit and vegetable crops hold a key position in smallholder agricultural production systems due to the number of farmers involved. Agricultural areas represent 70% of the total land area and approximately, around 2 million hectares are cultivated annually (FAO, 2018). Agriculture employed approximately 74.3% of the local population and accounted for 32% of the gross domestic products (GDP) in 2016 (FAO, 2018).
Malagasy farmers do not cultivate fruit crops on a large scale as monoculture. Most fruit trees are cultivated for local markets and are scattered around villages and hamlets, and referred to as ‘family agriculture’. More than 40 cultivated species (tropical and temperate) of fruits are grown in Madagascar, of which many species were introduced during the colonial era (Perrier de la Bathie, Reference Perrier de la Bathie1931). Smallholder farmers supply fruits and vegetables consumed in urban regions, most of which are sold through the informal sector. The majority produced is for local consumption. Few species of fruit or vegetables are of great economic significance as export products, such as cocoa Theobroma cacao Linnaeus (Malvaceae), coffee Coffea Linnaeus sp (Rubiaceae), lychee Litchi chinensis Sonnen (Sapindaceae), pepper Capsicum Linnaeus sp (Solanaceae) and spices.
Tephritidae are considered as one of the most destructive pest in horticulture in the continental island. Thirty-two species of frugivorous Tephritidae (De Meyer, Reference De Meyer1998; De Meyer et al., Reference De Meyer, Quilici, Franck, Chadhouliati, Issimaila, Youssoufa, Abdoul-Karime, Barbet, Attié and White2012) belonging to six genera were recorded from Madagascar: Carpophthoromyia Austen (one species), Ceratitis MacLeay (ten species), Neoceratitis Hendel (two species), Bactrocera Macquart (three species), Dacus Fabricius (11 species) and Trirhithrum Bezzi (five species). In addition to representatives of these main frugivorous genera, there are also other species belonging to lesser important genera: Celidodacus madagascariensis Hering, Taomyia marshalli Bezzi, Taomya mauritiana sp. nov., Taomyia ocellata Lamb and Taomyia pictipennis Hancock. Nevertheless, among this vast diversity, only four species are considered economically important. It includes three native species, Ceratitis malgassa Munro, Neoceratitis cyanescens Bezzi, Dacus demmerezi Bezzi (Dubois, Reference Dubois1965) and one exotic species Bactrocera dorsalis Hendel (Raoelijaona et al., Reference Raoelijaona, Raoelijaona, Ratovonomenjanahary, Brunet, De Meyer, Vayssières and Quilici2012). The Malagasy fruit fly species C. malgassa is endemic to Madagascar (De Meyer et al., Reference De Meyer, Quilici, Franck, Chadhouliati, Issimaila, Youssoufa, Abdoul-Karime, Barbet, Attié and White2012). Neoceratits cyanescens a major pest of Solanaceae and D. demmerezi, the Indian Ocean cucurbit fly, are probably native of Madagascar and were probably introduced from Madagascar to the Mascarenes (Etienne, Reference Etienne1982).
The invasive oriental fruit fly, B. dorsalis, previously recognized as Bactrocera invadens Drew, Tsura & White was first detected in Madagascar in 2010 near Toamasina (Raoelijaona et al., Reference Raoelijaona, Raoelijaona, Ratovonomenjanahary, Brunet, De Meyer, Vayssières and Quilici2012). This species was recorded in Africa mainland since 2003 (Lux et al., Reference Lux, Copeland, White, Manrakhan and Billah2003). Soon after its discovery in Kenya, B. dorsalis spread throughout Africa, as well as in the Indian Ocean Islands nearby Madagascar, where it was recorded in Comoro islands in 2005, then Mayotte in 2007 (De Meyer et al., Reference De Meyer, Robertson, Mansell, Ekesi, Tsuruta, Mwaiko, Vayssieres and Peterson2009, Reference De Meyer, Quilici, Franck, Chadhouliati, Issimaila, Youssoufa, Abdoul-Karime, Barbet, Attié and White2012). Other exotic polyphagous Tephritidae of major economic impacts are present on the island, most of the records date back from the 1950s such as the mango fruit fly Ceratitis cosyra Walker and the Mediterranean fruit fly Ceratitis capitata Wiedemann (Dubois, Reference Dubois1965; De Meyer, Reference De Meyer2000).
Fruit production losses by C. malgassa were previously estimated between 70 and 80% on plum Prunus domestica Linnaeus (Rosaceae), 80% on peach Prunus persica Linnaeus (Rosaceae) ‘paiso gasy’ variety, and 70% on citrus Citrus Linnaeus sp (Rosaceae) (Dubois, Reference Dubois1965). The only recent survey made was done by the local extension services (DPV, Direction de la Protection des Végétaux) that estimated direct damages due to B. dorsalis to reach up to 50% of the commercial production in five regions of Madagascar (Raoelijaona et al., Reference Raoelijaona, Raoelijaona, Ratovonomenjanahary, Brunet, De Meyer, Vayssières and Quilici2012), but nothing more precise had been conducted in the other regions of the country.
Despite the presence of fruit fly pests and quarantine species on the island, few studies have examined their ecology and distribution. Previous inventory results provided a list of Tephritidae species from Madagascar most often made by trapping systems or fruit surveys on a very small part of the country (Dubois, Reference Dubois1965; Hancock, Reference Hancock1984; De Meyer et al., Reference De Meyer, Quilici, Franck, Chadhouliati, Issimaila, Youssoufa, Abdoul-Karime, Barbet, Attié and White2012). The aim of this study was to obtain data on the host plant ranges, diversity and geographic distribution of all frugivorous tephritids throughout the different agroecological regions.
Materials and methods
Ecology and climatic conditions of the sampled regions
Madagascar (covering an estimated area of 592,000 km2) is a continental island situated in the Indian ocean separated from the east of Africa by the Mozambique Channel (about 400 km away). The country is divided into seven agroecological and climatic regions (R1–R7, fig. 1) ranging from humid eastern lowland forest to spiny thickets in the south west. The seventh agroecological zone (R7) is a mangrove covering around 320,000 ha (Iltis, Reference Iltis1995). The Highland stretches from the North to the South along more than 1000 km and occupies the interior of the island above 800 m asl of altitude. The climate is subtropical with a hot and rainy season between November to March (austral summer) and a cooler dry season from April to October (austral winter). Climate varies from one region to another (fig. 1). The day temperature varies from 5.5 °C (winter, on Highland) to 41.5 °C (summer in the South west lowlands), the minimum night temperature can reach −1 °C (during winter, but rarely) in the Vakinankaratra region. Annual rainfall ranges from 350 to 3500 mm. Administratively, Madagascar is divided into six provinces (fig. 1) which are sub-divided into 22 regions, about 119 districts and 800 municipalities.
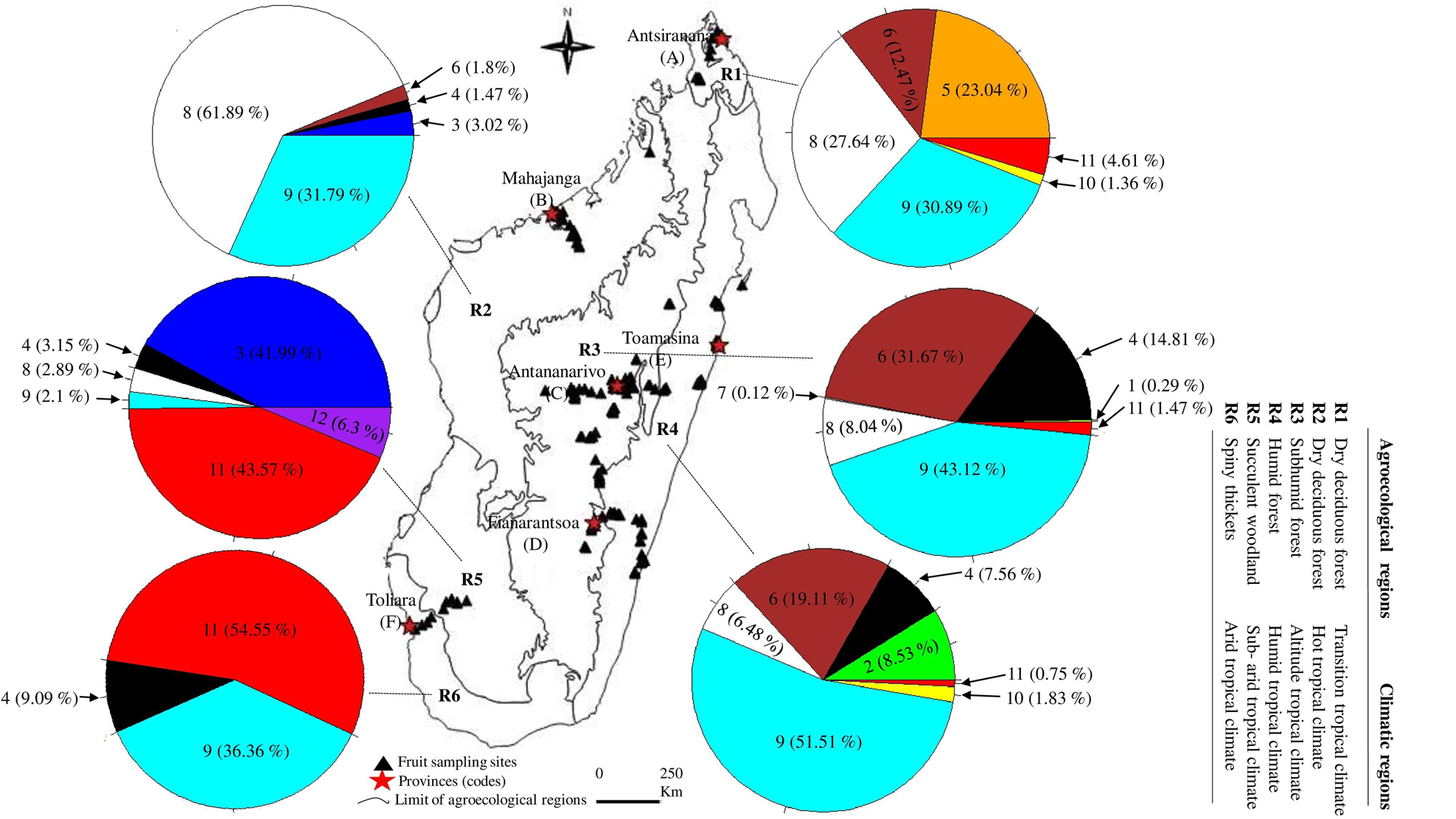
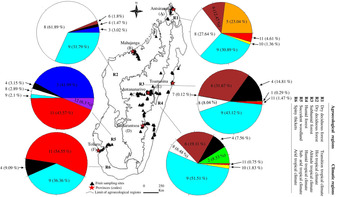
Figure 1. Proportion (% of adults) and geographic distribution of Tephritidae species across agroecological and climatic regions of Madagascar. (1) C. argenteostriata, (2) C. capitata, (3) C. cosyra, (4) C. malgassa, (5) C. pedestris, (6) N. cyanescens, (7) T. crescentis, (8) B. dorsalis, (9) D. demmerezi, (10) D. quilicii, (11) D. vertebratus, (12) D. xanthaspis.
Fruits and vegetables areas in Madagascar
Fruit and vegetable crops are distributed across all regions of Madagascar. Most fruit species can be grown on the Highland due to the subtropical climate. The most common fruit species grown in the region of Analamanga (Highland) are Japanese persimmon Diospyros kaki Thunberg (Ebenaceae), guava Psidium guajava Linnaeus (Myrtaceae), loquat Eriobotrya japonica (Thunberg) Lindley (Rosaceae), goldenberry Physalis peruviana Linnaeus, (Solanaceae). The temperate fruits are mostly cultivated in Vakinankaratra region (Highland) such as strawberry Fragaria Linnaeus sp (Rosaceae), apple Mallus domestica Borkhausen (Rosaceae), apricot Prunus armeniaca Linnaeus (Rosaceae), plum P. domestica, peach P. persica and pear Pyrus communis Linnaeus (Rosaceae). Grapes Vitis vinifera Linnaeus (Vitaceae) grown for wine production are mainly cultivated in Ambalavao (Fianarantsoa region, middle altitude). The eastern coastal region (Toamasina) is suitable for all tropical fruits, including bullock's heart Annona reticulata Linnaeus (Annonaceae), sugar-apple Annona squamosa Linnaeus (Annonaceae), jackfruit Artocarpus heterophyllus Lamarck (Moraceae), banana Musa Linnaeus sp (Musaceae), citrus species Citrus sp, lychee L. chinensis. Production of papaya Carica papaya Linnaeus (Caricaceae), banana Musa sp (available all year-round) and citrus Citrus sp are found both on the Highland and on the coast. The western and north west regions (Mahajanga, Antsiranana) has spontaneously cashew nut Anacardium occidentale Linnaeus (Anacardiaceae), mango Mangifera indica Linnaeus (Anacardiaceae), marula Sclerocarya birrea (A. Richard) Hochstetter (Anacardiaceae), jew plum Spondias dulcis Solander ex Parkinson (Anacardiaceae), tamarind Tamarindus indica Linnaeus (Fabaceae), monkey orange Strychnos spinosa Lamarck (Loganiaceae), common jujube Ziziphus jujuba Miller (Rhamnaceae), lemon Citrus limon (Linnaeus) Burman (Rutaceae) and others species of citrus Citrus sp. The main mango production areas are the western (Mahajanga), northern (Ambanja, Ambilobe) and southern (Toliary) regions. The south area is dominated by the wild marula S. birrea and especially the cactus Opuntia Miller sp (Cactaceae).
The family of Cucurbitaceae is represented by 27 genera of which eight are endemic (Keraudren-Aymonin, Reference Keraudren-Aymonin1966). Cultivation of cucurbits is practiced throughout the island with native seeds and available throughout the year. The south and west regions are very poor in vegetable crops, most of the production is made on the Highland. Cucurbitaceae are the main crops that are often intercropped with root crops and fruit trees. The Solanaceae, such as tomato Lycopersicum esculentum Miller (Solanaceae) and varieties of peppers are grown everywhere but mainly in the province of Antananarivo and Antsiranana and grown throughout the year.
Sampling sites and sampling fruits surveys
Fruit sampling was carried out from November 2016 to July 2018. Sampling covered all the different regions and agroecological zones of the island. Fruits of cultivated and wild plant species were collected from fields, orchards and wild areas and sites were chosen because of their accessibility (supplementary table S1).
Species fruiting period varies during the year. In order to maximize the number of different fruit species sampled, sampling were repeated twice or three times per year across the six agroecological regions. All sites were georeferenced with a GPS device (Garmin©). A sampling point (indicated by black triangle in fig. 1) corresponds to a location or site; two locations are at least 1 km apart. The number of fruits collected per sample was according to fruit availability and abundance during the sampling period. Fruits were sampled randomly in each site, most of them were collected from trees and only a few, very recently fallen (less than a day, as not rotten), were collected on the ground. Then, they were individually weighed and placed separately in perforated tagged transparent plastic bags. In order to avoid damage on fruits (or larvae inside the fruit that would need to pupate during the fruit transport to the lab) during transport, each bag was filled with sand at the bottom.
Fruit fly rearing
All samples were transported to the rearing unit at the DPV laboratory of Antananarivo, where they were labeled, and individually transferred to incubation buckets. Small size fruits of less than 5 g in weight were grouped together in batches and placed in plastic buckets covered by fine mesh (11 cm × 6 cm, diameter × depth). Larger fruits were kept in individual buckets (26 cm × 11 cm, diameter × depth). All fruits were weighed, counted and noted. The bottom of each incubation bucket was filled with a 2 cm layer of river sand, so that the last stage larvae could easily drop into the sand and pupate. Every 3 days, the sand was sieved to collect pupae. Pupae were then placed in small hatching cups covered by tissue with a moist cotton inside. The number of newly emerging fruit flies was recorded for all fruits; this procedure was repeated until no pupa was collected during approximately about 6 weeks after field sampling. Rearing was held at room condition.
Fruit fly and host plant identification
All adults were counted, sexed, and preserved in 96% ethanol for later identification. Different types of morphometric keys were used to identify the emerging fruit fly species, White and Elson-Harris (Reference White and Elson-Harris1992), De Meyer (Reference De Meyer2000) and Virgilio et al. (Reference Virgilio, White and De Meyer2014) accessed online (http://keys.lucidcentral.org/keys/v3/fruitflies/). In case of any doubts, sample specimens were sent to RMCA (Royal Museum of Central Africa, Belgium) for confirmation under the help of Dr M. De Meyer.
At the same time of sampling, photographs of tree, leaves, fruit and if possible inflorescences were taken for plant specimen identification. Indigenous plant species were identified by botanical expert using photographs and herbarium at the Biologie et Ecologie Végétale laboratory (University of Antananarivo). Botanical plant names corresponded to those found in the ‘Catalogue of the vascular plants of Madagascar’ (Phillipson et al., Reference Phillipson, Schatz, Lowry, Labat, Ghazanfar and Beentje2006).
Infestation level
Two parameters were evaluated for all the sampled fruit fly species. The infestation index was determined as the number of adult or pupa per kg of fruit, a fruit was considered infested when at least one pupa was found. The incidence was evaluated as the number of positive fruits per 100 (% F+ or infestation percentage) of incubated fruit samples, in this case, one sample was equal to one fruit incubated. Incidence was not calculated for small size fruits which were held together in batches and did not allow to have a precise incidence evaluation.
Statistical analysis
Statistical analysis was performed on the interaction between host plants, altitudes, provinces, agroecological and climatic divisions on the distribution and diversity of fruit fly species on the island. To visualize the web interaction between fruit fly species and host plant species, a network was drawn with ‘plotweb’ function in the package ‘bipartite’ (Dormann et al., Reference Dormann, Fründ, Blüthgen and Gruber2009). Altitudinal gradients were divided into three classes: low altitudes from 0 to 499 m asl, medium altitudes from 500 to 1000 m asl, high altitudes over 1000 m asl.
Incidence and infestation indexes were used to compare infestations level in different fruit species. Analysis of variance (ANOVA) was performed using the general linear model (glm function, quasipoisson family, link identity) procedure and mean separations were done using the Chi square test (Chisq function).
All the statistical analyses and graphs (all at 5% threshold) were performed with R software version 3.5.1 (R Core Team, 2018).
Results
Host range of fruit flies
Over the sampling period, 37,965 fruits in total, weighing 636.92 kg were collected from 198 plant species belonging to 59 families. A total of 191 georeferenced sites were sampled across six agroecological regions, with 8 sites in R1, 25 sites in R2, 83 sites in R3, 60 sites in R4, 7 sites in R5, and 8 sites in R6, respectively (fig. 1, supplementary table S1). Sample size consisted of two to 3401 fruits per plant species (supplementary table S2). The incubation of these fruits produced 10,302 pupae from which emerged 6021 fruit flies (table 1) corresponding to a rate of adult emergence of 58.42%.
Table 1. List of plant species identified as hosts of Tephritidae, infestation level, incidence, diversity and abundance of fruit flies
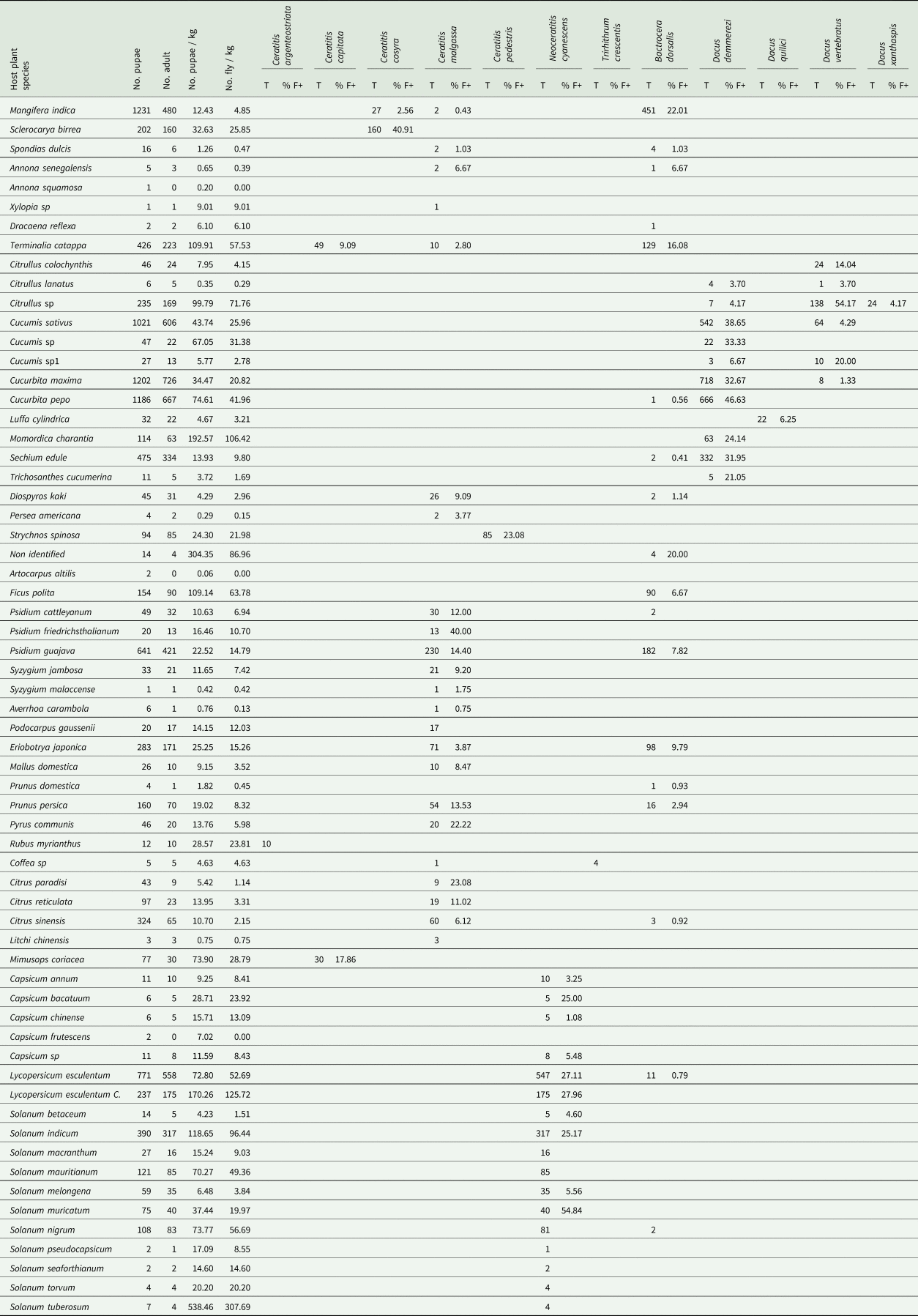
T: total number of adult of fruit fly species/host plant.
Fruit flies are ordered alphabetically by family, tribe and scientific name.
Positive fruits (% F+): percentage of infested fruit sample (one sample = one incubated fruit).
Among the 198 plant species (18,285 cultivated fruits and 19,680 wild fruits) inventoried, 63 species were identified as hosts for fruit fly species (table 1). Nevertheless, there was a significant difference (Deviance = 413,766, Df = 62, P < 2.2 × 10−16) between the number of pupa recovered from the 63 host plant species. Abundance of host plant varied from four to 41 host plant species and from three to seven fruit fly species between agroecological regions (fig. 1, supplementary table S2).
The infestation index ranged from 0.06 (Artocarpus altilis (Parkinson) Fosberg (Moraceae)) to 538.46 (Solanum tuberosum Linnaeus (Solanaceae)) pupae/kg. Among the 5627 samples incubated, 1085 were positive for fruit fly emergence, in some samples up to 54.84% (Solanum muricatum Aiton (Solanaceae)) tested positive for fruit flies (table 1).
No adult fruit fly was obtained from pupae of some plant species such as A. squamosa, A. altilis and Capsicum frutescens Linnaeus (Solanaceae) (table 1).
Ten plant species (Xylopia Linnaeus sp (Annonaceae), Dracaena reflexa Lamarck (Asparagaceae), Citrullus Schrad sp (Cucurbitaceae), Cucumis Linnaeus sp (Cucurbitaceae), Cucumis Linnaeus sp1 (Cucurbitaceae), non-identified species (Meliaceae), Ficus polita Vahl (Moraceae), Podocarpus gaussenii Woltz (Podocarpacae), Rubus myrianthus Baker (Rosaceae), S. tuberosum) were identified as new hosts to fruit flies, only one of which (fruit of the S. tuberosum) is an exotic species (table 1).
A reduced number of pupae was observed from fruit grown for export such as L. chinensis: 0.75 pupae/kg, among which only three adults were emerged from 309 fruits incubated (table 1).
Diversity of fruit fly species
In total 12 fruit fly species was identified in our samples; those 12 fruit fly species were collected in different sites and host plants and in different proportions (fig. 1 and 2, table 1, supplementary table S1): Ceratitis argenteostriata De Meyer & Freidberg (ten adults), C. capitata (79 adults), C. cosyra (187 adults), C. malgassa (605 adults), C. pedestris Bezzi (85 adults), N. cyanescens (1341 adults), Trirhithrum crescentis Hancock (four adults), B. dorsalis (1001 adults), D. demmerezi (2362 adults), D. quilicii White (22 adults), D. vertebratus Bezzi (245 adults) and D. xanthaspis Munro (24 adults). Fifty-six (56) adults were not Tephritidae species.
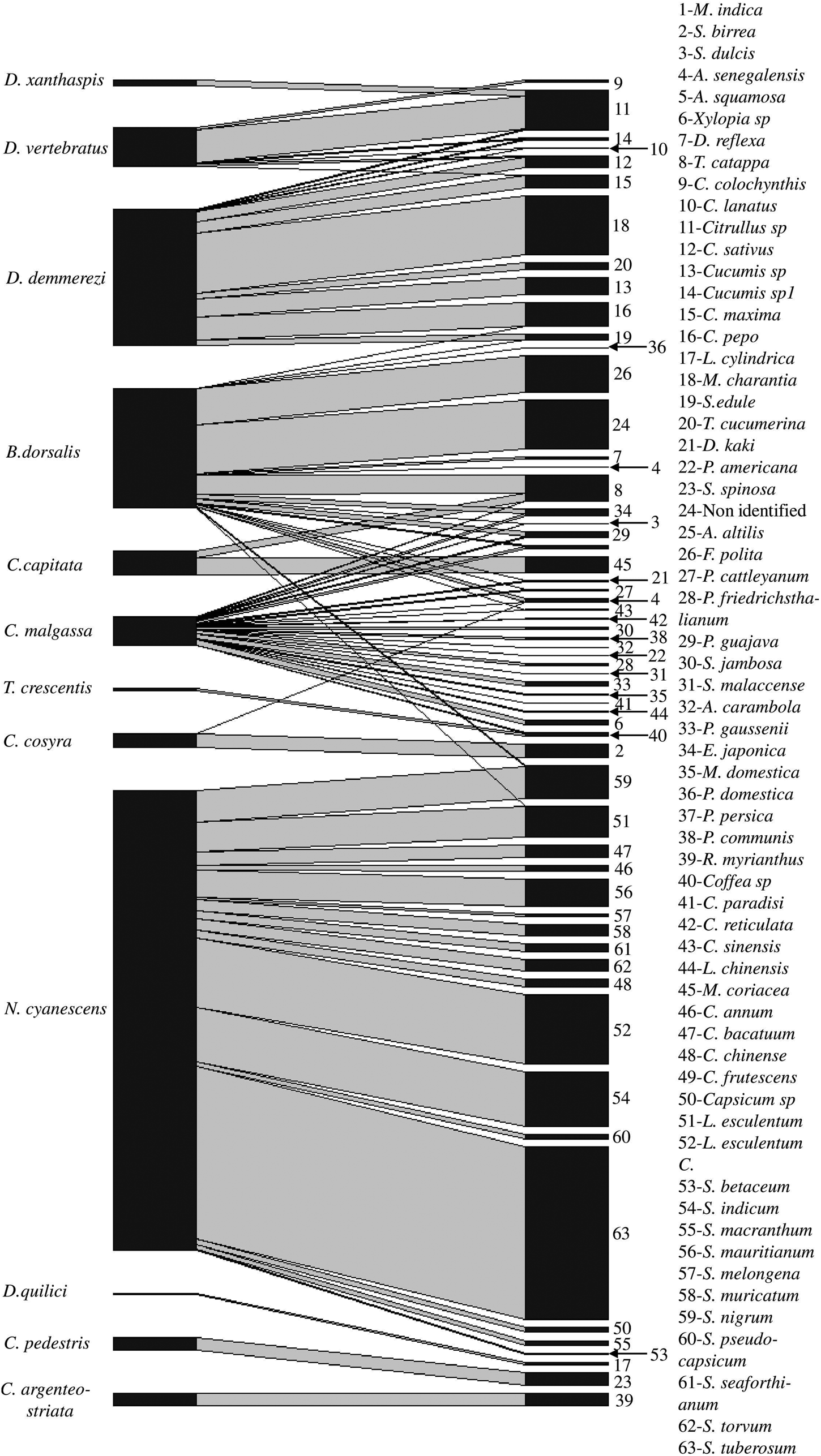
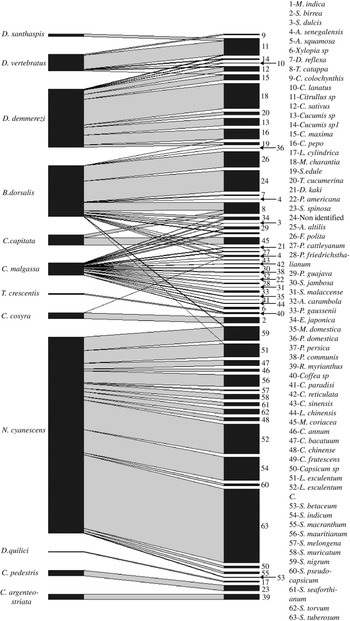
Figure 2. Network of interaction between fruit fly species and host plant species, line thickness represents the total number of adult of fruit flies/kg of fruits.
Ceratitis malgassa was retrieved from 12 families and 23 plant species (fig. 2), but none from vegetables. Bactrocera dorsalis was recorded from 18 species of 12 plant families (fig. 2), with few records (four species/two families) from vegetable species (one adult emerged from 183 fruits incubated of Cucurbita pepo Linnaeus (Cucurbitaceae), two from 245 fruits of Sechium edule (Jacquin) Swartz (Cucurbitaceae), 11 from 592 fruits of L. esculentum, two from 3401 fruits of Solanum nigrum Linnaeus (Solanceae)). Bactrocera dorsalis was also found in indigenous wild fruits: a non-identified plant species from Ankarafantsika Park; site B01 (86.96 adults/kg, 20% of positive fruits) and F. polita from urban area, site C23 and E34 (63.78 adults/kg, 6.67% of positive fruits).
Mango (M. indica, 22.01% of positive fruits) and tropical almond (Terminalia catappa Linnaeus (Combretaceae), 16.08% of positive fruits) were highly infested by B. dorsalis. Fruits belonging to the family of Myrtaceae such as Psidium friedrichsthalianum (Berg) Niedenzu (40% of positive fruits), the citrus Citrus paradisi Macfadyen (Rutaceae) (23.08% of positive fruits) and temperate fruits P. communis (22.22% of positive fruits) were infested by C. malgassa (table 1).
All species of Dacus sampled in this study were found in cucurbits species (table 1), such as, D. demmerezi found on species of Cucurbitaceae (ten species). Plant species of those families were very abundant on the island and cultivated throughout the year (supplementary table S2). Dacus vertebratus was found on six species of cucurbits and D. xanthaspis emerged only from a single species of wild cucurbits (Citrullus sp).
Neoceratitis cyanescens attacked 16 species, all belonging to the Solanaceae family (fig. 2, table 1).
The other species of Tephritidae were retrieved from a few host plants with short fruiting periods (1–5 months/year excepted T. catappa which fruiting throughout the year) in the year (table 1, supplementary table S2): C. argenteostriata found in R. myrianthus (Mar., June, Nov., Dec.), C. capitata only found in two plant species (Mimusops coriacea (A. de Candolle) Miquel (Sapotaceae) and T. catappa), C. cosyra only from the fruits of two Anacardiaceae species (M. indica and S. birrea), C. pedestris in S. spinosa (Feb.), T. crescentis in Coffea sp (Jan., Mars, Apr., July, Nov.), D. quilicii in Luffa cylindrica Roemer (Cucurbitaceae) (Feb., Apr., June), and D. xanthaspis in Citrullus sp (Mar. to June).
Geographic distribution of fruit flies
Infestation level varied between 169/191 infested sites (Deviance = 595,235, Df = 168, P = 2.2 × 10−16) and six provinces (Deviance = 55,838, Df = 5, P = 1.97 × 10−14) of sampling. The highest average of pupae/kg was retrieved from sites of the Antananarivo region (in average 66.48 ± 4.34 pupae/kg).
The overall sampling revealed that Tephritidae was spread across the six agroecological and climatic regions (fig. 1). High significant variations for pest infestation levels were observed among altitudinal gradients (Deviance = 49,780, Df = 2, P = 2.61 × 10−15) and agroecological regions (Deviance = 63,564, Df = 5, P < 2.2 × 10−16).
Five species (C. capitata, C. cosyra, C. pedestris, D. quilicii, and D. xanthaspis) were found only in coastal regions at altitudes below 500 m asl (figs 1 and 3). Ceratitis capitata was limited mainly on three sites (E18, E22, E38) from eastern warm and humid coast, the third site E38 was situated in Sainte-Marie Island. Ceratitis cosyra and D. quilicii were found below 500 m asl of altitude in two distinct climatic and agroecological regions; C. cosyra was distributed in R2 (site B14 of mango orchard) and R5 (site F02, F05 and F06) where climates are dry and sub-arid, and altitudes are ranking from 20 to 465 m asl; D. quilicii was found in sites R1 (A07 at 336 m asl) and R4 (E20 at 20 m asl) with dry and humid climates, respectively. Ceratitis pedestris was found in site (A08) from northern dry region at altitude 37 m asl and D. xanthaspis in the southern region in the sub-arid climate (site F02) at 465 m asl of altitude.
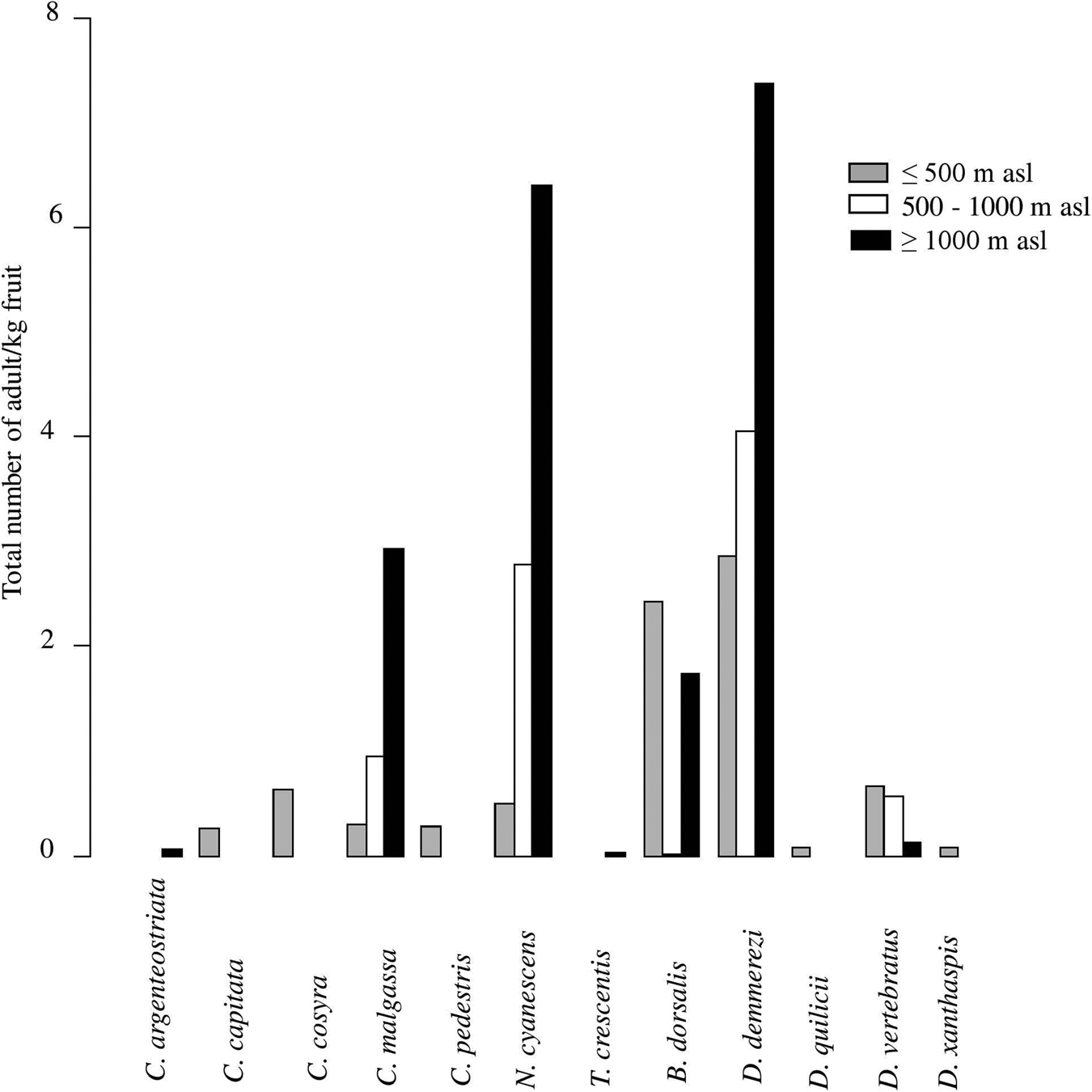
Figure 3. Infestation index (total number of adults/kg of fruits) of each of fruit fly species recorded in all host plant species (pooled data) in relation to the altitudinal gradient.
C. argenteostriata and T. crescentis were found only in Highland (Ambatolampy district) at high altitudes (1467 to 1556 m asl) with a tropical altitudinal climate (figs 1 and 3).
The other five species (C. malgassa, N. cyanescens, B. dorsalis, D. demmerezi, D. vertebratus) were widespread and infested fruits over an altitudinal range of 1–1634 m asl (figs 1 and 3).
Globally, C. malgassa and B. dorsalis were the two dominant species in fruit crops, their distributions were variable along the altitudinal gradients (Deviance = 9909.3, Df = 2, P = 1.13 × 10−14 and Deviance = 2777.1, Df = 2, P = 0.01, respectively). Ceratitis malgassa was found in 56 sites and B. dorsalis was found in 33 sites of the 191 monitored sites. Highest numbers of B. dorsalis were collected at low altitude (3.56% of C. malgassa and 29.61% of B. dorsalis at altitude ≤500 m asl). In contrast, C. malgassa appeared to be dominating at high altitude (15.01% of C. malgassa and 8.05% of B. dorsalis at altitude >500 m asl).
The species N. cyanescens had significatively higher infestation levels at high altitude (altitude ≥1000 m asl) than at lower altitude (altitude ≤500 m asl) (Deviance = 95,995, Df = 2, P < 2.2 × 10−16). Within southern regions (R5 and R6), no adult of N. cyanescens was obtained from 339 collected fruits of Solanaceae (fig. 1). Dacus demmerezi was found across the country in all regions without distinction (Deviance = 182.55, Df = 2, P = 0.84).
Dacus vertebratus occupied all regions (excepted R2) and all altitudinal gradients (from 13 to 1421 m asl) in 12 sites. The total number of adults collected from R5 (164/254 adults) was higher than in other regions (Deviance = 2250.4, Df = 2, P = 0.02).
Interaction between fruit fly species
Two or three fruit fly species were found emerging from the same fruit and this was the case of seven fruit fly species from 43 fruits of ten host plant species (fig. 4). The invasive species, B. dorsalis shared the same host plant fruit with other polyphagous species (figs 2 and 4): C. capitata (T. catappa, n = 2 fruits), C. cosyra (M. indica, n = 9 fruits) and C. malgassa (D. kaki, n = one fruit; P. guajava, n = 12 fruits; E. japonica, n = 6 fruits; P. persica, n = 2 fruits). In those fruits, the infestation indexes by B. dorsalis varied between 16.43 and 244.90 adults/kg, higher or equal to those of Ceratitis species (from 9.39 to 224.49 adults/kg).
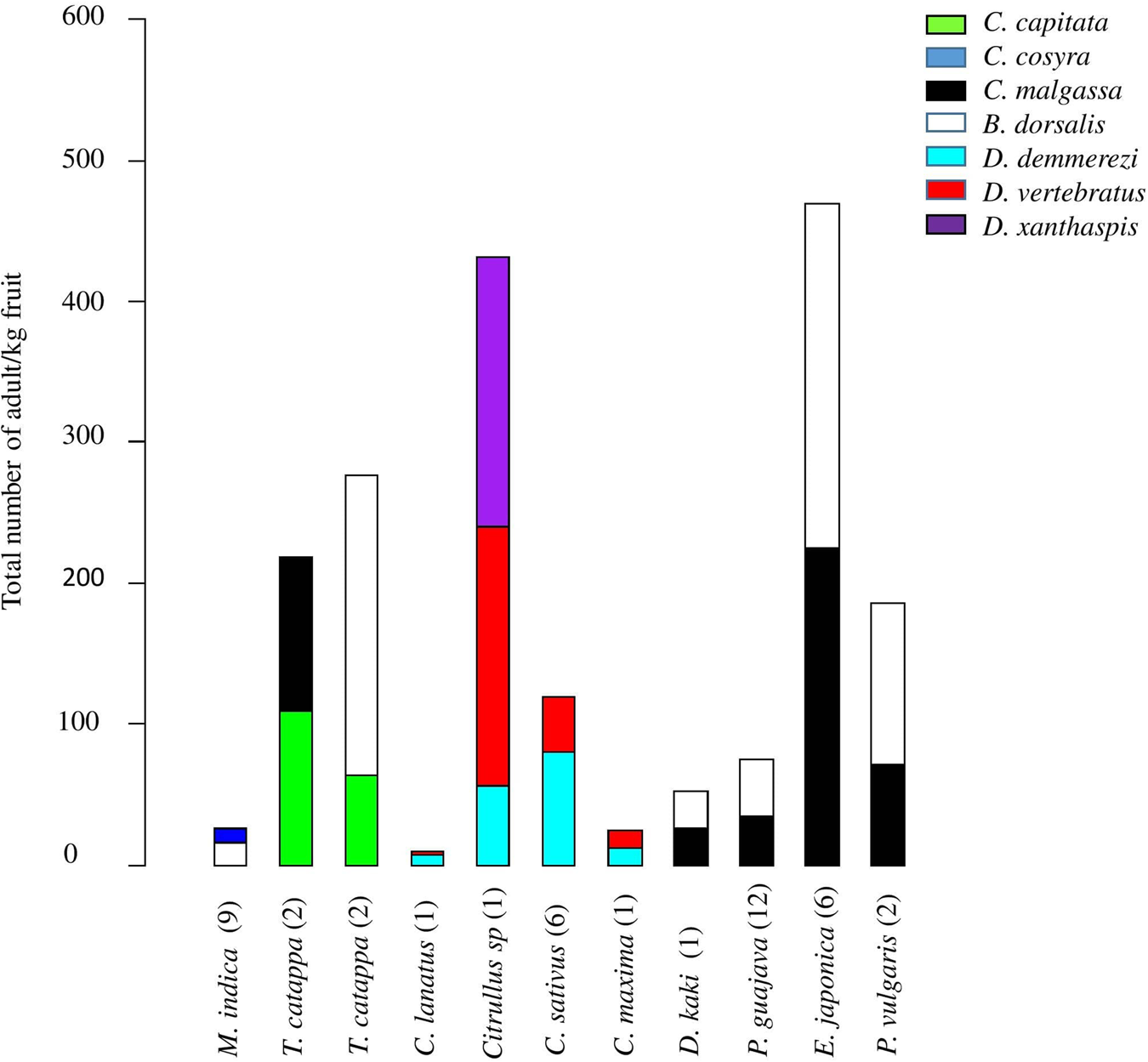
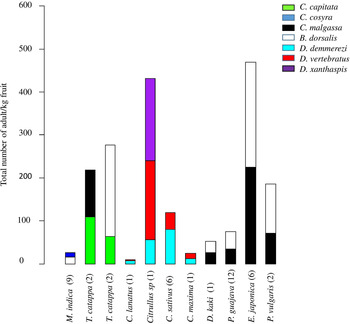
Figure 4. Total number of adults/kg of fruits for fruits of the ten plant species co-infested by two or three fruit fly species. Plants species of fruit and vegetable crops are ordered by family and species name. Numbers of positive fruits per species are presented in brackets.
A co-infestation of C. capitata and C. malgassa was also recorded from two samples of fruits of T. catappa with similar infestation indexes, 109.38 adults/kg.
Dacus demmerezi was found in co-infestation with D. vertebratus in four species of Cucurbitaceae (n = 10 fruits) and with D. xanthaspis in one species (n = one fruit). Generally, D. demmerezi was found dominant in cultivated fruits (Cucumis sativus Linnaeus (Cucurbitaceae), 38.65% positive fruits) and D. vertebratus in wild species (Citrullus sp, 54.17% positive fruits) (table 1).
Discussion
Diversity of frugivorous Tephritidae species in Madagascar
To our knowledge, this is the first study recording host range of Tephritidae of economic importance throughout all agroecological zones of Madagascar. This extensive survey made on 37,955 fruits was able to document 12 fruit fly species and their hosts, seven of which are endemic to Madagascar (C. argenteostriata, C. malgassa, N. cyanescens, T. crescentis, D. demmerezi, D. quilicii and D. xanthaspis) and the five remaining being non-indigenous (C. capitata, C. cosyra, C. pedestris, B. dorsalis and D. vertebratus). According to De Meyer et al. (Reference De Meyer, Quilici, Franck, Chadhouliati, Issimaila, Youssoufa, Abdoul-Karime, Barbet, Attié and White2012), the family of frugivorous Tephritidae comprised 32 species in Madagascar with 23 endemic species. The 20 species remaining were not retrieved during our survey as their host plants might not have been sampled, or because they might be rare or highly localized.
A comparison with the results from Afrotropical region suggests that the actual fruit fly diversity may be even lower. Fruit grown for export (T. cacao, Coffea sp, L. chinensis, Capsicum sp) were of minor importance as hosts with low infestation rate, with only one species per host plant observed. However, according to White and Elson-Harris (Reference White and Elson-Harris1992), Sub-Saharan Africa is a reservoir of 915 fruit fly species from 148 genera, nearly, 299 of these species are considered as pests by feeding on fruits of economic importance. Theobroma cacao and Coffea arabica Linnaeus (Rubiaceae) host three species of Ceratitis in Kenya with considerable damage (Copeland et al., Reference Copeland, Wharton, Luke, De Meyer, Lux, Zenz, Machera and Okumu2006). Three fruit fly species: C. capitata, C. cosyra and C. rosa Karsch are reported to attack L. chinensis in South Africa (Grové et al., Reference Grové, Steyn and De Beer2002); and in La Réunion, B. dorsalis and C. quilicii De Meyer, Mwatawala & Virgilio were also recorded as a pest on this plant (Moquet et al., Reference Moquet, Payet, Glenac and Delatte2021). Commercial species of pepper and chilies are known to host C. cosyra and B. dorsalis in west and central African countries (Goergen et al., Reference Goergen, Vayssières, Gnanvossou and Tindo2011; Badii et al., Reference Badii, Billah, Afreh-Nuamah and Obeng-Ofori2015); C. capitata, N. cyanescens and B. dorsalis in some of the islands of the Indian Ocean (Franck and Delatte, Reference Franck and Delatte2020).
Host plant use
Among the sampled fruits of the 198 plant species, 63 (31.66%) plant species from 23 plant families were positive to fruit fly, ten of which are indigenous species of the island and nine species are new fruit fly hosts compared with existing records in the Indian Ocean islands and in the world. Within the 99 collected plant species previously recognized as potential hosts of Tephritidae (supplementary table S2), 40 of them were found free of Tephritidae despite repeated sampling. Nevertheless, for some of them, the low number of fruits sampled (no. <10) does not allow any statement on their suitability as hosts in our sampling conditions.
Status of monophagous/polyphagous
Among the 12 species of Tephritidae found in this study, five species were widely found with high infestation indexes and can be considered as major pests in Madagascar: C. malgassa, N. cyanescens, B. dorsalis, D. demmerezi, and D. vertebratus.
Because of their wide range of host plants and occurrence on the island, C. malgassa (12 families/23 host species), and B. dorsalis (12 families/18 host species) can be considered the most ‘polyphagous’ species present in Madagascar. Ceratitis malgassa is native from Madagascar, it was recorded, but never found in the other Indian Ocean islands (Comoros, Mayotte and Mauritius) (Franck and Delatte, Reference Franck and Delatte2020), it could be particularly well adapted to the ecosystems of the island.
Fruit species belonging to major economic crops such as mango (M. indica), mostly occurring in low altitudes showed the highest infestation by B. dorsalis. Furthermore, temperate fruits, such as apple (M. domestica), peach (P. persica) and pear (P. communis) had very low infestation levels by B. dorsalis. Bactrocera dorsalis is known as a pest with the broadest host range of any known species of Bactrocera genus, Mwatawala et al. (Reference Mwatawala, De Meyer, Makundi and Maerere2006a) have shown that B. dorsalis only occurs in low numbers and only during a short seasonal period in the high-altitude areas where temperate fruit species are grown.
Not only exotic crops were found infested by B. dorsalis but also a few indigenous species with high rates of infestations showing its capacity to adapt to novel environments and hosts.
Bactrocera dorsalis was also found on cucurbit crops but in low number, confirming that the host status for certain members of the Cucurbitaceae is uncharacteristic of this fruit fly species as observed in Tanzania (Mwatawala et al., Reference Mwatawala, De Meyer, Makundi and Maerere2009). Furthermore, a few adults of B. dorsalis emerged from Solanaceae species, which is not corroborated in a study performed in Ghana, where bell pepper (Capsicum annum Linnaeus (Solanaceae)) and tomato (L. esculentum) were highly preferred by B. dorsalis (Badii et al., Reference Badii, Billah, Afreh-Nuamah and Obeng-Ofori2015). Despite its polyphagous trait, B. dorsalis preferred fruit crops other than Cucurbitaceae and Solanaceae in Madagascar, and this could be linked to interactions with other fruit fly species present on those crops.
Three fruit fly species could be classified as ‘oligophagous’, N. cyanescens associated with Solanaceae crops, D. demmerezi and D. vertebratus with Cucurbitaceae crops. Neoceratitis cyanescens was found only in Solanaceae species. This fruit fly species invaded also the islands surrounding Madagascar: Mauritius, Comoros, Mayotte and la Réunion, where N. cyanescens had been described attacking also only species of Solanaceae (Franck and Delatte, Reference Franck and Delatte2020).
Dacus demmerezi, is endemic to Madagascar, it was only found in cucurbits in Reunion Island and Mauritius (Franck and Delatte, Reference Franck and Delatte2020; Moquet et al., Reference Moquet, Payet, Glenac and Delatte2021).
Dacus vertebratus was also only found in cucurbits in our study. Following the wide distribution of D. vertebratus (12 sites spread in five agroecological regions) and its large host use of cucurbit hosts (50% of the cucurbits species sampled in this study), this species could be considered as one of the major pest species on cucurbit on the island. Dacus vertebratus is also known as an important pest of cucurbits in Africa where it is the dominant fruit fly species attacking water melon and cucumber varieties (Badii et al., Reference Badii, Billah, Afreh-Nuamah and Obeng-Ofori2015).
Four endemic species (C. argenteostriata, T. crescentis, D. quilicii and D. xanthaspis) occurred in relatively low abundances in terms of infestation of fruits (below around 10 adults/kg, excepted for C. argenteostriata found in R. myrianthus). They also have very narrow host range, with only one host plant species found per fruit fly species, for example, C. argenteostriata was found only once on an endemic and wild species R. myrianthus, which fruits are mostly eaten by lemurs or birds. For those reasons, these species could be considered as species with lesser economic importance, and are probably ‘monophagous’ species.
Distribution of fruit fly species
Five species (C. malgassa, N. cyanescens, B. dorsalis, D. demmerezi, and D. vertebratus) were found in all agroecological regions and able of infesting fruits over an altitudinal range of 1 to 1634 m asl. The highest infestations levels were found in Antananarivo, the capital of the island, which might be partly due to human transportation of infested fruits. The capital is very populated, receiving fruits and vegetables from all over the regions. A similar situation is often found for frugivorous pests being moved along with human activities (Putulan et al., Reference Putulan, Sar, Drew, Raghu and Clarke2004).
The polyphagous invasive fruit fly B. dorsalis was found in the highest number of sites of collected fruit species (33 sites) and has a wide host range (18 host plant species).
Both C. malgassa and B. dorsalis showed a similar regional distribution, but highest numbers of B. dorsalis were collected at low altitude (<500 m asl), while C. malgassa appeared to be dominating at higher altitudes (>1000 m asl). Similarly, Vargas et al. (Reference Vargas, Nishida and Beardsley1983) demonstrated that fruit infestation by B. dorsalis in native and exotic forests on Kauai Island was moderate at middle (579–800 m asl) altitude and low at high altitude (>800 m asl). A similar pattern was found in La Réunion with B. dorsalis, dominating all other species, but being less abundant at higher altitudes (Moquet et al., Reference Moquet, Payet, Glenac and Delatte2021).
The majority of minor occurrence fruit fly species (C. capitata, C. cosyra, C. pedestris, D. quilicii, and D. xanthaspis) were found in the coast region. Geurts et al. (Reference Geurts, Mwatawala, De Meyer and Heckel2012) observed a spatial increase in diversity and population density of Tephritidae species at lower altitude below (581 m asl) in the Morogoro mountains of Tanzania. Altitude by itself does not determine fruit fly distribution but associated with other factors such as weather and host plants availability play an important role (Mwatawala et al., Reference Mwatawala, De Meyer, Makundi and Maerere2006b; Geurts et al., Reference Geurts, Mwatawala, De Meyer and Heckel2012). Indeed, the diversity of Tephritidae was very poor in the dry southern region (only three species and five host plants recorded) with arid tropical climate and thickets of spiny plants. This very dry weather not allowing constant availability of potential host fruits may have been largely unfavorable for the establishment of a large diversity of fruit fly species.
Ceratitis argenteostriata and T. crescentis were collected above 1000 m asl, where the temperature can fall −1 °C at night during the austral winter. Both of these species are endemic of the island and might have developed specific adaptations to this kind of weather. This is the first record of the potential hosts of these two species, indeed no record from host existed for C. argenteostriata, it had only been described from trimedlure trapped specimens in Japanese persimmon (D. kaki) ochard from the Highland area (De Meyer and White, Reference De Meyer and White2016).
Trirhithrum is an afrotropical genus. Six species are now known from the Malagasy subregion, five of which are restricted to Madagascar (Hancock, Reference Hancock1984). Most of the species of Trirhithrum genus are monophagous, which is most probably the case for T. crescentis. Indeed, the availability of its main hosts Coffea sp was the most important factor contributing to the presence of T. crescentis in a given area in Madagascar.
The Strychnos fruit fly C. pedestris, was rare in our study on the country, it is widespread in the southern half of Africa, its country of distribution includes Angola, Zambia, Zimbabwe, South Africa and Madagascar and its hosts are various species of the genus Strychnos Linnaeus (Loganiaceae) (Hancock, Reference Hancock1984).
Ceratitis capitata, in our case, was only found on two plant species in low numbers from three sites restricted in the eastern region of Madagascar. In many countries this species is often considered as highly polyphagous with almost 400 host plants known worldwide (Liquido et al., Reference Liquido, Barr and Cunningham1998, Reference Liquido, McQuate and Suiter2015; Copeland et al., Reference Copeland, Wharton, Luke and De Meyer2002; Moquet et al., Reference Moquet, Payet, Glenac and Delatte2021). This limited distribution range could be due to environmental factors unfavorable to the establishment of this species (Vera et al., Reference Vera, Rodriguez, Segura, Cladera and Sutherst2002) and/or to a recent phenomenon of displacement following the invasion of B. dorsalis on the island. Unfortunately, we do not have any detailed records of its abundance before the invasion of B. dorsalis.
Ceratitis cosyra was found in a few areas at low altitude. However, studies done in Tanzania by Mwatawala et al. (Reference Mwatawala, De Meyer, Makundi and Maerere2006b) have shown that C. cosyra was the most abundant species at 781 m asl and 1105 m asl and has also been reported from Kenya at 700 m asl (Rwomushana et al., Reference Rwomushana, Ekesi, Gordon and Ogol2008), in equivalent latitudinal ranges. It was demonstrated that the distribution of C. cosyra, however, appears to be more related to host plants than climate, following a similar pattern of distribution of their host plant (De Villiers et al., Reference De Villiers, Manrakhan, Addison and Hattingh2013). Mango (M. indica) are known to be important hosts for C. cosyra. When mango is not available, C. cosyra shifts to alternative host plants including wild fruits such as marula (S. birrea) (Copeland et al., Reference Copeland, Wharton, Luke, De Meyer, Lux, Zenz, Machera and Okumu2006).
Co-occurrence between species of fruit flies in sampled fruit
In fruits of which C. malgassa and B. dorsalis species coexisted, the average number of B. dorsalis adults/kg of fruit was higher than that of C. malgassa. Those results might indicate the existence of potential interactions between both species. Nevertheless, further experiments in controlled conditions are needed.
In the coastal regions, B. dorsalis shared the same host fruit with other pre-established species such as C. capitata and C. cosyra. Nevertheless, high populations of both C. capitata and C. cosyra had never been reported, even in the first records made by Dubois (Reference Dubois1965), where he mentioned the absence of heavy damage attributed to both species on fruits, but with C. malgassa well developed in coast areas. The arrival of B. dorsalis might have negatively impacted both species that were already in low abundance.
Conclusion
Madagascar has a wide diversity of plant species that can support indigenous and exotic tephritid fruit fly species. More extensive sampling, targeting specific regions should be considered in order to complete host plant range of Tephritidae on the island, especially for the low abundance species. Five Tephritidae fruit fly species were found in all agroecological regions of Madagascar and considered as major pests of many horticultural crops among which B. dorsalis was the most abundant.
The majority of farmers do not apply any control against fruit flies despite the high presence of B. dorsalis in fruits (Rasolofoarivao, pers. comm.). Furthermore, little information is available on the natural enemies attacking Tephritidae pests in Madagascar. Historically, many hymenopteran parasitoids (Dirhinus gifardii Silvestri (Chalcididae), Fopius arisanus Sonan (Braconidae), Opius concolor Szépligeti (Braconidae), Opius longicaudatus Ashmead (Braconidae), Opius oophilus Fullaway (Braconidae)) were introduced into Madagascar from Hawaii for control of C. malgassa (Dubois, Reference Dubois1965; Fischer and Madl, Reference Fischer and Madl2008) but no updated studies to evaluate their installation or/and impacts on fruit fly populations have been made since their introduction. The future perspective of this study will be to identify native or exotic parasitoid species in order to provide a complete set of potential strategies for future biological control programs.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0007485321000511
Acknowledgements
The authors are grateful to the master II students (Herinandrasana P. and Rasolofondrainibe T.) from the University of Antananarivo, Département d'Entomologie and Mze Hassani I. from Institut National de Recherche pour l'Agriculture, la Pêche et l'Environnement (INRAPE) Comoros who assisted in the field and laboratory work. We would like to acknowledge the DPV for lending a laboratory to carry out fruit fly rearing. We thank De Meyer M. and Virgilio M. from RMCA, Belgium who gave a valuable support about fruit fly identification. This work was funded by Union Européenne (FEDER INTERREG V), Conseil Régional de la Réunion and CIRAD, under EPIBIO-OI ‘EPIdémiosurveillance et BIOcontrole dans le Sud-Ouest de l'Océan Indien’ project.