Introduction
Human activity has drastically altered the composition of the beetle fauna of North America through the establishment of nonnative (adventive) species. Adventive beetles have been detected in North America as early as 1620 (Prévost and Bain Reference Prévost, Bain, Bain, Chabot and Mousette2007), migrating with shipping traffic during European colonisation, and they continue to become established via the international trade of various organic products (reviewed in Klimaszewski et al. Reference Klimaszewski, Langor, Majka, Bouchard, Bousquet and LeSage2010). Rove beetles (Coleoptera: Staphylinidae) represent the greatest number of accidental beetle introductions, at least in Canada. As of 2019, 154 species had become adventive in Canada, about 8.6% of the roughly 1800 beetle species recorded at the time (Brunke et al. Reference Brunke, Bouchard, Douglas and Pentinsaari2019, incorporating the former Silphidae; see Sikes et al. Reference Sikes, Thayer and Newton2024).
Unlike plant-feeding groups, potential impacts of adventive rove beetles, mostly predators and recyclers of decaying organic matter, have received relatively little scientific attention. Nevertheless, the potential impact of these accidental introductions is particularly evident in the fragmented forests and wetlands of rural and urban northeastern North America, where adventive staphylinids tend to predominate over Nearctic ones. In a recent five-year study (2006–2010) of ground-dwelling beetles in an old field and forest fragment in southern Quebec (Scotstown), Canada, about 55% of all rove beetle specimens belonged to adventive species (Levesque and Levesque Reference Levesque and Levesque2024). These numbers may be even higher in areas with intense development, such as in southwestern Ontario and along the St. Lawrence River in Quebec. Klimaszewski and Brunke (Reference Klimaszewski, Brunke, Betz, Irmler and Klimaszewski2018) highlighted inadequate taxonomic knowledge and incomplete sequence reference libraries as two major impediments to the timely detection of new introductions and the tracking of established species as they expand their distributions in North America.
Recently, we have encountered specimens of Heterothops Stephens, Philonthus Stephens, and Quedius Stephens (Coleoptera: Staphylinidae: Staphylininae) collected in eastern Canada that could not be identified to species (Fig. 1). This was unexpected because all genera of Staphylininae in this region have modern revisions and taxonomic supplements available, including identification keys (e.g., Smetana Reference Smetana1971a, Reference Smetana1982, Reference Smetana1995; Frank Reference Frank1975, Reference Frank1981; Brunke et al. Reference Brunke, Newton, Klimaszewski, Majka and Marshall2011). These unknown taxa were recognised as such by ourselves or colleagues as early as 2005, 2009, and 2010 but have remained a mystery until now. In the process of re-examining Nearctic Heterothops specimens and associated barcode sequences, it was discovered that Palaearctic H. praevius Erichson has been adventive in eastern North America (Ontario, Canada) for some time and had been described subsequently as H. marmotae Smetana syn. nov. from the same region. Two Nearctic Heterothops were also newly discovered in eastern North America and are probably northern transcontinental species. Here, we newly report four rove beetle species as adventive and established in North America, and we provide illustrations of their diagnostic features, new or updated keys, barcode sequence data, and distribution maps. With the present study, 14 species of Heterothops, 114 Philonthus, and 92 Quedius species are now known to occur in North America.
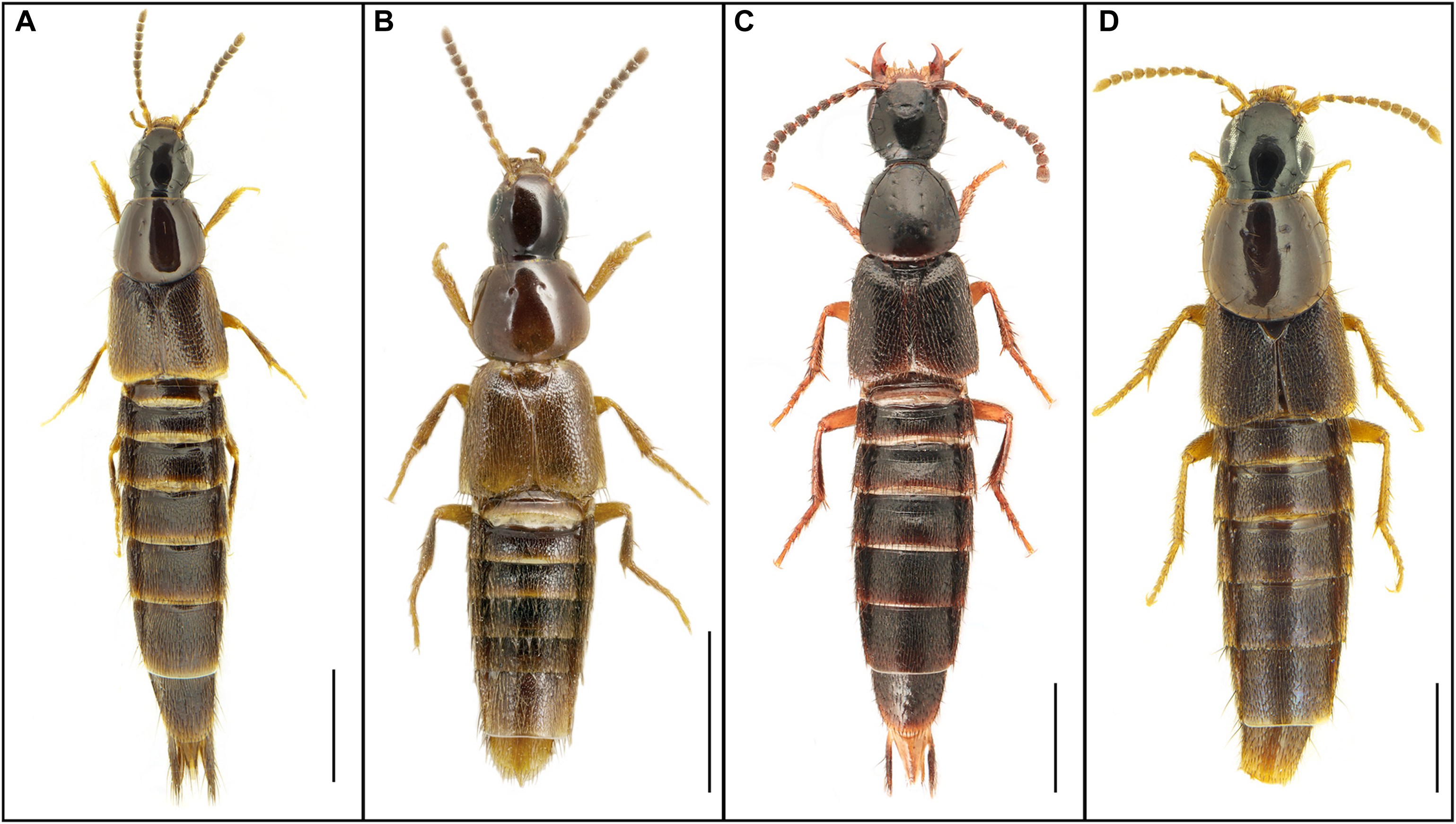
Figure 1. Habitus of each of A, Heterothops cognatus Sharp; B, H. praevius Erichson; C, Philonthus chujoi Dvořák; and D, Quedius maurorufus (Gravenhorst). Scale bars = 1 mm.
Methods
Depositories
BIO – Biodiversity Institute of Ontario, University of Guelph, Guelph, Ontario, Canada (M. Pentinsaari, A. Brown)
cCC – personal collection of Claude Chantal, Varennes, Quebec, Canada
cLC – personal collection of Ludovic Leclerc, Gatineau, Quebec, Canada
cNB – personal collection of Nicolas Bédard, City of Québec, Quebec, Canada
cPB – personal collection of Pierrick Bloin, City of Québec, Quebec, Canada
CNC – Canadian National Collection of Insects, Arachnids and Nematodes, Ottawa, Ontario, Canada
DEBU – University of Guelph Insect Collection, University of Guelph, Guelph, Ontario, Canada (S. Paiero)
LFC – Laurentian Forestry Centre, Canadian Forest Service, Natural Resources Canada, City of Québec, Quebec, Canada
NFC – Northern Forestry Centre, Canadian Forest Service, Natural Resources Canada, Edmonton, Alberta, Canada (G. Pohl)
Specimen examination
Non-type label data were standardised to improve clarity. Specimens were georeferenced using Google Earth or Google Maps, and GPS coordinates were included in the materials examined as verbatim. Specimen localities were mapped using SimpleMappr (Shorthouse Reference Shorthouse2010). Specimens were examined dry using a Leica M80 stereomicroscope (Leica, Wetzlar, Germany). Genitalia and terminal segments of the abdomen were dissected and placed in glycerine-filled vials, pinned with their respective specimens. Several specimens were dissected previous to this study, and genitalia were sometimes mounted in Canada Balsam or Euparal. Line drawings were made from images and then digitally inked in Adobe Illustrator, Creative Cloud 2024 (Adobe Canada, Ottawa, Ontario, Canada). All imaging, including photomontage, was accomplished using a motorised Nikon SMZ25 microscope (Nikon Canada, Mississauga, Ontario, Canada) and NIS Elements Basic Research, version 4.5 (Nikon; https://www.microscope.healthcare.nikon.com/products/software/nis-elements/nis-elements-basic-research). Photos were postprocessed in Adobe Photoshop, Creative Cloud 2024 (Adobe Canada).
Molecular data
Extraction, amplification, and sequencing of the barcoding fragment of cytochrome c oxidase subunit 1 (CO1) mitochondrial DNA were performed by the Canadian Centre for DNA Barcoding, Biodiversity Institute of Ontario (BIO; University of Guelph, Guelph, Ontario, Canada). The DNA was extracted from both dried museum specimens and specimens preserved in 95% alcohol. Sequences were uploaded to the Barcode of Life Data System, version 4 (BOLD; https://v4.boldsystems.org/index.php), and those sequences deemed to be barcode compliant by BOLD were assigned BINs (barcode index numbers; Ratnasingham and Hebert Reference Ratnasingham and Hebert2013) and were considered as tentative species hypotheses. Using the Taxon-ID tree tool in the workbench of BOLD, barcodes and their associated BINs were visualised in a neighbour-joining tree using the BOLD aligner and Kimura 2-parameter (K2P) distances. The DNA barcode sequences studied here, including both previously unpublished data and the sequences published in earlier studies, have been compiled into a publicly available dataset on BOLD (DS-ADSTAPH1, https://dx.doi.org/10.5883/DS-ADSTAPH1), along with collecting data, images of the specimens (if available), and other metadata related to the specimens and sequences.
Results
Staphylininae Latreille, 1802
Amblyopinina Seevers, 1944
Amblyopinina can be recognised among the Nearctic Staphylininae by a combination of the following characters: antennal bases closer to eyes than each other; superior and inferior marginal lines of the pronotum separated throughout their length; apical maxillary palpomere aciculate; and dorsal rows of pronotum located just lateral of midline. Only Heterothops occurs in North America.
Heterothops Stephens, 1829
Recently, some Heterothops species (including the Nearctic Occidentis and Pusio Groups of Smetana Reference Smetana1971a) were moved to a new genus (Chiquiticus Reyes-Hernández and Solodovnikov), based on the results of comprehensive phylogenomic analyses that showed these to be only superficially similar to true Heterothops (Reyes-Hernández and Solodovnikov Reference Reyes-Hernández and Solodovnikov2024). Chiquiticus is currently a member of the newly erected Ctenandropina (Reyes-Hernández et al. Reference Reyes-Hernández, Brunke, Hansen, Qinghao, Jenkins Shaw, Newton and Solodovnikov2025).
Heterothops cognatus Sharp, 1874

Figure 2. A, Habitus of Chiquiticus pusio (LeConte). Dorsal heads or forebodies of the following Heterothops species: B, H. cognatus Sharp; C, H. minor Smetana, indicating puncture of dorsal row; D, H. praevius Erichson; E, H. conformis Smetana, indicating second antennomere; and F, H. sordidus Smetana, indicating second antennomere. Basal abdominal tergites, indicating presence or absence of posterior transverse basal ridge, on the following Heterothops species: G, H. fumigatus LeConte; and H, H. sordidus. Asterisks indicate parocular punctures; afp, anterior frontal puncture; pfp, posterior frontal puncture. Scale bars = 1 mm (A); 0.2 mm (B–H).

Figure 3. Male genitalia of Heterothops cognatus Sharp and H. praevius Erichson: A, H. cognatus, non-type male, internal sac in situ, and B, internal sac everted; and C, H. praevius, non-type male from Slovakia, internal sac in situ, and D, H. marmotae Smetana syn. nov. (= H. praevius) holotype male, internal sac partly everted. Lowercase letters refer to internal sac sclerites following the scheme of Israelson (Reference Israelson1979). Scale bars = 0.1 mm.
BIN BOLD: AAU7313
Heterothops cognatus Sharp Reference Sharp1874: 20; Nakane et al. Reference Nakane, Ohbayashi, Nomura and Kurosawa1963 (habitus photo, distribution in Japan); Li Reference Li, Li and Chen1993 (distribution in China); Cho Reference Cho1996 (diagnosis, aedeagus illustration, distribution in South Korea); Shibata et al. Reference Shibata, Maruyama, Hoshina, Kishimoto, Naomi and Nomura2013 (distribution in Japan); Cho Reference Cho2019 (habitus illustration, additional records)
Diagnosis. In eastern North America, H. cognatus can be identified by the following combination of characters: eyes large, distinctly longer than the temple; first antennomere paler than other antennomeres, sometimes darkened dorsally (Fig. 2B). The internal sac of this species is distinctive for the heavily sclerotised bases of the short pair of sclerites (sclerite “c” of Israelson Reference Israelson1979; Fig. 2B), which appear as two small arcuate markings in situ (Fig. 2A).
Type material. The type material of H. cognatus was not examined for this study, but one male was dissected from material identified by David Sharp and collected by G. Lewis in Japan before accession in 1910 (exchange between the Natural History Museum, London, United Kingdom, and the CNC). Additional non-type males from Japan were dissected, and their aedeagi correspond exactly with the species now established and widespread in eastern North America.
Non-type material. JAPAN: no locality, G. Lewis, 1910–320. (2, CNC); Hokkaido: Oshamanbe, Toyotsu, seashore, 27.vii.1991, A. Smetana (1, CNC); Honshu: Ibariki Pref.: Tsukuba, pan and flight traps, 6–26.v.1989, M. Sharkey (1, CNC); “Yatabeho,” 12.vii.1980, A. and Z. Smetana (1, CNC); Kyoto Pref.: “Seryo-loge,” 500–700 m, 6.viii.1980, A. and Z. Smetana (1, CNC); Nagano Pref.: Shibu Onsen, 800 m, 23.vii.1980, A. and Z. Smetana (4, CNC); Komoro, 800 m, 19.vii.1980, A. and Z. Smetana (1, CNC); Shimane Pref.: Matsuda, 12.iv.1957, Y. Higo (1, CNC); Shikoku: Ehime Pref.: Hiromi [= Kihoku], 20.vi.1957, K. Kagami (1, CNC).
CANADA: Ontario: Brant County: Brantford Water Works Park, 43.14 N, 80.3 W, 3.vii.2013, 2015PHS IFT-039060 (1, CNC); Chatham–Kent County: Rondeau Provincial Park, near entrance, temporary pool with oak and poplar, dead leaves, 30.v.1985, L. LeSage (1, CNC); Essex County: Harrow, Harrow Research and Development Centre, 42° 01′ 34.2″ N, 82° 5′ 59.9″ W, Malaise trap in hedgerow, 2–5.vi.2021, L. Des Marteaux (1, CNC); same except 5–8.vi.2021 (1, CNC); same except Malaise trap in forest, 8–11.vi.2021 (2, CNC); Niagara Region: Welland, St. George Park, 42.968, –79.274, 16.vii.2014, IAS forest pest trapping (2, CNC); Norfolk County: Verhoeve Site, 42° 52′ 30.5″ N, 80° 29′ 52.0″ W, Malaise trap in forest, 8–11.vi.2021, L. Des Marteaux (1, CNC); same except 2–5.vi.2021 (1, CNC); same except Malaise in hedgerow, 8–11.vi.2021 (2, CNC); Hodgsen site, 42° 49′ 43.3″ N, 80° 38′ 49.0″ W, Malaise in hedgerow, 5–8.vi.2021, L. Des Marteaux (1, CNC); same except 2–5.vi.2021 (2, CNC); Lammens Site, 42° 43′ 45.7″ N, 80° 42′ 10.9″ W, Malaise trap at field margin, 30.v–2.vi.2021, L. Des Marteaux (2, CNC); same except 17–20.vi.2021 (1, CNC); same except 5–8.vi.2021 (1, CNC); same except Malaise in hedgerow, 20–23.vi.2021 (1, CNC); same except Malaise in hedgerow, 30.v–2.vi.2021 (6, CNC); same except Malaise trap in forest, 30.v–2.vi.2021 (2, CNC); Timpf site, 42° 39′ 01.0″ N, 80° 33′ 25.0″ W, Malaise trap in hedgerow, 2–5.vi.2021, L. Des Marteaux (1, CNC); same except Malaise trap in forest, 30.v–2.vi.2021 (1, CNC); same except Malaise trap at field margin, 5–8.vi.2021 (1, CNC); same except Malaise trap at field margin, 2–5.vi.2021 (1, CNC); Boothby site, 42° 39′ 57.5″ N, 80° 41′ 58.6″ W, Malaise trap in forest, 30.v–2.vi.2021, L. Des Marteaux (2, CNC); same except Malaise trap at field margin, 5–8.vi.2021 (1, CNC); Chanyi site, 42° 34′ 55.7″ N, 80° 34′ 34.1″ W, Malaise trap at field margin, 2–5.vi.2021, L. Des Marteaux (1, CNC); Csoffi site, 42° 52′ 18.1″ N, 80° 32′ 37.8″ W, Malaise trap in forest, 5–8.vi.2021, L. Des Marteaux (2, CNC); same except 2–5.vi.2021 (1, CNC); LLON 6 site, 42° 46′ 57.1″ N, 80° 37′ 01.3″ W, Malaise trap in hedgerow, 2–5.vi.2021, L. Des Marteaux (1, CNC); same except Malaise trap in forest, 2–5.vi.2021 (1, CNC); Bonnieheath site, 42° 52′ 55.0″ N, 80° 15′ 28.5″ W, Malaise trap in hedgerow, 5–8.vi.2021, L. Des Marteaux (1, CNC); same except 8–11.vi.2021 (1, CNC); Gilvesy site, 42° 47′ 24.5″ N, 80° 40′ 19.7″ W, Malaise trap in hedgerow, 5–8.vi.2021, L. Des Marteaux (2, CNC); same except 30.v–2.vi.2021 (1, CNC); VanTil site, 42° 49′ 30.8″ N, 80° 14′ 17.4″ W, Malaise trap in forest, 5–8.vi.2021, L. Des Marteaux (1, CNC); Blake site, 42° 48′ 25.2″ N, 80° 17′ 45.1″ W, Malaise at field margin, 30.v–2.vi.2021, L. Des Marteaux (1, CNC); same except 8–11.vi.2021 (2, CNC); Ottawa Region: Ottawa, Britannia Bay, Mud Lake, 45.367 N, 75.793 W, sifting forest edge, 7.iv.2021, A. Brunke (5, CNC); Quebec: Deux-Montagnes: Oka National Park, 45.476, –74.054, white tulle intercept trap at compost site, 11.v.2023, R. Vigneault (1, cNB); Vallée-du-Richelieu: Carignan, Goyer Island, 45.476, –73.278, mature oak forest, sifting dead leaves, grass and riverside debris, 6.v.2022, N. Bédard (2, cNB). UNITED STATES OF AMERICA: Alabama: Lee County: Auburn, 6.iv.1977, E.J. Kiteley (1, CNC); Massachusetts: Middlesex County: Townsend, pasture 15.iv.2010, T. Murray (2, DEBU); Groton, 30.iv.2010, T. Murray (1, DEBU); Wayland community garden on Rt. 27, 25.iii.2010, T. Murray (1, DEBU); Northampton: Northampton, 9.viii.1974, E.J. Kiteley (1, CNC); same except 1.v.1971 (2, CNC); same except 14.viii.1982 (2, CNC); New Jersey: Monmouth County: Eatontown, 11–21.vi.1991, M. Schülke (2, CNC); Pennsylvania: Dauphin County: Harrisburg, North Cameron St. and Maclay St., 40° 17′ 03″ N, 76° 52′ 42″ W, sifting leaf litter, 18.v.2010, S.M. Paiero (3, DEBU).
Photo only records. CANADA: Ontario: Essex County: Amherstburg, Texas Rd., 13.iii.2024, user russjones, iNaturalist record 202358793; Oxford County: 11.vii.2024, user: wrenwraith, iNaturalist record 228748478. UNITED STATES OF AMERICA: Massachusetts: Middlesex County: Cambridge, 27.iii.2010, user name: tom murray, bugguide.net record 380272; Suffolk County: Boston Nature Centre, 18.iii.2023, user: JaredAd, bugguide.net record 2227639; Michigan: Calhoun County: Springfield, 9.vi.2009, user: Sharon Warner, bugguide.net record 297778; North Carolina: Wake County: Raleigh, 9.iii.2023, user: David Guzman, bugguide.net record 750221; Pennsylvania: Allegheny County: Alison Park, 26.iv.2015, user: John Rosenfeld, bugguide.net record 1060056.
DNA-only records. CANADA: Ontario: Essex County: Point Pelee National Park, 41.939, –82.516, Cedar/Savannah, cactus field, 9.v.2012, H. Brown, BIOUG02913-B02 (1, CBG); Hamilton Region: Waterdown, St. Thomas the Apostle Catholic School, 43.331, –79.894, 3.v.2013, K. Armstrong, BIOUG05621-E07 (1, CBG); Middlesex County: London, Oakridge Secondary School, 42.978, –81.312, Malaise trap, 8.v.2015, S. Smith, BIOUG21997-D01 (1, CBG); Oxford County: Woodstock, St. Michael’s Catholic School, Malaise trap, 43.1464, –80.732, 5.v.2014, S. Cox, BIOUG13090-C09 (1, CBG); Toronto Region: Toronto, East York Collegiate Institute, 43.696, –79.326, Malaise trap, 8.v.2015, I. Lolja, BIOUG21923-F02 (1, CBG); Waterloo Region: Cambridge, Southwood Secondary School, 43.3502, –80.336, Malaise trap, 3.v.2013, S. Gray, BIOUG05555-D09 (1, CBG); Kitchener, Huron Heights Secondary School, 43.392, –80.466, Malaise trap, 22.iv−3.v.2013, C. Roth, BIOUG05652-G08 (1, CBG); Wellington County: Arkell Research Station, Malaise trap, 43.5187, –80.1709, 26.vi.2015, BIOUG31800-D11 (1, CBG); Guelph, 25 Division St., 43.554, –80.264, 1.v.2010, A. Smith, BIOUG00818-G05 (1, CBG). UNITED STATES OF AMERICA: New York: Westchester County: Pocantico Hills, Stone Barns Center for Food and Agriculture, 41.104, –73.825, Field 40, Malaise trap, 27.viii.2021, BIOUG74646-E08 (1, CBG). CHINA: Beijing: Beijing, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, 40.0217, 116.279, 9.ix.2016, Chenxi Liu, BIOUG37506-F05 (1, CBG).
Distribution. Adventive: Canada (Ontario, Quebec); United States of America (Alabama, Massachusetts, Michigan, North Carolina, New Jersey, New York, Pennsylvania). Native: Japan (Hokkaido, Honshu, Kyushu, Shikoku, Ryukyu Islands); South Korea; China (Beijing, Liaoning, Shaanxi).
Heterothops cognatus is an East Palaearctic species widespread in Japan (Shibata et al. Reference Shibata, Maruyama, Hoshina, Kishimoto, Naomi and Nomura2013) and is also reported from South Korea (Cho Reference Cho1996, Reference Cho2019) and northern China (Li Reference Li, Li and Chen1993). Here, we confirm its presence in China, based on a barcoded specimen from urban Beijing. Heterothops cognatus has become widespread in eastern North America, from southern Canada south to Alabama.
Bionomics. This species has been collected in both disturbed and forested areas by sifting leaf litter and other debris. The species has also been readily collected in Malaise traps. In North America, it is active from early spring to the end of summer.
Comments. The earliest record of this adventive species in North America is based on two specimens from Northampton, Massachusetts, United States of America, collected in 1971. By 1977, it had reached as far south as Alabama, United States of America, and is known from southern Canada (Ontario) as early as 1985. This species is now quite common in eastern North America and can be readily collected from sifted litter in urbanised and rural areas.
Heterothops praevius Erichson, 1839
BIN BOLD: AAX0829
Heterothops praevius Erichson (Reference Erichson1839: 480); Israelson Reference Israelson1979 (diagnosis in West Palaearctic); Assing and Schülke Reference Assing and Schülke2012 (diagnosis in Central Europe); Schülke and Smetana Reference Schülke, Smetana, Löbl and Löbl2015 (distribution, H. niger Kraatz as synonym)
Heterothops niger Kraatz (Reference Kraatz1868: 352)
Heterothops marmotae Smetana (Reference Smetana1971b: 1837) new synonym
Diagnosis. In eastern North America, H. praevius can be identified by the following combination of characters: eyes distinctly shorter than temples, head transverse, with a broad neck, and very sparse microsculpture of the head and pronotum, with spaces many times broader than lines (Fig. 2D).
Type material. The type material of Palaearctic Heterothops praevius was not studied because the species is well-known and illustrated in detail (e.g., Israelson Reference Israelson1979).
Heterothops marmotae Smetana, 1971 new synonym
Holotype (male, CNC): Ontario, Ottawa, Kanata, 26.iv.69, Smetana [type label]/Marmota burrow [typed label]/HOLOTYPE Heterothops marmotae Smetana, 1968 CNC No. 4463 [red typed and handwritten label]/CNC1589889 [identifier]. Paratype (male, CNC): CANADA: Ontario: Kanata, Marmota burrow, 25.iv.1969, A. Smetana.
The everted internal sac of the male paratype of H. marmotae bears the characteristic pair of long sclerites of Palaearctic H. praevius, which are expanded at the apex into a circular or ovoid flange (illustrated in Smetana Reference Smetana1971b, Fig. 3, for H. marmotae; everted sclerite “d” illustrated by Israelson Reference Israelson1979, Fig. 10). If the sclerites are viewed in situ or in a partly everted state such as that of the male holotype, the apices of sclerite “d” may instead appear as thin, laterally pointing hooks (e.g., Fig. 3C and 3D; Israelson Reference Israelson1979, Fig. 10). It was also possible to view the paired short sclerites (sclerite “c” of Israelson Reference Israelson1979) on the holotype preparation (Fig. 3D), which each bear a median tooth and together form a basal “H”-shape when the internal sac is in situ or partly everted. These short sclerites are identical to those found in H. praevius (Fig. 3C); it was not possible to match the “triangular plate” illustrated in Smetana (Reference Smetana1971b) with the sclerites of the holotype. This may be an artefactual amalgamation of overlapping, paired sclerite “c.” A non-type male (the only other Nearctic male available) collected at the same site as the type series was observed to have the same morphology of the internal sclerites as the holotype of H. marmotae and non-type European specimens of H. praevius.
Non-type material. CANADA: Ontario: Ottawa Region: Kanata, Marmota burrows, 12.v.1972, A. Smetana (3, CNC); Prince Edward County: 20.vi.1962, J.F. Brimley (1, CNC). CZECHIA: Bohemia, Plačice, Smetana 1947 (1, CNC); Bohemia, Kukleny, 1947, Smetana (1, CNC). DENMARK: Sealand [= Zealand], Boserup, mole nest, E.C. Rosenburg (1, CNC).
DNA-only record. CANADA: Ontario: Kawartha Lakes: east of Orange Corners, Meadowview Rd., farm, 44.296, –78.452, 290 m, Malaise trap, 24.vi.2016, B. McClenaghan (ProcID: BARSM1095–17) (1, CBG).
Distribution. Adventive: Canada (Ontario). Native: Trans-Palaearctic; Europe (including European Russia), North Africa (Algeria), Middle East (Afghanistan, Iran), Mongolia, Uzbekistan, and eastern Russia, including the Far East.
Bionomics. In the Palaearctic, H. praevius occurs in a variety of anthropogenic habitats, especially in hay and straw piles but also in a variety of other types of decaying vegetation and wood (Assing and Schülke Reference Assing and Schülke2012). The species also occurs frequently in mammal nests, including those of badgers and moles (Lott Reference Lott2008). In North America, H. praevius has been found in groundhog burrows, and one recent specimen was collected in a Malaise trap placed on a farm.
Comments. Before the present study, Heterothops marmotae was an eastern species known only from a single site near Ottawa, Ontario, and collected exclusively in groundhog burrows. In addition to having the same internal sclerites and shape of the median lobe, all specimens of H. marmotae are externally indistinguishable (microsculpture, eye size, colouration, subapical antennomere shapes) from the pale form of Palaearctic H. praevius. We continue to treat the darker form of H. praevius (previously as H. niger Kraatz) as colour polymorphism (as in Assing and Schülke Reference Assing and Schülke2012), but this question was outside of the scope of the present study. A partial CO1 barcode sequence was obtained from a non-type, female specimen of H. marmotae (307 bp; BOLD ProcID: CNCCJ3027-14), collected by A. Smetana from the same site as the type series (records in Smetana Reference Smetana1973) and during the same collecting event as the examined dissected male (see above). This partial sequence matched sequences of H. praevius in BOLD with 100% identity. Recently, a specimen from Kawartha Lakes, Ontario was collected in a Malaise trap and produced a full-length barcode that also matched H. praevius with 100% identity, consistent with the hypothesis that the species known in North America as H. marmotae is indeed H. praevius and continues to be established there (at least in northeastern Ontario), despite few records. All undetermined Heterothops from eastern North America at the CNC were screened for additional records of H. praevius, which resulted in the discovery of one female collected from Prince Edward County, Ontario, in 1962. This site is just east of the most recent record of the species and represents the oldest known occurrence of H. praevius in North America, pre-dating the type series of H. marmotae.
Heterothops conformis Smetana, 1971
Fig. 2E
BIN BOLD: ACT2650
Heterothops conformis Smetana (Reference Smetana1971a: 30); Smetana Reference Smetana1973 (additional records); Smetana Reference Smetana1978 (additional records)
Diagnosis. In eastern North America, Heterothops conformis can be recognised by the following combination of characters: eyes at least slightly smaller than temples; head longer than wide; antennomere 2 distinctly paler at base (Fig. 2E).
Type material. B.C., 8 mi. W. Creston, VI.10.1968, Campbell & Smetana [printed label]/ex river debris [printed label]/HOLOTYPE Heterothops conformis Smetana, 1968, CNC No. 10849 [red printed label, partly handwritten].
Non-type material. CANADA: British Columbia: Vancouver Island, 21 km SW Campbell River, 49° 51′ 55” N, 125° 27′ 51″ W, “Balsam CrLT-1B,” 29.vi–9.vii.1996 (1, CNC); same except “Balsam CrLT-1A,” 22.v–6.VI.1996 (1, CNC); Ontario: Sudbury District: ∼22 km SW Chapleau, Island Lake Biomass, Clearcut (2011), 47° 41.66′ N, 83° 35.60′ W, pitfall, 1–14.v.2012, L. Venier (2, CNC); same except 11–25.vi.2012 (1, CNC); Thunder Bay District: Manitouwadge, Caramat Side Rd., Rudder Lake Creek, under old grass beside creek, 22.vii.1992, T. Bakker (1, CNC); Manitouwadge, #1 rail crossing, under pine log, 20.vi.1990, T. Bakker (1, CNC); Manitouwadge, Wowan Lake Road, under poplar bark, 18.v.1987, T. Bakker (1, CNC). UNITED STATES OF AMERICA: California: Shasta County: Lassen National Park, Manzanita Lake, 1783 m, 15.vii.1979, J.M. Campbell and B.A. Campbell (1, CNC).
DNA-only records. CANADA: Alberta: NE of Peace River, chip residue pile 2002, 56.635, –116.933, 551 m, 11.vi.2008, ProcID: COLNF2002–15 (1, NFC); same except 57.239, –116.738, 457 m, 7.viii.2008 (1, NFC); British Columbia: Spences Bridge, Highland Valley Copper Mine, 50.4793, –121.0264, 1261 m, Malaise trap, 9.viii.2017, L. Fraser, ProcID: LFBC1028-18 (1, CBG).
Distribution. Native: Canada (Alberta, British Columbia, Ontario); United States of America (Arizona, California, Colorado, Idaho, New Mexico, Nevada, Oregon, Washington).
Heterothops conformis is a classic boreomontane species that has been previously overlooked outside of the western cordilleras. It is here newly reported from Alberta (east of the Rockies) and Ontario, and its distribution is very likely transboreal across Canada.
Bionomics. This species has been collected in river debris (Smetana Reference Smetana1971a), leaf litter, and under pieces of wood in a montane forest and in wet debris near a stream (Smetana Reference Smetana1971b), willow and aspen litter along canal and swamp edges (Smetana Reference Smetana1973), moss along a waterfall, and under stones near springs (Smetana Reference Smetana1978). The Alberta specimens were collected in the chip residue piles left after forest cutting. The Ontario specimens were collected in a clear-cut, in old grass near a creek, under a log, and under bark.
Comments. Until the present study, H. conformis was considered to be a western montane species. An examination of the available barcode data revealed two specimens from boreal Alberta, east of the Rockies, that belong to the same BIN as the examined specimen listed above from Shasta County, California, United States of America. Six additional specimens from two localities in northern Ontario (all females) correspond perfectly with the above diagnosis and are considered conspecific with H. conformis. Although it can be reliably separated from H. fumigatus by the colouration of the basal antennomeres (unlike H. sordidus), H. conformis is likely its sister species, based on the shared central sclerotised area of the internal sac that is basally positioned in situ (see Smetana Reference Smetana1971a) and the observation that these species’ barcode sequences form sister clusters.
Heterothops sordidus Smetana, 1971
BIN BOLD: ACU5289
Heterothops sordidus Smetana (Reference Smetana1971a: 32); Smetana Reference Smetana1973 (additional records); Smetana Reference Smetana1981 (additional records)
Diagnosis. In eastern North America, Heterothops sordidus can be identified using the following combination of characters: eyes smaller than temples (Fig. 2F); antennomeres 1–3 uniformly pale (Fig. 2F); abdominal tergites III–IV without posterior transverse basal line (Fig. 2H). In eastern North America, it is the only species to entirely lack the posterior transverse basal line.
Type material. B.C., 8 mi. W. Creston, VI.10.1968, Campbell & Smetana [printed label]/ex river debris [printed label]/HOLOTYPE Heterothops sordidus Smetana, 1968, CNC No. 10 851 [red printed label, partly handwritten].
Non-type material. CANADA: Northwest Territories: 10 miles NW Fort Simpson, Martin River, 20.vi.1972, A. Smetana, ProcID: CNCCJ3020-14 (1, CNC). UNITED STATES OF AMERICA: Alaska: Prudhoe Bay Road, Bonanza Creek, 66.667, –150.667, 274 m, 9.vii.1978, J.M. Campbell, ProcID: CNCCJ3022-14 (1, CNC).
DNA-only records. CANADA: Manitoba: Winnipeg, Ecole Precieux-Sang, 49.878, –97.12, Malaise trap, 20.iv.2015, S. Hamilton, ProcID: SMTPL5848-15 (1, CBG); Winnipeg, Landmark Collegiate, 49.669, –96.818, 240 m, Malaise trap, 20.iv.2015, S. Hiebert, ProcID: SMTPM603–15 (1, CBG). Saskatchewan: Yorkton, M.C. Knoll Elementary School, 51.217, –102.433, 499 m, Malaise trap, 8.v.2015, S. Muir, ProcID: SMTPL8062-15 (1, CBG).
Distribution. Native: Canada (British Columbia, Manitoba, Northwest Territories, Saskatchewan); United States of America (Alaska).
Heterothops sordidus is a northern species that is newly reported here from Saskatchewan and Manitoba. As it occurs in the Taiga and south to Winnipeg, it is likely transcontinental.
Bionomics. This species has been collected from river flood debris (Smetana Reference Smetana1973), mouldy piles of straw containing mice nests, and in pitfall traps in a spruce forest (Smetana Reference Smetana1981).
Comments. Heterothops sordidus was originally described from British Columbia (Smetana Reference Smetana1971a) and later found in the Taiga of interior Alaska and Northwest Territories (Smetana Reference Smetana1973, Reference Smetana1981). Here we report it from much farther south and east of its prior-known distribution and propose that it is a widespread transcontinental species that has been overlooked in the east.
Key to Heterothops of eastern North America
With the recent transfer of two eastern Heterothops to genus Chiquiticus by Reyes-Hernández and Solodovnikov (Reference Reyes-Hernández and Solodovnikov2024), the detection of two adventive species, and the discovery of two western species in eastern Canada, the composition of Heterothops in eastern North America now differs drastically from that depicted in Smetana (Reference Smetana1971a) and Smetana’s (Reference Smetana1978) updated Nearctic key. We here provide a novel key below, based entirely on stable external differences. The key should work for Heterothops within an area including Manitoba, south to eastern Texas, and eastwards.
-
1. Head with only one interocular puncture; pronotum with dorsal rows very widely spaced (Fig. 2A); apical antennomere conspicuously elongate, distinctly more than twice as long as penultimate antennomere (Fig. 2A) ……………… ………………………………………………Chiquiticus Reyes-Hernández and Solodovnikov (previously H. campbelli and H. pusio; see Reyes-Hernández and Solodovnikov Reference Reyes-Hernández and Solodovnikov2024)
-
– Head with two or, rarely, three interocular punctures (e.g., Fig. 2B); pronotum with dorsal rows in usual position along either side of midline (as in Fig. 2C); apical antennomere less than twice as long as penultimate antennomere (Fig. 1A and 1B)…………………2
-
3. Minute, narrower-bodied species (3.4–4.0 mm), pronotum only about 1.2 times as wide as head (Fig. 2C); antennomeres entirely dark, except for base of antennomere 2; elytra without paler apex; northern, boreal species, occurring in bogs in the southernmost part of its distribution………………………H. minor Smetana (BOLD BIN: ACU3133)
-
– Much larger, more strongly fusiform species (>4.0 mm), pronotum about 1.4 times as wide as head (Fig. 1A); antennomere 1 much paler than 2–11, at least ventrally (Fig. 2B); elytra with distinctly paler apex (Fig. 1A); East Palaearctic species, widespread adventive, and common in a variety of habitats in eastern North America………………………………………………………H. cognatus Sharp (BOLD BIN: AAU7313)
-
4. Head transverse, with temples weakly converging to a broader neck (Fig. 2D); microsculpture of the head and pronotum with lines markedly sparse (Fig. 2D); Palaearctic species, adventive in at least Canada (Ontario)………H. praevius Erichson (= H. marmotae Smetana syn. nov.) (BOLD BIN: AAX0829)
-
– Head elongate, with temples more strongly converging to narrower neck (Fig. 2E and 2F); microsculpture of the head and pronotum with lines much denser (Fig. 2E and 2F)……5
-
5. Antennomere 2 distinctly paler at base, antennomere 1 usually as pale as base of antennomere 2 (Fig. 2E); pronotum dark reddish to brownish (Fig. 2E); boreomontane species that is probably transcontinental in the north, east to at least Ontario…………………………………………H. conformis Smetana (BOLD BIN: ACT2650)
-
– Antennomeres 1–3, or rarely 1–2, uniformly coloured and distinctly paler than other antennomeres (Fig. 2F); pronotum pale orange to yellow, rarely darkened medially……6
-
6. Abdominal tergites III–IV with at least medial fragment of posterior transverse basal line (in line with spiracles; Fig. 2G); widespread in North America, species in need of revision…………………………………………………………H. fumigatus LeConte (previously as H. fusculus LeConte) (BOLD BIN: ACU7125, BIN: ACU5449)
-
– Abdominal tergites III–IV without posterior transverse basal line (Fig. 2H); boreomontane species that is probably transcontinental in the north, east at least to southeast Manitoba…………………………………H. sordidus Smetana (BOLD BIN: ACU5289)
Philonthina Kirby, 1837
Philonthus Stephens, 1829
Rufipes group
In the most recent revision of Nearctic Philonthina (Smetana Reference Smetana1995), P. debilis was included in the Immundus Group following the concept of Coiffait (Reference Coiffait1967). The group was diagnosed based on the sharply acute apex of the paramere, the two regular rows of peg setae that do not reach the apex, and the position of a pair of normal setae basal to the basalmost peg setae (Coiffait Reference Coiffait1974). Since then, the Palaearctic species previously known as P. immundus Gyllenhal is now referred to as P. rufipes (Stephens) (Smetana and Herman Reference Smetana and Herman1999), so the species group should now be known as the Rufipes Group. In addition to West Palaearctic species (P. debilis, P. immundus, P. aculeatus Coiffait, and P. gagates Mulsant and Rey), the group also has a close relative in the East Palaearctic, P. chujoi Dvořák (Schillhammer Reference Schillhammer2024). Unlike the others, P. chujoi has a rounded apex of the median lobe but otherwise shares the acute apex of the paramere, arrangement of peg setae into two rows, eyes shorter than the temples, five punctures in each dorsal row, sparse microsculpture of the head and pronotum (space between lines distinctly greater than line width), and the distinct transverse microsculpture on the abdominal tergites (Figs. 4 and 5). Some of these characters were used to identify the pair P. debilis and P. rufipes in Central Europe, where they are the only members of the group (e.g., Assing and Schülke Reference Assing and Schülke2012). The Rufipes Group is strictly Palaearctic, although P. debilis and P. chujoi have become adventive in the Nearctic (see below), likely as a result of their affinity to rapid decay microhabitats like compost.

Figure 4. Dorsal heads of Philonthus debilis (Gravenhorst) (A, male and C, female) and P. chujoi Dvořák (B, male and D, female); pronota of E, P. chujoi and F, P. sericans (Gravenhorst); G, microsculpture of abdominal tergite VII in P. debilis; and H, metatarsus of P. chujoi. Scale bars = 0.2 mm.

Figure 5. Male genitalia of the following species: A, C, E, and G, Philonthus chujoi Dvořák; B, D, F, and H, P. debilis (Gravenhorst); I and K, Quedius (Raphirus) maurorufus (Gravenhorst); and J and L, Q. (R.) sublimbatus Mäklin; A and B, aedeagi in ventral view; C, D, I, and J, aedeagi in lateral view; E, F, K, and L, underside of parameres; G and H, male sternites IX. Scale bars = 0.1 mm.
Together, P. debilis and P. chujoi can be readily distinguished from the superficially similar, common Nearctic species P. sericans (Gravenhorst), which occurs in the same microhabitats, by the sparse microsculpture of the head and pronotum alone (Fig. 4E; lines and spaces of about equal width in P. sericans (Fig. 4F)). Most specimens of the Rufipes Group have five punctures in the dorsal row of the pronotum, whereas P. sericans has at least six, but rare individuals of P. chujoi have been seen with six in one row. Other somewhat similar species in compost include adventive P. ventralis (Gravenhorst) and P. discoideus (Gravenhorst), but these species have the first metatarsomere slightly shorter than the apical one, whereas the members of the Rufipes Group have the first metatarsomere slightly longer (Fig. 4H).
Philonthus chujoi Dvořák, 1958
Figs. 1C, 4B, 4D, 4G, 5A, 5C, 5E, 5G, 7C
BINS BOLD: AAU6969 and BOLD: ACU4940
Philonthus chujoi Dvořák (Reference Dvořák1958: 138); Shibata et al. Reference Shibata, Maruyama, Hoshina, Kishimoto, Naomi and Nomura2013 (distribution in Japan); Cho Reference Cho2014 (distribution in South Korea); Schillhammer Reference Schillhammer2024 (synonymy, species chosen as senior synonym as first reviser)
Philonthus azabuensis Dvořák (Reference Dvořák1958: 137); Schillhammer Reference Schillhammer2024 (synonymy)
Philonthus yokohamus Dvořák (Reference Dvořák1958: 138); Schillhammer Reference Schillhammer2024 (synonymy)
Diagnosis. In the Nearctic fauna, P. chujoi is most similar to P. debilis and can be distinguished by the more elongate head that is subquadrate in males and slightly elongate (∼0.95 width/length) in females (Fig. 4B and 4D). The aedeagus is markedly different in ventral view, with a rounded apex of the median lobe and a longer paramere bearing longer rows of peg setae (Fig. 5A and 5E). The shape of female tergite X was found to overlap in the two species.
Type material. Photos of the male genitalia and head of a Nearctic specimen were found to correspond well with the type series of P. chujoi deposited in the Slóvenske Národné Múzeum (Bratislava, Slovakia) and studied by Harald Schillhammer (Schillhammer, personal communication). Recently and in preparation for the present study, Schillhammer (Reference Schillhammer2024) synonymised P. azabuensis Dvořák and P. yokohamus Dvořák with P. chujoi and reported that the differences shown in the illustrations of the aedeagi for these species (Dvořák Reference Dvořák1958) were not observed on the actual specimens.
Non-type material. JAPAN: Honshu: Ibariki: Tsukuba, pan and flight traps, 6–26.v.1989, M. Sharkey (2, CNC).
CANADA: Ontario: Toronto Region: Toronto, John Polanyi Collegiate Institute, 43.7176 N, 79.4396 W, 180 m, 20.iv.–8.v.2015, N. Anthony, BIOUG21939-D03, BIOUG21939-E04 (2, CBG); Middlesex County: London, St. Thomas Aquinas Catholic Secondary School, 42.9708 N, 81.3371 W, 3.v.2013, L. McAdam, BIOUG05630-F06 (1, CBG); Ottawa Region: Ottawa, 1305 Normandy Crescent, 1–15.v.2010, H. Goulet (3, CNC); Ottawa, Britannia Conservation Area, Mud Lake, 45.367 N, 75.793 W, forest edge, sifting litter, 4.iv.2021, A. Brunke (11, CNC); Wellington County: Puslinch Township Concession 11, 43.54, –80.14, 320 m, 10.vii.2010, P. Hebert, BIOUG01144-D11 (1, CBG); same except 22.v.2010, 10PHMAL-0285 (1, CBG). Quebec: Agglomération de Québec: St-Augustin-de-Desmaures, Ch. Cabouron, 46.7445 N, 71.5523 W, sifting old hay in an open field, 31.x.2024, N. Bédard (2, cNB); municipalité régionale de comté des Deux-Montagnes: Parc National d’Oka [collected with permit], 45.4764 N, 74.0542 W, compost site, white tulle flight-interception trap, 16.vi.2023, R. Vigneault (1, cNB); same but 1.vi.2024, L. Leclerc (3, cLC); municipalité régionale de comté de Marguerite-D’Youville: Varennes, flying, 27.iv.2009, C. Chantal (1, cCC); municipalité régionale de comté de Portneuf: Pont-Rouge, Rue Paradis, 46.7526 N, 71.7196 W, sandpit, sifting dead leaves and vegetation, 22.iv.2023, N. Bédard (3, cNB); Ville de Gatineau: Aylmer, [Ouest Forêt Boucher], Berlese of porcupine dung in a hollow base of a large maple tree, in a mixed forest, 6.iv.2010, V. Théberge and L. LeSage (18, CNC); same except 15.iv.2010 (5, CNC); same except 3.v.2010 (6, CNC); same except 12.v.2010 (3, CNC); same except 18.vi.2010 (24, CNC); same except 31.iii.2010 (1, CNC); Ville de Québec: Québec, La Cité-Limoliou, 46.7950 N, 71.2284 W, compost site, sifting wood chips, 9.ix.2023, L. Leclerc (4, cLC); Ste-Foy, Cité-Universitaire, 46.7863 N, 71.2686 W, compost site, sifted from a compost heap, 27.iv.2023, L. Leclerc (7, cLC); same but white tulle flight-interception trap, 5.v.2023 (3, cLC); Rue Frank-Carrel, 46.7921 N, 71.2285 W, sifted from dried vegetal debris, 12.v.2023, L. Leclerc (1, cLC); Rue Carré-Pijart, 46.7874 N, 71.2915 W, sifting compost, 22.ix.2022, N. Bédard (1, cNB); same except 1.x.2022 (3, cNB); same except L. Leclerc (4, cLC); same except P. Bloin (6, cPB; 2, LFC); same except 27.iv.2023, L. Leclerc (2, cNB): same except 29.iv.2023, N. Bédard (3, cNB).
Distribution. Adventive: Canada (Ontario, Quebec). Native: Japan (Honshu), South Korea.
Philonthus chujoi is native to the East Palaearctic, including Japan and South Korea, and has become adventive and quite common in southern Ontario and Quebec. It is likely broadly distributed in eastern North America but overlooked.
Bionomics. Nothing has been previously published about this species’ microhabitat preferences. In the Nearctic, it lives in a similar way to P. debilis: in compost and other rapid decay microhabitats but also in litter at the edge of a forest in early Spring. In Quebec, both species have been frequently collected together in compost.
Comments. Philonthus chujoi is here reported from North America for the first time, based on numerous specimens collected in southern Ontario and Quebec. The earliest known record is dated 2009 from southern Quebec. This species is native to the East Palaearctic, including Japan and South Korea (Shibata et al. Reference Shibata, Maruyama, Hoshina, Kishimoto, Naomi and Nomura2013; Cho Reference Cho2014). Unlike P. debilis, which has become extremely widespread in North America since becoming established sometime before 1857 (Smetana Reference Smetana1995; “1957” listed in Klimaszewski and Brunke Reference Klimaszewski, Brunke, Betz, Irmler and Klimaszewski2018 is a typo for 1857), P. chujoi seems to be a much more recent addition to the Nearctic fauna. Currently, the available barcode data from North America (all from southern Ontario) forms two clusters, each of which is assigned a BIN. A male from each of these BINs was dissected, and no differences could be found in the male genitalia or externally. The distance between BINs is approximately the same as that observed within the single BIN corresponding to P. debilis, and it is likely that further sequencing, especially in its native, Palaearctic range, will reveal additional haplotype diversity and collapse these two BINs into one.
Philonthus debilis (Gravenhorst, 1802)
Figs. 4A, 4C, 4G, 5B, 5D, 5F, 5H
BIN BOLD: ABV1529
Staphylinus debilis Gravenhorst (Reference Gravenhorst1802: 35)
Philonthus debilis (Gravenhorst): Smetana Reference Smetana1995 (summary of important references, Nearctic distribution, diagnosis)
Diagnosis. In the Nearctic, P. debilis is most similar to P. chujoi and can be distinguished by the shape of the head, which is transverse in males (∼1.1 width/length) and subquadrate in females (Fig. 4A and 4C). The pointed apex of the median lobe also distinguishes it immediately from P. chujoi. The aedeagus and external morphology of Nearctic P. sericans are superficially similar, but this species can be distinguished easily by the denser microsculpture and six punctures of the dorsal row (Fig. 4F).
Non-type material. CANADA: Yukon: Whitehorse, Granger, compost, 5.ix.2005, ‘EP-Yukon (2, CNC).
Distribution. Adventive: Canada (Alberta, British Columbia, Manitoba, Ontario, New Brunswick, Newfoundland and Labrador, Nova Scotia, Quebec, Prince Edward Island, Saskatchewan, Yukon Territory); United States of America (Arizona, California, Colorado, Connecticut, District of Columbia, Florida, Idaho, Illinois, Indiana, Iowa, Kansas, Maine, Maryland, Massachusetts, Michigan, Minnesota, Montana, New Hampshire, New Jersey, New York, Ohio, Oregon, Pennsylvania, Rhode Island, South Dakota, Utah, Virginia, Washington, West Virginia, Wisconsin, Wyoming). Native: Trans-Palaearctic species, from Europe and North Africa to the Russian Far East, Mongolia, northeastern China, and Japan (Newton Reference Newton, Bánki, Roskov, Döring, Ower, Hernández Robles and Plata Corredor2025).
Philonthus debilis is an extremely widespread adventive species in North America but is somewhat limited by the climate of the far north. However, it likely can persist farther north in urban compost piles that are perpetually much warmer than their surroundings. The new record above from Whitehorse, Yukon Territory, likely represents such an occurrence.
Bionomics. Philonthus debilis lives in a variety of litter-based microhabitats in disturbed habitats and along ecotones and can be quite abundant in rapid decay microhabitats such as compost.
Comments. The identification key in Smetana (Reference Smetana1995) contains an error that would not allow P. debilis to key correctly. Couplet 39 should lead to couplet 42, not 41, and couplet 40 should lead to 41 in the second half. The only way to arrive at P. debilis is to select “eyes at least as large as temples.” This is clearly a mistake because the eyes are correctly stated elsewhere in Smetana (Reference Smetana1995) to be shorter than the temples. We correct this below and modify the couplets to accommodate Philonthus chujoi.
Updated, corrected couplets to the Nearctic Philonthus key of Smetana (Reference Smetana1995)
-
39. Eyes small, each seen from above shorter than temples (ratios 0.74–0.85)…………40
-
– Eyes larger, each from above at least as long as temple………………………42
-
40. Punctures on head and pronotum conspicuously large and deep, pit-like. Pronotum narrowed posteriad, lateral margins each distinctly concave posteriorly in front of basal margin. Aedeagus as in figs. 1405–1407. Length 7.0–9.1 mm…Nudus Group…………………………………………………….Philonthus nudus Sharp
-
– Punctures on head and pronotum fine. Pronotum narrowed anteriad, lateral margins each slightly arcuate or parallel to each other posteriorly in front of basal margin. Aedeagus different………………………………………………………41a
-
41a. Small, length not exceeding 5.7 mm. Head and pronotum with fine and rather sparse microsculpture, interspaces between striae distinctly wider than diameters of striae. Pronotum slightly iridescent. Paramere of aedeagus entire (fig. 258). Length 4.2–5.7 mm…Rufipes Group [previously Immundus Group]……………………41b
-
– Large, length over 5.7 mm. Head and pronotum with exceedingly fine and dense microsculpture, interspaces between striae not wider than diameters of striae. Pronotum not iridescent. Paramere of aedeagus bilobed (fig. 344). Length 5.8–8.2 mm…Furvus Group…………………………………P. umbrinus (Gravenhorst) (pars)
-
41b. Head transverse in males, subquadrate in females; median lobe of aedeagus with pointed apex in ventral view………………………………P. debilis (Gravenhorst)
-
– Head subquadrate in males, elongate in females; median lobe of aedeagus with rounded apex in ventral view……………………………………P. chujoi Dvořák
Quediina Kraatz, 1857
Quedius (Raphirus) Stephens, 1829
A phylogenomic study of Quediina by Brunke et al. (Reference Brunke, Hansen, Salnitska, Kypke, Predeus and Escalona2021a) revealed that the subgenus Raphirus was highly polyphyletic and requires revision. The subgenus has never had a robust morphological definition that works for all included species, and this is even a problem within specific regions, such as the Nearctic (e.g., Smetana Reference Smetana1971a). Some very distantly related lineages, previously associated with Raphirus, have recently been elevated to genus level (Brunke Reference Brunke2022), but much taxonomic work remains to be completed. Brunke et al. (Reference Brunke, Hansen, Salnitska, Kypke, Predeus and Escalona2021a) proposed that their Clade X would represent a suitable future concept for Raphirus, but it is still not possible to provide a global, morphological diagnosis of this group or of the Raphirus lineage in its entirety without novel morphological characters (A.J.B., unpublished data). In the meantime, we provide a short key below, limited in scope to the two eastern North American members of Clade X sensu Brunke et al. (Reference Brunke, Hansen, Salnitska, Kypke, Predeus and Escalona2021a), which can be recognised among other Quediina by the following combination of characters: head with single basal puncture, without interocular punctures; labrum with median notch; scutellum impunctate; and elytra with evenly distributed punctation, never with meshed microsculpture but sometimes with irregular micropunctures between the setose punctures. Clade X is quite poorly represented in the Nearctic, although one West Palaearctic species has recently become adventive (see below).
Quedius (Raphirus) maurorufus (Gravenhorst, 1806)
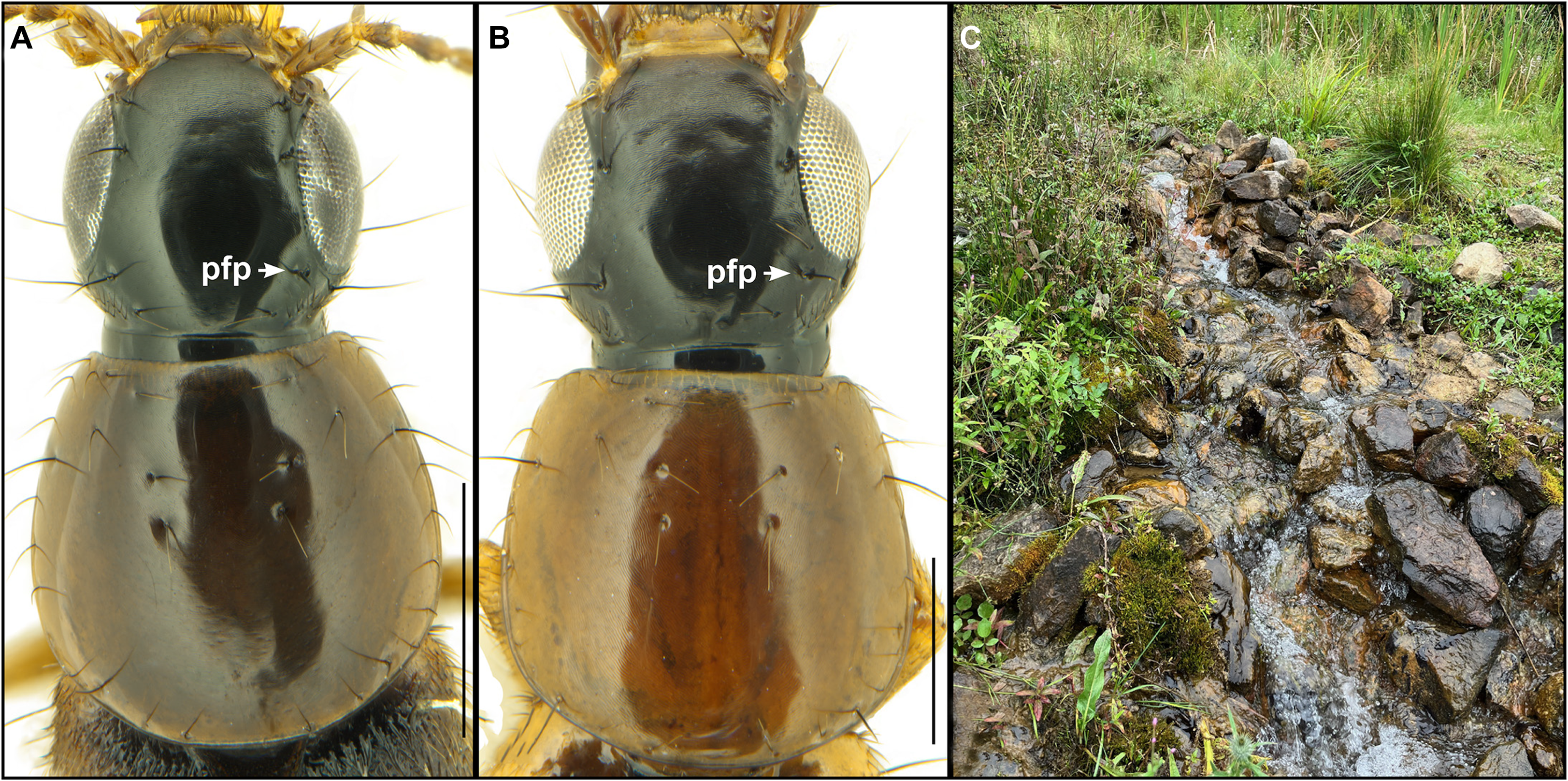
Figure 6. Dorsal forebodies of the following species: A, Quedius (Raphirus) maurorufus (Gravenhorst); and B, Quedius (Raphirus) sublimbatus Mäklin. C, Microhabitat of adventive Q. maurorufus, a groundwater-fed creek near Barrie, Ontario, Canada (photo by K. Brunke, used with permission). pfp = posterior frontal puncture. Scale bars = 0.5 mm.

Figure 7. Adventive Nearctic distribution of the following species: A, Heterothops cognatus Sharp (white circles indicate photo records, black circles indicate specimen records); B, H. praevius Erichson; C, Philonthus chujoi Dvořák; and D, Quedius (Raphirus) maurorufus (Gravenhorst).
BIN BOLD: AAX9863 and BOLD: ACZ1264
Staphylinus maurorufus Gravenhorst (Reference Gravenhorst1806: 56)
Quedius (Raphirus) maurorufus Gravenhorst; Assing and Schülke (Reference Assing and Schülke2012) (diagnosis, biology); Salnitska and Solodovnikov (Reference Salnitska and Solodovnikov2019) (diagnosis, Russian records doubtful)
Diagnosis. In eastern North America, Q. maurorufus can be recognised as a member of Quedius (Raphirus) Clade X sensu Brunke et al. (Reference Brunke, Hansen, Salnitska, Kypke, Predeus and Escalona2021a) based on the following characters: head with single basal puncture, without interocular punctures; labrum with median notch; scutellum impunctate; and elytra with evenly distributed punctation, never with meshed microsculpture but sometimes with irregular micropunctures between the setose punctures. Quedius maurorufus can be externally distinguished from the only other eastern North American member of Clade X, Q. sublimbatus Mäklin, by the larger eyes and longer and wider pronotum (Fig. 6A). More obvious differences may be found on the male genitalia (Fig. 5).
Type material. The type material of this well-known and illustrated (e.g., Assing and Schülke Reference Assing and Schülke2012) species was not studied.
Non-type material. CANADA: Ontario: Northumberland County: Peter’s Woods Provincial Nature Reserve, 44.12417, –78.03917, moss, 6.x.2011, A. Brunke, ProcID: QUEDB084–19 (1, DEBU); same except cold wet moss on rocks in stream and at edge of spring, 15.ix.2011, A. Brunke (7, DEBU); Oxford County: 17 km NE Tillsonburg, Milldale, oak litter by stream in ravine, 4.x.2005, A. Davies (1, CNC); Milldale, 45° 56′ 08″ N, 80° 35′ 08″ W, seepage area at stream in deep ravine, 25.v.2011, A. Davies (4, CNC); same except pine and beech litter by stream in ravine (3, CNC); Simcoe County: Minesing, forest N Barrie Sports Complex, 44° 27′ 0.36″ N, 79° 46′ 23.88″ W, spring outflow to gravel pit pond, treading moss, 24.viii.2024, A. Brunke (2, CNC); Toronto Region: Rouge National Park, 43.7948, –79.124, pitfall traps, 15.ix.2015, BIO Team, ProcID: RBINA341–13 (1, CBG).
Distribution. Adventive: Canada (Ontario). Native: Europe.
Quedius maurorufus is a West Palaearctic species that is widespread across Europe, but records from any part of Russia are doubtful (Salnitska and Solodovnikov Reference Salnitska and Solodovnikov2019). In North America, it has been detected only in southern Ontario.
Bionomics. In its native range, Q. maurorufus is commonly found in a variety of moist microhabitats (Assing and Schülke Reference Assing and Schülke2012). At the time of publication, adventive populations in North America have been mostly found in or near wet, cold moss along the edges of seepages and springs and on mossy boulders in a cold, fast-flowing stream. The only other specimen was collected in pitfall traps placed in forested suburbs near the Rouge River (Toronto area) at its outflow to Lake Ontario, but the microhabitat is unknown.
Comments. Here, we report Quedius (Raphirus) maurorufus from North America for the first time as an adventive species, based on dissected males and several sequenced specimens that cluster amongst European specimens. Despite a complete screening of the CNC staphylinids, few North American specimens are available, and all are quite recent, with 2005 as the earliest date of detection. Nearly all specimens were collected in or near cold, wet moss associated with running groundwater-based microhabitats (Fig. 6C) that are very local on the low plains of southern Ontario and are usually quite poor in rove beetle diversity, either naturally or due to fragmentation from intense development in this region. In all three of the groundwater-associated sites, these were either the only rove beetles collected or there were only litter generalists present (e.g., Philonthus asper Horn in the deep ravine at the Milldale locality). It is possible that Q. maurorufus has taken advantage of an empty or nearly empty niche in southern Ontario. The species will certainly be detected elsewhere in southern Canada and eastern United States of America, but it may be extremely local and require targeted collecting. Two barcode clusters, each representing a BIN cluster in BOLD, are only 1.28% different and are associated with this species; both sequenced Nearctic specimens belong to BOLD: AAX9863.
Key to the species of Quedius Clade X sensu Brunke et al. (Reference Brunke, Hansen, Salnitska, Kypke, Predeus and Escalona2021a) in eastern North America
-
1. Eyes larger, such that the posterior frontal puncture is in line with hind margin of eye (Fig. 6A); pronotum distinctly wider and longer than head (Fig. 6A); median lobe in lateral view with apical tooth (Fig 5I); paramere with short fields of peg setae (Fig. 5K); West Palaearctic species, adventive in eastern North America…………………………………..Q. maurorufus Gravenhorst (BIN BOLD:AAX9863 and BOLD:ACZ1264)
-
– Eyes smaller, such that the posterior frontal puncture is just posterior to hind margin of eye (Fig. 6B); pronotum only slightly wider and longer than head (Fig. 6B); median lobe in lateral view with subapical tooth (Fig. 5J); paramere with long fields of peg setae (Fig. 5L); Holarctic species with boreomontane and Arctic distribution, unknown from southern Canada but occurring in high elevation refugia of New York and New Hampshire, United States of America……………..…………Q. sublimbatus Mäklin (BIN BOLD: ACA9274)
Discussion
After the present study, 60 species of Staphylininae have been confirmed as adventive in North America, slightly more than the 56 species currently reported from Canada (Newton Reference Newton1987; Rood et al. Reference Rood, Brunke and Solodovnikov2015; Klimaszewski and Brunke Reference Klimaszewski, Brunke, Betz, Irmler and Klimaszewski2018; Bédard et al. Reference Bédard, Brunke, Bloin and Leclerc2024). Philonthus and Quedius are diverse genera with 17 and 8 species adventive in North America, respectively, some of which are strongly synanthropic and have become widely distributed around the world (Klimaszewski et al. Reference Klimaszewski, Brunke, Assing, Langor, Newton and Bourdon2013). Before the present study, no species of Heterothops had been reported as adventive, although Nearctic Chiquiticus pusio, previously Heterothops, was recently detected in Germany (Schülke and Renner Reference Schülke and Renner2020). Although Heterothops cognatus was present in North America before 1971, it was probably overlooked because rigorous identification of Heterothops species typically relies on difficult-to-observe, minute internal sclerites of the male genitalia. In the case of West Palaearctic H. praevius, this species was technically detected but described instead as a Nearctic species (Smetana Reference Smetana1971b), synonymised herein. It is important to note that Israelson’s detailed and standardised study of the internal sclerites in West Palaearctic Heterothops was not published until 1979, and H. praevius (as H. marmotae) was not collected again in North America until 2016, providing little opportunity for reassessment. Fortunately, eastern North America is relatively poor in Heterothops spp., which can now be identified using external characters (see key above). We hope that this makes the genus more accessible.
The species detected as adventive in North America were first recognised through modern biomonitoring efforts, which consist of both detailed morphological study (including microdissections and curating records on citizen science–based websites) and CO1 barcode sequencing. The latter can be conducted on authoritatively identified specimens for building reference libraries or on a larger scale for faunistics (e.g., metabarcoding of entire Malaise trap samples, as in Buchner et al. Reference Buchner, Sinclair, Ayasse, Beermann, Buse and Dziock2024). Through insect survey work undertaken in southern Quebec by the second and third authors of the present paper (N.B. and L.L.), with the help of collaborators, Philonthus chujoi was first detected after microdissections revealed male genitalia that were strongly incongruent with related and externally similar P. debilis. Corresponding CO1 barcodes were identified in BOLD after borrowing specimen vouchers from CBG and examining the database for sequences clustering near P. debilis. The true identity of H. marmotae syn. nov. and the detection of Heterothops praevius were first indicated through routine barcode clustering performed by research staff (including A.J.B.) at the Canadian National Collection of Insects, Arachnids and Nematodes. A partial sequence of H. marmotae had been generated several years before as part of a large reference library–building effort for Canadian Coleoptera (Agriculture and Agri-Food Canada, Genomics Research and Development Initiative) and was flagged for taxonomic follow-up. Finally, the detection of Heterothops cognatus began when live images of a species that did not match any known eastern Heterothops at the time were posted on bugguide.net in 2010. A similar integrative taxonomic approach was successful in the novel detection of adventive aleocharine rove beetles in both North America and Central Europe (Brunke et al. Reference Brunke, Pentinsaari and Klimaszewski2021b), and online citizen science observations continue to be critical for producing accurate distributions, including that of an adventive staphylinine that is rapidly expanding its range in eastern North America (Brunke Reference Brunke2016).
Accurate and timely detection of adventive species can be challenging in a group as diverse as Staphylinidae. It is fortunate that nearly all adventive staphylinids in North America have originated from Central Europe (Klimaszewski and Brunke Reference Klimaszewski, Brunke, Betz, Irmler and Klimaszewski2018), one of the taxonomically best-known regions of the planet with identification resources such as Assing and Schülke (Reference Assing and Schülke2012) and the well-illustrated Danish website https://danbiller.dk. A Eurocentric adventive fauna is a general trend in Coleoptera that are strongly connected to soil and other organic matter such as Carabidae, with 54 of 55 adventive species in Canada originating from the West Palaearctic (Klimaszewski et al. Reference Klimaszewski, Langor, Batista, Duval, Majka, Scudder and Bousquet2012), and many groups of Elateridae and Chrysomelidae, whose larvae develop in the soil and litter (Douglas Reference Douglas2011; Douglas et al. Reference Douglas, Dumont, Savard and Chantal2021, Reference Douglas, Hammond, Smith, Mutz and Konstantinov2024). Both P. chujoi and Q. maurorufus appear to be recent introductions (2009 and 2005, respectively) and indicate that soil invertebrates continue to establish adventive populations in North America despite rigorous, no-soil phytosanitary requirements for importation of plants by both Canada and the United States of America (Eschen et al. Reference Eschen, Britton, Brockerhoff, Burgess, Dalley and Epanchin-Niell2015). Douglas et al. (Reference Douglas, Dumont, Savard and Chantal2021, Reference Douglas, Hammond, Smith, Mutz and Konstantinov2024) proposed that recent detections of soil Chrysomelidae and Elateridae may represent populations that have remained undetected at low densities since establishing in North America during 1960–1965, via imported plants with soil. However, trade of nonphytosanitary plants during this period was primarily with central Europe (e.g., the Netherlands; Douglas et al. Reference Douglas, Dumont, Savard and Chantal2021), and this historical pathway therefore cannot account for the recent detections of soil invertebrates native to the East Palaearctic.
Compared to the West Palaearctic, the East Palaearctic soil invertebrate fauna is poorly known taxonomically, identification resources are generally scattered or absent, and little representation (identified or otherwise) exists in sequence reference libraries. For Philonthus chujoi, morphological taxonomic work (Schillhammer Reference Schillhammer2024) was needed to establish a reliable species concept and corresponding valid name before an identification could be made and published. Another case was recently solved for an adventive carabid beetle in the United States of America (Harden and Boyd Reference Harden and Boyd2024), when sequenced specimens were integrated into an in-progress phylogenetic dataset, which directed the authors’ attention to the East Palaearctic fauna and, luckily, a recent modern description of the matching species. The situation is even more fraught for the terrestrial hammerhead flatworms (Tricladida: Geoplanidae: Bipalium Stimpson, Diversibipalium Kawakatsu et al.) that have become adventive in North America from the East Palaearctic (reviewed by Skvarla Reference Skvarla2022). Due to a lack of existing taxonomic knowledge, four of the five species were described from their area of first detection (western North America, the Caribbean, the United Kingdom’s Kew Botanical Gardens, etc.), even though the area of origin for all these species is known or thought to be in Asia (Skvarla Reference Skvarla2022). For other pathways of introduction, including solid wood packing materials and shipping containers, the East Palaearctic has been the source of several, well-known adventive insects, including emerald ash borer, Agrilis planipennis Fairmaire (Coleoptera: Buprestidae), Asian longhorn beetle, Anoplophora glabripennis (Motschulsky) (Coleoptera: Cerambycidae), and soybean aphid, Aphis glycines Matsumura (Hemiptera: Aphididae), some of which have had devastating economic and ecological impacts (reviewed by Wheeler and Hoebeke Reference Wheeler, Hoebeke, Foottit and Adler2017). In recognition of the above and potential impacts of similar introductions, the governments of Canada and the United States of America have responded by directly supporting taxonomic research on these genera in the East Palaearctic (Lingafelter and Hoebeke Reference Lingafelter and Hoebeke2002; Jendek and Grebennikov Reference Jendek and Grebennikov2011). Additional detections of East Palaearctic rove beetles in North America are in various stages of confirmation by ourselves and collaborators, further emphasising the importance of improving taxonomic knowledge of the East Palaearctic invertebrate fauna and its representation in North American biological collections, especially for poorly known soil-dwelling taxa.
Acknowledgements
The authors thank Harald Schillhammer (Vienna, Austria) for comparing detailed images of Canadian Philonthus chujoi with the Dvořák type material and for more than a decade of productive collaborations with A.J.B. Mikko Pentinsaari and Hume Douglas (both Agriculture and Agri-Food Canada (AAFC), Ottawa, Canada) provided important insights into East Palaearctic introductions and potential pathways. Dr. Sandrine Picq and Abdelmadjid Djoumad (LFC, Quebec, Canada) are thanked for their help in obtaining sequences for two P. chujoi specimens from Quebec. Pierrick Bloin, Robert Vigneault, and Claude Chantal (City of Québec, Quebec, Canada) generously shared data or donated specimens of P. chujoi from their collections. A.J.B. thanks Lauren Des Marteaux (AAFC, Harrow, Ontario) for providing numerous specimens of H. cognatus from southern Ontario Malaise trap surveys as part of AAFC’s Living Labs project. The authors thank Kevin Brunke (Midhurst, Ontario, Canada) for photographing the microhabitat of Q. maurorufus.
Funding statement
This study received funding to A.J.B. from AAFC (Systematics of Beneficial Arthropods, J-002276).
Competing interests
The authors declare that they have no competing interests.










