In the late 1990s and early 2000s, cases were described of previously healthy young women who presented with a benign ovarian teratoma and subacute limbic encephalitis, which reversed at least partially following teratoma resection and immunosuppression.Reference Taylor, Mason, Kong and Wennberg 1 In 2007, Dalmau et al.Reference Dalmau, Tüzün and Wu 2 coined the term “anti-N-methyl-D-aspartate-receptor (NMDAR) encephalitis” after discovering that these patients had antibodies against the NMDAR. Most patients initially present with a non-specific prodrome of headaches, fever, nausea, and psychiatric symptoms. Many also develop short-term memory impairment, seizures, and movement disorders, often progressing to decreased level of consciousness and autonomic instability requiring intensive care unit admission.Reference Dalmau, Tüzün and Wu 2 - Reference Wang, Li and Hu 4 Poor outcome, usually defined as a modified Rankin Scale of 3-5, occurs in ~10% of patients and death in another 10%. Early identification and treatment of the disorder is thought to be of prime importance in improving outcomes.Reference Wang, Li and Hu 4 , Reference Titulaer, McCraken and Iñigo 5 Aphasia as a sign of anti-NMDAR encephalitis has been poorly characterized and is neither typically thought to be a localizing sign nor a presenting feature of this disorder. We describe a case of anti-NMDAR encephalitis that presented as a progressive non-fluent aphasia.
A 29-year-old English-speaking female with no significant past medical history reported a 6-day history of progressive word-finding difficulties followed by a 3-day history of multiple stereotypic episodes. The latter episodes lasted a few minutes each and were characterized by a prodrome of metallic taste, and a “scratching” sensation in her teeth, which then evolved into right-sided facial twitching, right hand cramping, and right face and arm paresthesiae without loss of consciousness, presumably representing simple partial seizures. She was brought to the emergency department following a first witnessed generalized tonic-clonic seizure.
Initial neurological examination revealed agrammatic non-fluent speech and writing (see Figure 1A). She could name only one of the six objects on the National Institutes of Health (NIH) stroke scale. She had no spontaneous speech and could only provide single-word answers. She made frequent phonemic paraphasic errors (e.g., “tea” for “key”), demonstrated apraxia of speech, articulatory groping, and impaired sentence repetition. She was unable to read any of the NIH stroke scale sentences. She could obey commands, including “take this paper in your hands and fold it in half and put it on the bed”. There was no obvious ideomotor apraxia as she could use her smartphone. The rest of the neurological examination was normal.
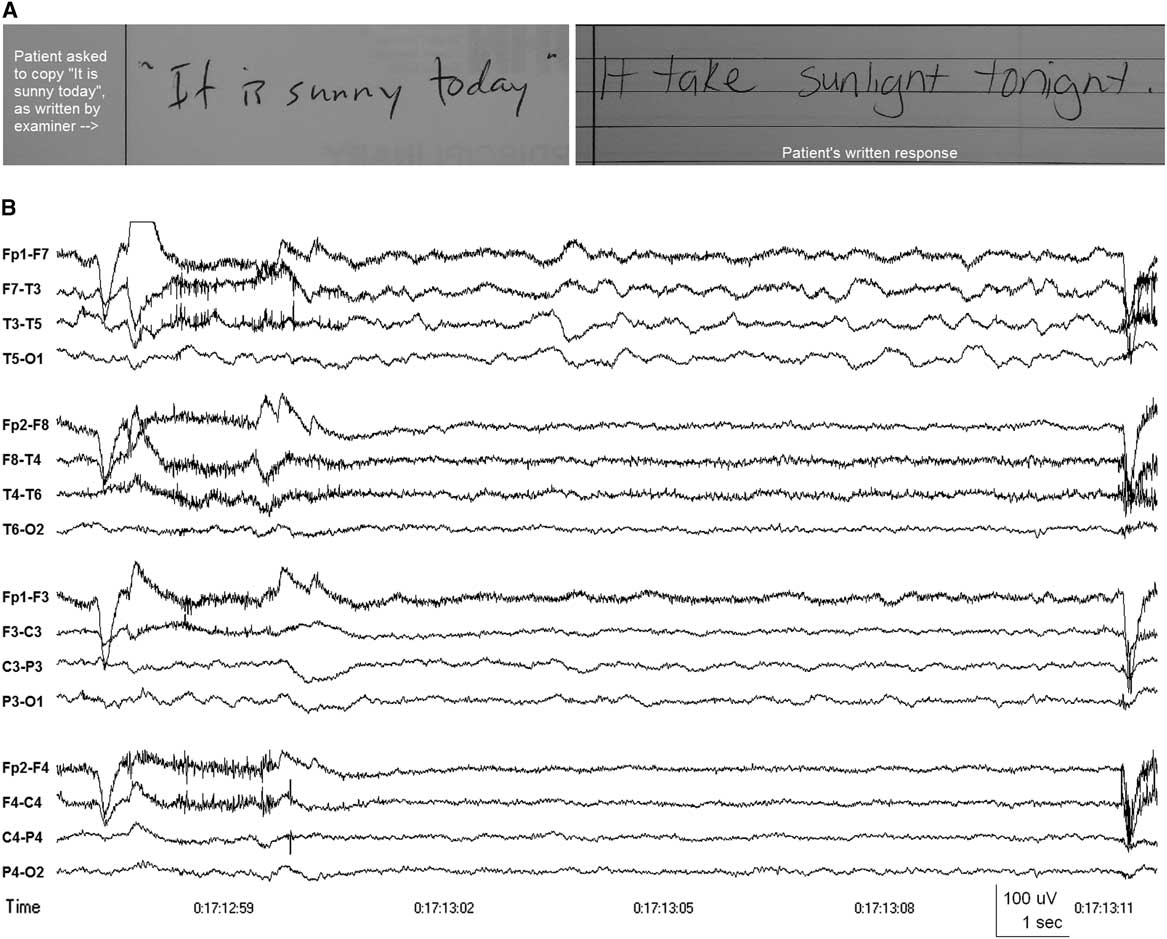
Figure 1 (A) Patient's attempt to copy the written phrase “It is sunny today,” demonstrating multiple phonemic paraphasic errors. (B) EEG showing intermittent slow wave activity in the delta and theta frequency bands over the left frontotemporal region (maximal at T3 or F7-T3), indicating dysfunction affecting the left mid temporal and anterior Sylvian regions. Patient’s eyes were open at the time and she was attempting to write a response to a question, having just been unable to answer the questions verbally. Anterior-posterior longitudinal bipolar montage. Time constant 0.3 sec. High frequency filter 70 Hz.
CT angiogram of the head and MRI of the brain with gadolinium obtained at presentation were normal. CSF analysis was within normal limits (two white blood cells×106/L, 95% lymphocytes, protein 0.20 g/L, glucose 3.7 mmol/L) with normal cytology. Electroencephalography (EEG) showed abundant intermittent polymorphic slow wave activity over the left lateral frontotemporal area (see Figure 1B). She was started on acyclovir initially, which was discontinued after a negative CSF Polymerase Chain Reaction for herpes simplex virus, varicella-zoster virus, human herpesvirus-6, cytomegalovirus, and Epstein-Barr virus. Her generalized and partial seizures were controlled with levetiracetam and carbamazepine, and no seizures were recorded during 48 hours of continuous video-EEG obtained 5 days after presentation to the hospital. Fluctuating sensory symptoms of right-sided numbness (leg more than arm) and a sensation of something “cutting” her were not associated with EEG changes apart from the left frontotemporal slow wave activity. There was no EEG evidence of the extreme delta brush pattern and no definite interictal epileptiform activity was recorded. Her aphasia progressed with increasing phonemic errors and agrammatism to eventual mutism 22 days after symptom onset, by which time she could obey only simple one-step commands. By then, she had also developed prolonged periods of confusion and emotional lability.
Her CSF anti-NMDR NR-1 antibody (Cell-based assay; Mitogen Advanced Diagnostic Laboratory, Calgary, Alberta, Canada) was positive and an MRI of her abdomen revealed a 5.3 cm right adnexal cystic teratoma (confirmed on pathology). She underwent a right oophorectomy 19 days after symptom onset without appreciable clinical improvement postoperatively. Thirty days after symptom onset, she received a 2-day course of 2 mg/kg of intravenous immunoglobulin and there was subsequent gradual improvement of her aphasia. Sixty-three days after symptom onset, she was discharged to a neurocognitive rehabilitation facility on carbamazepine and clobazam. At that time, she could produce short sentences such as “it’s good to see you”, but was still making frequent phonemic errors. Her comprehension was intact.
One hundred and twenty days after symptom onset, she had a normal routine EEG and she was successfully tapered off her anti-epileptic medications. Ten months after symptom onset, formal neurocognitive assessment showed complete resolution of her language impairments, but she did have impaired recollection of the events surrounding her hospitalization.
To our knowledge, this is the first detailed description of adult-onset anti-NMDAR encephalitis presenting primarily as a progressive non-fluent aphasia affecting left frontal and opercular structures. Lesser associated sensory disturbances and focal seizures without loss of awareness (before admission and commencement of antiepileptic medication) were also compatible with a localization to the left opercular region, and EEG provided neurophysiological evidence of dysfunction restricted to the same left frontotemporal area.
A review of the literature revealed only four cases of adult anti-NMDAR encephalitis possibly presenting with aphasia, but described only as “speech disturbance”. Neither a detailed description nor a timeline of symptom progression were provided for those cases, and it is uncertain whether the speech disturbance presented in isolation or with other symptoms.Reference Wang, Li and Hu 4 , Reference Titulaer, McCraken and Iñigo 5 Aphasia has been more commonly described as a symptom that evolves following an initial presentation with psychiatric symptoms or seizure, rather than as the main presenting symptom. Dalmau et al.Reference Dalmau, Gleichman and Hughes 3 found that 6% of patients with anti-NMDAR encephalitis developed “mumbled unintelligible words”, “echolalia”, or “transient aphasia” within 3 weeks of symptom onset. Vahter et al.Reference Vahter, Kannel, Sorro, Jaakmees, Talvik and Gross-Paju 6 described a case of anti-NMDAR encephalitis that developed “sensorimotor aphasia” approximately 1 month after initial presentation with generalized tonic-clonic seizure. In the paediatric literature, Florance et al.Reference Florance, Davis, Lam, Szperka, Zhou and Ahmad 7 reported that, in a case series of 32 children with a median age of 14 years, 17 (53%) developed “reduction of verbal output or mutism, mumbling, or perseveration”, but this was the presenting symptom in only one case. Deiva et al.Reference Kumaran, Pera, Maurey, Chrétien, Archambaud and Bouilleret 8 reported on a 4-year-old female who presented with right partial motor seizures and fever and developed Broca’s aphasia 8 days after symptom onset. Recently, Leon-Salas et al.Reference Leon-Salas, Rosas, Diaz-Victoria, Flores, Ojeda-Lopez and Espinola-Nadurille 9 described a 17-year-old female who suffered a relapse of anti-NMDAR encephalitis with aphasic features that included, similar to our patient, reduced non-fluent spontaneous speech, impaired naming and repetition, and phonemic paraphasias, in addition to orofacial, ideomotor, and constructive apraxias, the cortical aphasia and apraxias developing 15 days after initial presentation with headache, dizziness, nausea, and vomiting (Table 1).
Table 1 Reported cases of anti-N-methyl-D-aspartate receptor encephalitis associated with aphasia
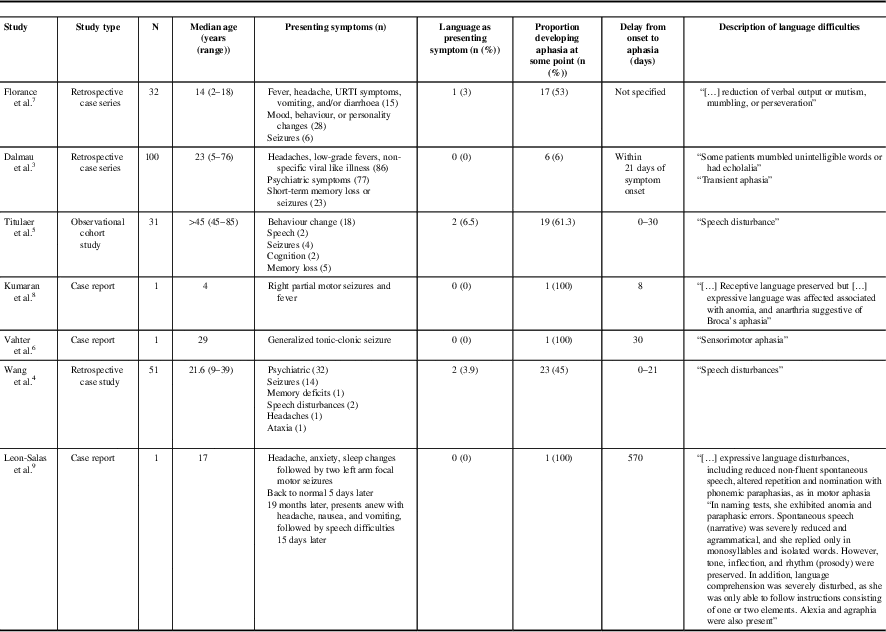
URTI=upper respiratory tract infection.
This study is limited in that it is a single case report, but the case clearly demonstrates that anti-NMDAR encephalitis can present as a focal cortical disturbance, such as a non-fluent aphasia, without associated features of limbic encephalitis. Consideration should therefore be given to including focal cortical deficits as a possible presentation of this autoimmune condition to ensure early identification and treatment of future cases.
Acknowledgement
The authors would like to thank the patient and her family.
Statement of Authorship
Julien Hébert contributed to the drafting of the manuscript. Farah El-Sadi contributed clinical information on the case. Catherine Maurice contributed clinical information on the case and to the critical revision of the manuscript. Richard A. Wennberg contributed clinical information on the case and to the critical revision of the article. David F. Tang-Wai contributed to the critical revision of the manuscript and original conception and design of the manuscript. All authors approved the final version of the manuscript to be published.
Disclosure Information
Julien Hébert, Farah El-Sadi, Catherine Maurice, Richard A. Wennberg, and David F. Tang-Wai have nothing to disclose.
Financial Support
None.
Conflicts of Interest
The authors declare no conflicts of interest.




