Spasticity is a sensory motor control disorder that results from an upper motor neuron lesion and presents as an intermittent or sustained involuntary contraction of muscles. Reference Pandyan, Gregoric and Barnes1 Patients who develop focal spasticity include populations, such as multiple sclerosis, cerebral palsy, spinal cord injury, traumatic brain injury, and stroke, with focal spasticity affecting from 35% to 85% of these populations. Reference Pereira, Richardson, Mehta, Teasell and Miller2 When spasticity is left untreated, rheologic changes occur in the affected muscles, compounded due to immobilization and disuse, leading to contractures. Reference Gracies3 Contracture, in turn, causes pain, limb deformity, and severe limitations in activities of daily living. Reference Gracies3 The financial burden of poststroke patients with spasticity is often cited as four times the medical costs as those without spasticity. Reference Lundström, Smits, Borg and Terént4 Additionally, the 2019 Canadian Stroke Best Practices Recommendations (CSBPR) notes that barriers to funded services for spasticity care can lead to an incomplete recovery. Reference Teasell, Salbach and Foley5
Treatment of spasticity is dependent on presentation, severity, and the goals of the patient and healthcare team. Reference Graham6 The CSBPR states that botulinum toxin A (BoNT-A) is recommended first-line therapy for focal spasticity – a treatment approved by Health Canada. Reference Teasell, Salbach and Foley5,Reference Foley, Pereira and Salter7 BoNT-A injections for focal spasticity are typically administered at 3 to 4 months intervals. Reference Foley, Pereira and Salter7 In addition, the CSBPR recommends an interdisciplinary approach to managing spasticity. Reference Teasell, Salbach and Foley5 Thus, function-oriented therapies and interventions – such as stretching, bracing, and strengthening exercises – are typically prescribed.
Referral for spasticity management is first limited by recognition of the disorder and the need for expert care. BoNT-A access may be limited by individual provincial formularies and by the number of physicians available to administer injections. Adjuvant therapies are also subjected to provincial and private insurance policies. To date, little data exist outlining provincial differences in access to comprehensive focal spasticity care. Therefore, it is unclear whether significant health inequities exist across Canada for patients with spasticity and whether these individuals have “reasonable access to health services without financial or other barriers,” as mandated by the Canada Health Act.
The purpose of this study is to assess the provision of comprehensive focal spasticity treatment across Canada. This cross-sectional study examined two main topics: (1) variations in coverage and accessibility of BoNT-A and (2) differences in availability of non-pharmacological spasticity treatment, including physiotherapy (PT), occupational therapy (OT), and bracing/orthotics care. This survey aims to assist decision-makers and healthcare administrators in establishing a national standard practice guideline for spasticity care.
We surveyed practicing physiatrists who treat focal spasticity in outpatient clinics. Inclusion criteria for recruited physiatrists included a minimum 3-year experience providing focal spasticity care. Potential respondents were identified through three separate methods. First, email contact was made with the department head of every Canadian Physical Medicine and Rehabilitation academic program (n = 13) to request study participation of physicians treating focal spasticity. An additional list of physiatrists was generated from each individual provincial physician directory. Physiatrists from both lists were contacted (n = 73) by telephone to determine whether they fit the study’s inclusion criteria. Additionally, all study participants were asked to identify other physiatrists directly involved in treating patients with focal spasticity. These recommended physicians (n = 12) were also invited to participate in the study.
This study was approved by the Research Ethics Board of the Vancouver Island Health Authority. A 22-question survey (Supplementary Material 1) was developed to assess three domains of focal spasticity treatment: (1) BoNT-A, (2) PT and OT, and (3) orthotic bracing. This questionnaire was subsequently reviewed and approved by a panel of five physicians involved with focal spasticity care. An email including the survey, study protocol, and consent form was sent to all participants.
Forty-eight surveys were distributed to eligible physicians; of these, 34 (71%) were completed and returned. All completed surveys were used for analysis. Completed surveys were defined based on the guidelines of the American Association for Public Opinion Research: if ≥80% of the questions were answered by the respondent.
The respondents were evenly distributed throughout each province by population: Alberta (7), British Columbia (4), Manitoba (3), New Brunswick (2), Newfoundland (1), Nova Scotia (2), Ontario (7), Prince Edward Island (1), Quebec (4), and Saskatchewan (3). Table 1 contains the demographic information.
Table 1: Respondent demographic
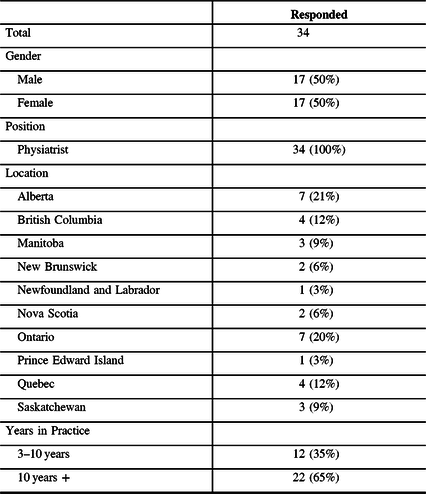
Wait time was classified as the length of time between the initial referral and the first treatment (i.e., BoNT-A injection). The average wait time for an initial outpatient assessment by a physician for focal spasticity was 12.7 weeks (median 12 weeks) and ranged from 3 weeks to 32 weeks between provinces. Wait time variations also existed between centers in the same province, for example, the wait time ranged from 3 weeks to 12 weeks in different cities in Ontario (Table 2).
Table 2: Wait time for initial spasticity treatment by province
All respondents indicated that the delay for spasticity treatment and the length of wait time was primarily due to a lack of physicians able to provide BoNT-A injection.
The second most common contributor to wait time was due to patient financial limitations. Twenty-one out of 34 (62%) individuals with representation from every province claimed that the cost of BoNT-A was a restriction and a deterrent for focal spasticity treatment. Five respondents (15%) indicated that the delay in timely treatment was due to the lack of recognition, thus late referral from the patient’s treating physician. As one respondent explained “spasticity is an often underrecognized disorder in patients with many accompanying issues.” All respondents indicated that there were no delays for patients who required re-injection at the 3–6-month interval.
Drug coverage was inconsistent between provinces for different diseases that cause spasticity and for patients of different age and income brackets. Alberta was the only province that provided regular toxin coverage (not requiring special provincial authorization) to treat all indications (i.e., multiple sclerosis, cerebral palsy, stroke). Canada has no guaranteed Pharmacare program for all, thus those that have coverage under provincial programs vary widely and are dependent on patient demographic factors (such as age and income). All respondents reported no issues with timely access to BoNT-A from their local pharmacy. A detailed review of the requirements for BoNT-A coverage by province is listed in Table 3.
Table 3: Provincial coverage for BoNT-A and adjuvant therapies
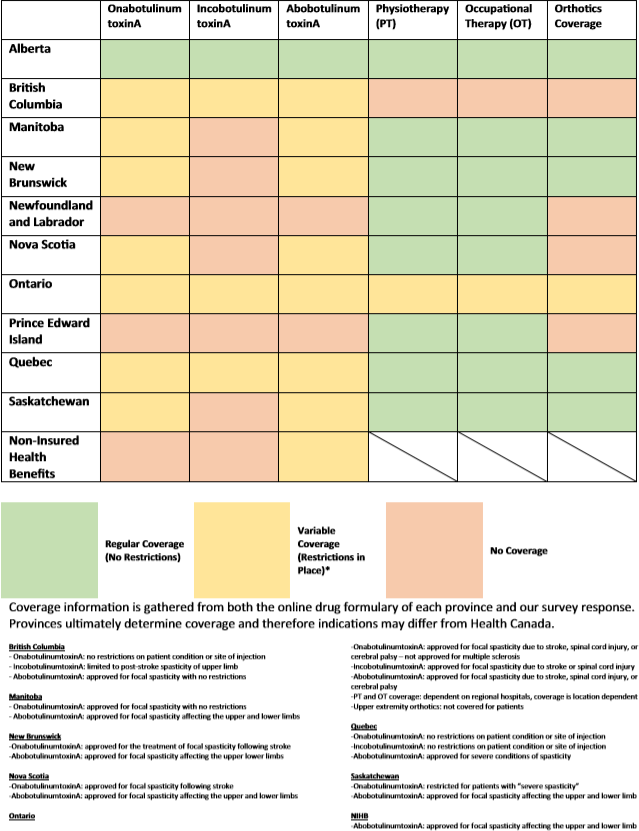
Appropriate and timely adjuvant treatments (PT, OT, and bracing) are only reported as covered in Alberta. Twenty-six out of 34 (76.5%) respondents (including those from Alberta) reported that the lack of coverage prevented patients from obtaining the necessary prescribed orthoses. For PT and OT coverage, 29/34 (85%) respondents indicated that a long wait time was a significant issue for their patients. These results suggest that adjuvant care for focal spasticity remains poor across Canada.
Eleven out of 34 (33%) respondents worked with patients covered under the Non-Insurance Health Benefits (NIHB) program, a coverage plan for (1) First Nations individuals who are registered under the Indian Act, or (2) Inuks recognized by an Inuit land claim organization. These respondents experienced significant delays in obtaining coverage for this patient population. Five respondents cited that this was due to the failure of the NIHB to cover for spasticity medications.
In conclusion, patients with spasticity receive varying levels of care across Canada, given the variations in wait time for BoNT-A injections, coverage, and access to post-injection care. Delays to spasticity treatment may have serious consequences for patients, leading to increased pain and immobility, which contribute to decreased quality of life. Untreated spasticity may lead to the formation of contracture by shortening the affected muscle, resulting in a permanent loss of joint motion. Reference Teasell, Salbach and Foley5,Reference Brainin, Norrving and Sunnerhagen8,Reference Zorowitz, Gillard and Brainin9
Progresses in wait times must also be paired with improvements in the lack of interprovincial agreement concerning coverage for BoNT-A. While BoNT-A is the established standard for focal spasticity care in Canada and abroad, Reference Teasell, Salbach and Foley5,Reference Wissel, Ward and Erztgaard10 significant interprovincial variations in drug coverage programs exist for BoNT-A. Notably, registered First Nations and recognized Inuit may receive inferior spasticity care due to the NIHB program. First Nations patients with spasticity often require extensive paperwork to obtain coverage in some provinces. British Columbia has since moved to covering patients previously with NIHB plans under their provincial formulary.
We acknowledge the limitations that, as an email survey, our results are vulnerable to voluntary response bias. No responses in the territories of Canada were collected as there are no permanent practicing physiatrists in these regions. Our data did not explore issues related to spasticity care from the patients’ perspective nor does it address the delay in the identification of spasticity from the initial presentation to referral for care.
Optimizing treatment for all patients with spasticity has important implications for quality of life and healthcare spending. Spasticity is known to have a significant impact on not only patients but also their caregivers. Reference Zorowitz, Gillard and Brainin9 These improvements must be rooted in not only improved BoNT-A coverage but also better and more equitable access to multidisciplinary, adjuvant care. As noted in our study findings, adjuvant therapy between provinces is variable and may further deteriorate spasticity care.
By comparing the current state of care, access, and coverage across the provinces, our study highlights a need to elevate and equalize the standard of practice to bridge treatment gaps and improve the care and quality of life for patients living with focal spasticity. A national consensus on treatment is necessary to address these gaps to ensure optimal treatment for all Canadians.
Take-Home Points:
Eighty-five percent of patients face barriers to timely physical therapies.
Seventy-six percent of patients face challenges in the funding of orthotic devices.
Financial barriers to BoNT-A lead to delays in treatment.
First Nations covered under Non-Insurance Health Benefits face increased barriers to BoNT-A treatment compared to those on provincial formularies.
Acknowledgments
Dr. Winston and his hospital have unrestricted educational grants and honoraria from Allergan, Merz, and Ipsen. We thank Dr. Theodore Wein for his contribution to the editing of this paper.
Disclosures
Dr. Winston reports grants, personal fees, non-financial support and other from Allergan Canada, grants, personal fees, non-financial support and other from Ipsen Canada, grants, personal fees, non-financial support and other from Merz, outside the submitted work; and Dr. Winston and his hospital have unrestricted educational grants and honours from Allergan, Merz, and Ipsen. All funds are managed by Island Health, and Dr. Winston does not receive financial benefit from these grants.
Statement of Authorship
All authors contributed to the study design and implementation. KL collected the results, performed the analysis, and drafted the manuscript. All authors revised the manuscript and approved the final version.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2020.108.





