Article contents
Long-term suitability of an alternative host for rearing the sugarcane stalk borer parasitoid Tetrastichus howardi
Published online by Cambridge University Press: 17 April 2024
Abstract
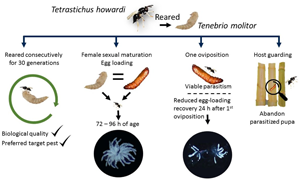
The continuous utilisation of an alternative host may influence parasitoid performance across successive generations due to conditioning in natal hosts. Tetrastichus howardi (Olliff) has successfully been reared using Tenebrio molitor L. pupae as a feasible alternative host. However, the extended rearing of T. howardi on this alternative host may impact the biological features of the parasitoids. Parasitoids were reared using T. molitor pupae for 30 consecutive generations. Quality criteria were assessed during the generations F5, F15, and F30, offering pupae of the target pest, Diatraea saccharalis (Fabr.), and compared with the F0 generation (parasitoids reared in D. saccharalis pupae). Criteria included assessments of parasitism performance, host selection, and wing form variation in the parasitoid wasps. Additionally, we examined the fecundity of T. howardi females that emerged from both hosts, considering their age, egg loading before and after one oviposition, as well as parasitism of sugarcane stalk borer pupae. Rearing T. howardi using pupae of T. molitor did not affect its biological traits or preference for the target pest for 30 generations. After parasitism, the parasitoid left the host pupa inside the stalk, and one oviposition was enough to kill D. saccharalis pupae and obtain viable parasitoid progeny. Female sexual maturation and egg loading occurred 72 and 96 h after parasitoid emergence. Egg-loading recovery after parasitism did not happen within 24 h. T. howardi can be reared for up to 30 generations using alternative hosts without compromising its parasitism performance or egg loading.
Keywords
Information
- Type
- Research Paper
- Information
- Copyright
- Copyright © Universidade Federal Rural de Pernambuco, 2024. Published by Cambridge University Press
References
- 1
- Cited by


