INTRODUCTION
The 2018 update of the Heart and Stroke Foundation of Canada’s Canadian Stroke Best Practice Recommendations for Acute Stroke Management, Sixth Edition (CSBPR, 6th Ed.)Reference Boulanger, Lindsay and Gubitz 1 was developed with input from the Canadian Association of Emergency Physicians (CAEP) Stroke Practice Committee and approved by the CAEP Executive Board. It provides comprehensive, evidence-informed recommendations for the management of acute ischemic stroke (AIS) and transient ischemic attack (TIA). This article aims to highlight recommendations from the CSBPR, 6th Ed. that are particularly relevant to stroke care in the prehospital and emergency department (ED) settings, including several significant changes for endovascular thrombectomy (EVT) extended treatment time windowsReference Nogueira, Jadhav and Haussen 2 , Reference Albers, Marks and Kemp 3 and revisions for the triage and management of TIA based on recent evidence.
This article is a condensed synthesis of the CSBPR, 6th Ed. reflecting solely the opinions of the authors through a review outside of CSBPR methodology. Readers are encouraged to refer to the primary CSBPR documents, freely available online at www.strokebestpractices.ca, for full details of the peer-reviewed recommendations. If specific language from the CSBPR, 6th Ed. is conveyed, it is indicated by quotation marks with the source recommendation location identified by a section number (e.g., Section 3.3.ii). Otherwise, unquoted text represents the commentary of the authors alone in summarizing the CSBPR, 6th Ed. recommendations. All tables taken or modified from the CSBPR, 6th Ed. and presented here are shared with permission from the Heart and Stroke Foundation of Canada, SAGE, and the International Journal of Stroke. (Tables 1 and 3)
Table 1 Summary of criteria for levels of evidence reported in the CSBPR, 6th Ed., updated 2018 (Adapted from Guyatt et al.Reference Boulanger, Lindsay and Gubitz 1 , Reference Guyatt, Cook and Jaeschke 4 )

Recommendations from the CSBPR, 6th Ed. are organized into the following major sections:
∙ Stroke awareness, recognition, and response (CSBPR, 6th Ed., Section 1)
∙ Emergency medical services on-scene response (CSBPR, 6th Ed., Section 3)
∙ ED evaluation of acute stroke and TIA (CSBPR, 6th Ed., Section 4)
∙ Treatment of acute stroke (intravenous thrombolysis and endovascular therapy; CSBPR, 6th Ed., Sections 5-7)
∙ Outpatient management of TIA and non-disabling minor stroke (CSBPR, 6th Ed., Sections 2 and 6)
PUBLIC STROKE AWARENESS, RECOGNITION, AND RESPONSE
(CSBPR, 6th Edition, Section 1)
Emphasis remains on promoting public awareness campaigns to increase early recognition of stroke symptoms and signs by both healthcare providers and members of the public.
∙ “Public and healthcare provider education should include information that stroke can affect persons of any age, including newborns, children, and adults. Education should also emphasize the benefits of early emergency treatment” (Evidence Level B; Section 1.1.i/v).
∙ “Public awareness campaigns and education should include use of the FAST 5 (Face, Arms, Speech, Time) acronym to facilitate memory recognition of [stroke] signs” (Evidence Level B; Section 1.1.iii).
∙ “Public and healthcare provider education should emphasize the need to respond immediately by calling 9-1-1 or their local emergency number, even if symptoms resolve” (Evidence Level B; Section 1.1.iv).
EMERGENCY MEDICAL SERVICES ON-SCENE MANAGEMENT
(CSBPR, 6th Edition, Section 3)
Stroke systems of care are being reorganized to screen and direct patients potentially eligible for EVT to EVT-capable hospitals. Emergency medical services (EMS) plays a critical role in screening for these patients. New recommendations in the CSBPR, 6th Ed. include a two-stage stroke EMS screening process to identify signs of stroke and further select the subset of patients with severe stroke who are most likely to be eligible for EVT.
∙ EMS on-scene assessment should include the use of validated out-of-hospital diagnostic tools for a two-stage screening process (Evidence Level B; Section 3.2.i):
ο Stage one : screening for signs of stroke with a tool that includes the components of FAST.
ο Stage two : patients who screen positive in stage one then undergo a second screen to assess for stroke severity (which can assist with the identification of EVT candidates for potential large vessel occlusions and inform transport destination decisions).
∙ “On-scene time with suspected stroke patients should be as short as possible” with a target median on-scene time of “20 minutes or less for patients presenting within 4.5-hours” of symptom onset or last seen normal time (Evidence Level C; Section 3.2.iii).
∙ “Initial assessment by paramedics on-scene should include capillary blood glucose measurement” (Evidence Level B; Section 3.2.iv).
∙ Whenever possible prior to transport, the patient’s family and/or alternate decision-makers should be directed by EMS to “accompany the patient to hospital or be accessible by phone for [treatment] decision-making, as well as confirming time last known well, and providing required information about existing health conditions, current medications, and other information as needed” (Evidence Level C; Section 3.2.v).
TRANSPORT FOR SUSPECTED STROKE PATIENTS
∙ Regional direct transport protocols must be in place to ensure the timely transfer of patients potentially eligible for acute stroke treatment (within 4.5 hours of known or presumed symptom onset for alteplase [tPA], 6 hours for EVT, and up to 24 hours for EVT in patients with highly favourable neuroimaging) to treatment-capable stroke centres (Evidence Level C; Section 3.3.i). Optimal protocols remain the subject of ongoing research and are expected to vary across regional stroke systems based on local geographical factors, such as the distribution of primary stroke centres (PSC) and comprehensive stroke centres (CSC), transport times, hospital-specific times from ED arrival to initiating acute stroke reperfusion therapy, and hospital-specific door-in/door-out times for transferred patients. Recommendations for elements of transfer protocols are provided in Section 3.3.ii and include the following:
ο Designation of a PSC and CSC based on criteria summarized in Table 2.
ο Patients potentially eligible for intravenous alteplase (tPA) may be directed to the closest stroke centre (PSC or CSC) (Clinical Consideration; Section 3.1).
ο Patients potentially eligible for EVT may proceed directly to an EVT-enabled CSC or to the nearest PSC first for alteplase (tPA) consideration prior to transfer to an EVT-enabled CSC (Clinical Consideration; Section 3.1).
∙ “Patients with suspected stroke should be triaged . . . as Canadian Triage Acuity Scale (CTAS) Level 2 in most cases and as a CTAS Level 1 for patients with compromised airway, breathing, or cardiovascular function” (Evidence Level B; Section 3.3.iii).
∙ EMS prenotification should be provided to the receiving ED, including sufficient details for “Code Stroke” activation (Evidence Level B; Section 3.3.iv).
ο “Information required includes: time of stroke onset or time of symptom recognition or time when last known well (as accurate as possible), total symptom duration at anticipated arrival in the emergency department, presenting signs and symptoms of stroke, Glasgow Coma Scale (GCS) score, CTAS triage score, patient age, current use of antithrombotic drugs, and expected time of arrival at the receiving hospital.”
Table 2 Recommendations for regional primary and comprehensive stroke centre designations

ED EVALUATION OF ACUTE STROKE AND TRANSIENT ISCHEMIC ATTACK (TIA)
(CSBPR, 6th Edition, Section 4)
Patients presenting to the ED with suspected stroke or TIA require immediate clinical evaluation and investigations, including appropriate neuroimaging consideration. In the authors’ opinion, immediate imaging indicates imaging without waiting for the return of any bloodwork or creatinine results (with the exception of capillary or blood glucose). Geography may present unique challenges for access to timely imaging. Therefore, for strokes occurring in rural or remote settings, the recommended time windows should take into account necessary transfer time to centres with imaging, alteplase (tPA), and/or EVT capabilities, respectively.
NEUROIMAGING (BRAIN IMAGING)
∙ All patients with suspected acute stroke should undergo neuroimaging with non-contrast computed tomography (NCCT) or magnetic resonance imaging (MRI) (Evidence Level A; Section 4.2.i). The following imaging recommendations are based on presentation from the time of symptom onset or when last seen normal:
ο <4.5 hours: potentially alteplase (tPA) eligible and should undergo immediate NCCT (Evidence Level A; Section 4.2.ii).
ο <6 hours: potentially EVT eligible and should undergo immediate NCCT and CT angiography (CTA) from aortic arch to vertex, including extracranial and intracranial circulation (Evidence Level A; Section 4.2.iii).
ο 6-24 hours: potentially EVT eligible (including late presentation and stroke on awakening) and should undergo immediate brain imaging with NCCT, CTA, and CT perfusion or MRI with MR angiography (MRA) and MR perfusion (MRP) (Evidence Level B; Section 4.2.iv).
ACUTE ISCHEMIC STROKE BLOOD PRESSURE MANAGEMENT
∙ Ideal blood pressure targets in the hyperacute phase of ischemic stroke management are unknown at present, but care should be taken to avoid precipitous falls in blood pressure (Evidence Level C; Section 4.3.i). Hemorrhagic stroke blood pressure targets will be addressed in a separate upcoming Canadian Stroke Best Practice Guidelines update.
∙ Ischemic stroke patients eligible for thrombolytic therapy: blood pressure target <185/110 mm Hg prior to alteplase (tPA) (Evidence Level B, Section 4.3.ii) and <180/105 mm Hg for 24 hours after alteplase (tPA) administration (Evidence Level C; Section 4.3.ii).
∙ Ischemic stroke patients NOT eligible for thrombolytic therapy: no routine blood pressure lowering in the absence of reperfusion therapy (i.e., without alteplase [tPA] or EVT). However, extreme blood pressure elevation (>220/120 mm Hg) should be treated to a target reduction of 15% to 25% over the first 24 hours with a gradual reduction thereafter (Evidence Level C; Section 4.3.iii).
∙ Choice of agents for managing blood pressure should be based on current Hypertension Canada Blood Pressure treatment guidelines (www.hypertension.ca) (Section 4.3.vi).
ADDITIONAL ED INVESTIGATION AND MANAGEMENT CONSIDERATIONS
∙ Hyperthermia (>37.5 Celsius) and hypoxemia should be avoided (Evidence Level B; Section 4.6.iv, 9.3.i). Supplemental oxygen is not required for patients with normal oxygen saturation levels (Evidence Level C; Section 4.6.v).
∙ Additional investigations for patients with suspected ischemic stroke or TIA include bloodwork (including random glucose, complete blood count, electrolytes, coagulation studies such as international normalized ratio (INR) and aPTT, and creatinine), 12-lead electrocardiogram, chest X-ray, and non-urgent echocardiography (Evidence Level B; Sections 4.4.i/iii, 4.5.i, 4.6.i). Unless patients are clinically unstable, these investigations should not delay neuroimaging or acute treatment (Evidence Level C; Section 2.2.1.iia).
∙ Patients should remain nil per os (NPO, no oral intake) until a swallowing assessment has been performed, ideally within 24 hours of hospital arrival (Evidence Level B; Section 4.6.ii).
ACUTE ISCHEMIC STROKE (AIS) TREATMENT
(CSBPR, 6th Edition, Sections 5, 6, and 7)
Rapid delivery of alteplase (tPA) and EVT in eligible patients remains the mainstay of AIS treatment. New extended time windows should not be interpreted to mean that time to treatment can be slowed in any way, because outcomes are optimized with earlier treatment.
PATIENT SELECTION FOR ACUTE ISCHEMIC STROKE TREATMENTS
∙ “All ischemic stroke patients not already on an antiplatelet agent and not receiving alteplase (tPA) should be given at least 160 mg of acetylsalicylic acid (ASA) immediately as a one-time loading dose after brain imaging has excluded intracranial hemorrhage” (Evidence Level A; Section 6.i). “ASA (81 to 325 mg daily) should then be continued indefinitely or until an alternative antithrombotic regimen is started” (Evidence Level A; Section 6.i.a.).
∙ All patients with disabling acute stroke presenting within eligible treatment time windows must be screened without delay through appropriate clinical evaluation and neuroimaging by a physician with stroke expertise (either on-site or by telemedicine/telestroke consultation) (Evidence Level A; Section 5.1.i/ii).
∙ The following eligibility time windows are based on presentation from time of symptom onset or when last seen normal:
ο <4.5 hours: potentially alteplase (tPA) eligible (Evidence Level A; Section 4.2.ii).
ο <6 hours: potentially EVT eligible (Evidence Level A; Section 4.2.iii).
ο 6-24 hours: potentially EVT eligible (including late presentation and stroke on awakening) in highly selected patients with appropriate neuroimaging criteria and stroke expert consultation (Evidence Level B; Section 4.2.iv).
INTRAVENOUS THROMBOLYSIS WITH ALTEPLASE (tPA)
∙ “All eligible patients with disabling ischemic stroke should be offered intravenous alteplase (tPA). Eligible patients are those who can receive intravenous alteplase (tPA) within 4.5 hours” of symptom onset time or last seen normal (Evidence Level A; Section 5.3.i).
ο Inclusion and exclusion criteria for alteplase (tPA) eligibility can be reviewed in the CSBPR, 6th Ed. (Box 5B).
ο The decision of the CAEP Stroke Committee to support a 4.5-hour treatment time window for alteplase (tPA) arises from a consensus agreement that the benefits of collaboration, including partnership in improved regional EVT pathways, outweigh the harms of alteplase (tPA) within the 3- to 4.5-hour treatment time window.
∙ “All eligible patients should receive alteplase (tPA) as soon as possible after hospital arrival” (Evidence Level A; Section 5.3.ii), with a target median door-to-needle time of 30 minutes (Evidence Level B; Section 5.3.ii).
∙ “Alteplase (tPA) should be administered using a dose of 0.9 mg/kg to a maximum of 90-mg total dose, with 10% (0.09 mg/kg) given as an intravenous bolus over one minute and the remaining 90% (0.81 mg/kg) given as an intravenous infusion over 60 minutes” (Evidence Level A; Section 5.3.iib).
∙ “In patients treated with intravenous alteplase (tPA), antiplatelets should be delayed until after the 24-hour post-thrombolysis [neuroimaging] has excluded intracranial hemorrhage” (Evidence Level B; Section 6.iii).
∙ Alteplase (tPA) should not be routinely administered to patients on anticoagulation therapy presenting with AIS, except when the patient is taking warfarin and the INR is subtherapeutic (less than or equal to 1.7) (Section 5, Box 5B). There is currently no evidence to support routine reversal of anticoagulation to administer alteplase (tPA) (Clinical Consideration; Section 4.6). EVT may be considered in these patients if they are otherwise eligible for treatment (Clinical Consideration; Section 5.3).
ACUTE ENDOVASCULAR THROMBECTOMY (EVT)
∙ “EVT should be offered within a coordinated system of care,” including EMS, ED, stroke teams, radiology, and local neurointerventional experts. Access to rapid neuroimaging and a stroke unit is critical (Evidence Level A; Section 5.4.i).
∙ EVT is indicated in eligible patients whether or not they are also eligible to receive or have already received alteplase (tPA) (Evidence Level A; Section 5.4.iii).
ο Whenever possible, patients who are eligible for both alteplase (tPA) and EVT should have alteplase (tPA) administered while simultaneously preparing the angiography suite for EVT (Evidence Level A; Section 5.4.iv).
∙ EVT is indicated in patients based upon the following (Evidence Level A; Section 5.4.v/vi):
ο Presentation <6 hours from symptom onset or when last seen normal time in a patient with an anterior circulation large vessel occlusion (Section 5.4.v)
ο Presentation 6-24 hours from symptom onset or when last seen normal time in highly selected patients based on advanced neuroimaging (Section 5.4.vi and Box 4C)
∙ Posterior circulation large vessel occlusions (e.g., basilar artery occlusions) may be EVT eligible in consultation with a stroke expert and under appropriate consideration of the potential risks/benefits (Evidence Level C; Section 5.4.vii).
OUTPATIENT MANAGEMENT OF TIA AND NON-DISABLING MINOR STROKE
(CSBPR, 6th Edition, Sections 2 and 6)
Many patients with TIA or non-disabling minor stroke can be safely managed in an outpatient setting. However, the risk of a recurrent stroke is highest within the first 90 days following a TIA or non-disabling minor stroke event (12% to 20%). The goal of outpatient management is to appropriately risk stratify patients and reduce modifiable cardiovascular risk factors. Early recognition, risk factor management, and referral to stroke specialty clinics have been shown to considerably reduce the risk of recurrent stroke. Two randomized controlled trials have now established a role for dual antiplatelet therapy with aspirin and clopidogrel in the first few weeks following a TIA or minor ischemic stroke.Reference Wang, Wang and Zhao 6 , Reference Johnston, Easton and Farrant 7
RISK STRATIFICATION FOR TIA AND NON-DISABLING MINOR STROKE
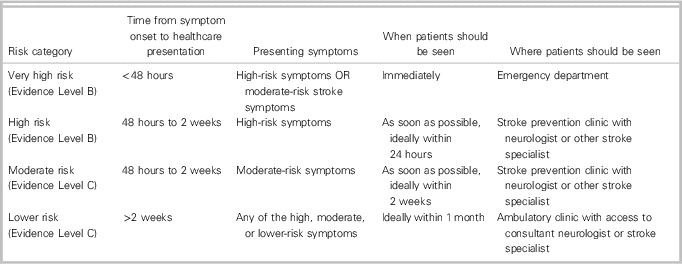
∙ The CSBPR, 6th Ed. outlines a risk stratification approach for a suspected TIA or non-disabling minor stroke based on presenting symptoms and the timing of symptom onset.
∙ Presenting symptoms may be categorized as follows:
ο High-risk symptoms
▪ Transient, fluctuating, or persistent unilateral weakness (face, arm, and/or leg) or
▪ Transient, fluctuating, or persistent language/speech disturbance
ο Moderate-risk symptoms
▪ Fluctuating or persistent symptoms, including hemibody sensory changes, painless monocular vision loss, hemifield vision loss, binocular diplopia, dysarthria, dysphagia, or ataxia without motor weakness or language/speech disturbance
ο Lower-risk symptoms
▪ All other symptoms not outlined in the high-risk or moderate-risk categories
∙ Urgency of initial assessment by a healthcare provider with stroke expertise is determined by overall risk stratification, as summarized in Table 3.
Table 3 Risk stratification levels for suspected TIA and non-disabling minor stroke with initial management recommendations. Adapted from CSBPR, 6th Ed., secondary prevention of stroke Table 2 Reference Boulanger, Lindsay and Gubitz 1 Reference Wein, Lindsay and Cote 8
ANTIPLATELET MANAGEMENT FOR TIA AND NON-DISABLING MINOR STROKE
∙ “All TIA and non-disabling minor stroke patients not already on an antiplatelet agent should be given at least 160 mg of ASA immediately as a one-time loading dose after brain imaging has excluded intracranial hemorrhage” (Evidence Level A; Section 6.i). “ASA (81 to 325 mg daily) should then be continued indefinitely or until an alternative antithrombotic regimen is started” (Evidence Level A; Section 6.ia).
∙ Patients at very high risk with suspected non-cardioembolic origin of stroke should be initiated on a dual-antiplatelet combination of ASA and clopidogrel for a duration of 21 to 30 days followed by antiplatelet monotherapy (such as ASA or clopidogrel alone)4,5 (Evidence Level B; Section 6.ii).
CONCLUSION
This CAEP-endorsed summary of the CSBPR, 6th Ed. was developed as a collaboration between Canadian emergency physicians, stroke neurology experts, and the Heart and Stroke Foundation of Canada. It is meant as a tool for knowledge translation to familiarize practicing clinicians with important updates in the management of an acute stroke or TIA. It also serves as a means to promote the importance of partnership between emergency providers, EMS personnel, diagnostic imaging teams, stroke neurology experts, advocacy groups, non-governmental organizations, and system leaders in the management of acute stroke and TIA.Reference Harris, Lang, Perry and Morrison 9 Finally, it should be emphasized that an effective interdisciplinary approach to stroke care requires engagement from all stakeholders and must be tailored to meet local needs.
Competing interests: None declared.
Acknowledgements and financial disclosures statement: The Canadian Stroke Best Practice Recommendations initiative is developed and updated under the leadership of the Heart and Stroke Foundation of Canada. The development of the Canadian Stroke Best Practice Recommendations is led by and funded in its entirety by the Heart and Stroke Foundation of Canada. No funds for the development of the original guidelines were received from commercial interests, including pharmaceutical or medical device companies. We acknowledge the Heart and Stroke Acute Stroke Management writing group leaders and members who volunteered their time and expertise to update the Canadian Stroke Best Practice Recommendations. Members of the Canadian Stroke Consortium were involved in all aspects of the development of the source recommendations, and members of the Canadian Association of Emergency Physicians (CAEP) were involved in the development of recommendations relevant to their practice. CAEP physicians involved in guideline development included Alix Carter, Crystal Doyle, Charles Duffy, Nadder Sharif, Kevin Lobay, Bilal Mir, Eddy Lang, Jeffrey J. Perry, Anthony Shearing, and Etienne van der Linde.





