The John Cunningham virus (JCV), a common polyomavirus, is known to cause progressive multifocal leukoencephalopathy (PML), an infrequent demyelinating disease of the central nervous system.Reference Major 1 , Reference Ferenczy, Marshall and Nelson 2 The primary infection with JCV is typically clinically unapparent, and the virus resides latent in the urinary tract, bone marrow, and lymphoid tissue.Reference Bag, Cure, Chapman, Robertson and Shah 3 , Reference Tan and Koralnik 4 Development of PML occurs as a result of viral reactivation, dissemination to the central nervous system, and interactions between host and viral factors. Immunosuppression is a frequent host factor, with PML occurring most commonly in patients infected with HIV.Reference Ferenczy, Marshall and Nelson 2 However, immunomodulatory therapies, particularly monoclonal antibodies (e.g. natalizumab, efalizumab, rituximab), also have been associated with the development of PML.Reference Major 1 , Reference Ferenczy, Marshall and Nelson 2
Various assay methodologies have been employed to detect anti-JCV antibodies and thereby confirm exposure to JCV. Use of a validated two-step enzyme-linked immunosorbent assay (ELISA) has consistently detected anti-JCV antibodies in approximately 50% to 60% of multiple sclerosis (MS) patients receiving or considering natalizumab treatment across the United States and European countries.Reference Gorelik, Lerner and Bixler 5 - Reference Warnke, Ramanujam and Plavina 12 Detection of anti-JCV antibodies is a known risk factor for development of PML in natalizumab-treated patients and thus an important aspect of the benefit-risk discussion.Reference Bloomgren, Richman and Hotermans 13
The JEMS (JCV Epidemiology in MS) study was conducted to describe the prevalence of anti-JCV antibodies in a geographically diverse cohort of MS patients and to examine the effects of demographic and disease-related factors on anti-JCV antibody prevalence. This report details the findings from the Canadian cohort of patients participating in JEMS.
Methods
Study Design
The JEMS study was a cross-sectional, multicenter, multinational epidemiological study (NCT01185717).Reference Bozic, Subramanyam, Richman, Plavina, Zhang and Ticho 14 Eligible patients had the ability to understand the purpose of the study, provided informed consent, and had a diagnosis of MS of any type without any restrictions regarding their treatment. The study involved one visit, in which participating sites’ personnel collected patient information (birth date, sex, and race) and asked patients to list all therapies they received for MS treatment, including last dose date and treatment duration. Prior or current immunosuppressant therapy was categorized by any use of mitoxantrone, azathioprine, methotrexate, mycophenolate mofetil, or cyclophosphamide. A single 8.5-ml blood sample was collected, stored, and shipped frozen to Focus Diagnostics (Cypress, CA) to be evaluated using STRATIFY JCV, a two-step anti-JCV antibody ELISA for analysis of anti-JCV antibodies that has been previously described.Reference Gorelik, Lerner and Bixler 5 Briefly, anti-JCV antibodies were captured using JCV-like particles coated on ELISA plates. Bound anti-JCV antibodies were detected spectrophotometrically at 450 nm. Determination of positive or negative anti-JCV antibody status was based on a statistically derived cut-point (normalized optical density [nOD450] values <0.10= anti-JCV antibody negative; nOD450 >0.25= anti-JCV antibody positive).Reference Gorelik, Lerner and Bixler 5 Samples with results in the indeterminate zone of the assay (nOD450=0.10 to 0.25) were repeat-tested in the inhibition assay, in which samples were preincubated with JCV-like particles before evaluation with ELISA. An inhibition >40% confirmed the presence of JCV-specific antibodies.
Study approval was obtained from an ethics committee or institutional review board at each site before the start of the study, and all participating patients signed the informed consent forms.
Statistical Analysis
Patients with anti-JCV antibody test results and demographic data were included in the analyses. Data were collected and analyzed overall for Canada and by region as follows: Quebec (sites in Quebec); Ontario (sites in Ontario); Atlantic (sites in Nova Scotia, New Brunswick, and Newfoundland); and Western (sites in Manitoba, Saskatchewan, Alberta, and British Columbia). Background data were summarized by frequency distribution and/or summary statistics. The prevalence of anti-JCV antibodies was estimated as the number of patients with anti-JCV antibodies detected in serum divided by the total number of patients with a serum sample that was evaluated by the two-step ELISA. The prevalence of anti-JCV antibodies also was described by age, sex, race, region, type of MS, duration of MS, and treatment status using descriptive statistics. Chi-square test and logistic regression model (univariate and multivariate analyses) were used to compare anti-JCV antibody prevalence by geographic and demographic factors, as well as MS type, disease duration, and treatment status.
Results
Patients
A total of 4198 MS patients from 29 sites in Canada had demographic and disease information and serum anti-JCV antibody test results (Table 1). Sites in Ontario and Quebec accounted for the majority of patients (76%). Patient demographics were similar across regions. Approximately 72% of patients were female and the average age was approximately 44 years, with a range of 15 to 83 years. Most patients were white (92.8%).
Table 1 Patients enrolled by region
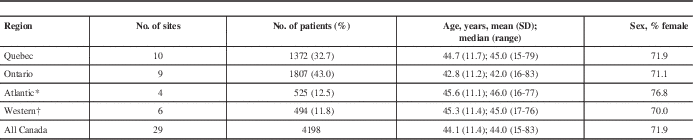
* Comprises sites in Nova Scotia, New Brunswick, and Newfoundland.
† Comprises sites in Manitoba, Saskatchewan, Alberta, and British Columbia.
SD: standard deviation.
Disease characteristics are summarized for all patients in Table 2. The median disease duration was 10 years (range: 0 to 62 years) and relapsing-remitting MS was the most common type (78.6%). Other MS types included clinically isolated syndrome (4.0%), progressive-relapsing MS (1.4%), and progressive MS forms (primary and secondary progressive MS; 16.1%). A small proportion of patients was treatment-naive (16.6%). Of the 83.4% of patients who reported MS treatment, the mean and median treatment durations were 5.5 and 4.6 years, respectively, with a range of 0 to 35.3 years. The use of prior or current immunosuppressant therapy was reported by 5.1% of patients.
Table 2 MS disease and treatment characteristicsFootnote *

* n=4198 unless otherwise noted.
† Includes primary and secondary progressive MS.
CIS: clinically isolated syndrome; MS: multiple sclerosis; SD: standard deviation.
Prevalence of Anti-JCV Antibodies
The overall prevalence rate of anti-JCV antibodies was 56.3% (95% confidence interval [CI]: 54.8% to 57.8%) (Figure 1). Multivariate analysis showed that anti-JCV antibody prevalence was significantly associated, with age (p<0.0001), sex (p<0.0001), and region (p=0.005) as independent factors. Across the four regions of Canada, anti-JCV prevalence rates ranged from 50.6% in the Western region to 59.3% in Quebec, with rates for the Atlantic and Ontario regions being 55.4% and 55.8%, respectively. Anti-JCV antibody prevalence increased with age from 45.0% in patients aged 15 to 29 years to 61.2% for those aged 60 years or older (Figure 2). Females had a lower rate of seropositivity (54.3%) than males (61.5%) (Figure 2). Rates of anti-JCV antibody prevalence varied by race, but because of a predominance of white patients, no significant difference was observed (Figure 2).

Figure 1 Anti-JCV antibody prevalence overall and by region; p value comparing seroprevalence across all regions was determined by logistic regression model adjusted for age, sex, and immunosuppressant use. The number of patients enrolled overall and for each region is listed in the column and vertical bars represent 95% confidence intervals. Quebec: sites in Quebec; Ontario: sites in Ontario; Atlantic: sites in Nova Scotia, New Brunswick, and Newfoundland; Western: sites in Manitoba, Saskatchewan, Alberta, and British Columbia. JCV=John Cunningham virus.

Figure 2 Anti-JCV antibody prevalence by patient demographics. *p value comparing seroprevalence across all age categories and all race categories was determined by chi-square test. **p value comparing seroprevalence between female and male patients was determined by logistic regression model adjusted for age. The number of patients in each group is listed in the column, and vertical bars represent 95% confidence intervals. JCV=John Cunningham virus.
Anti-JCV antibody prevalence rates increased with increasing MS disease duration, but the differences were not significant after adjusting for age (p=0.928; Figure 3). There was also no significant difference in anti-JCV antibody prevalence rates by MS disease type after adjusting for age, sex, and region (p=0.296; Figure 3). The number of prior and current MS therapies did not significantly influence anti-JCV antibody seroprevalence, with rates ranging from 54.6% to 58.7% (p=0.463; Figure 4). However, there was a significant association between the use of immunosuppressant therapy and anti-JCV antibody prevalence rate after adjusting for age, sex, and region (p=0.040). Anti-JCV antibodies were detected in 63.4% of patients who reported use of immunosuppressant therapy compared with 55.9% of patients who reported no use of immunosuppressants (Figure 4). The length of time that patients had received MS treatment did not significantly affect anti-JCV antibody prevalence, with rates ranging from 54.5% to 58.8% (p=0.316; Figure 4).

Figure 3 Anti-JCV antibody prevalence by MS disease characteristics. **p value comparing seroprevalence across all MS duration categories was determined by logistic regression model adjusted for age. ***p value comparing seroprevalence across all MS type categories was determined by logistic regression model adjusted for age, sex, and region. The number of patients in each group is listed in the column, and vertical bars represent 95% confidence intervals. Relapsing MS includes relapsing-remitting MS and progressive-relapsing MS; progressive MS includes primary and secondary progressive MS. CIS: clinically isolated syndrome. JCV=John Cunningham virus; MS=multiple sclerosis.

Figure 4 Anti-JCV antibody prevalence by treatment history. *p value comparing seroprevalence across all categories for the number of prior and current MS therapies and across all treatment duration categories was determined by chi-square test. ***p value comparing seroprevalence between patients with and without IS use was determined by logistic regression model adjusted for age, sex, and region. The number of patients in each group is listed in the column and vertical bars represent 95% confidence intervals. JCV=John Cunningham virus; IS=immunosuppressant.
Discussion
The Canadian cohort of MS patients participating in the JEMS study had an overall anti-JCV antibody seroprevalence of 56.3%. These results are consistent with previous reports of anti-JCV antibody seroprevalence rates in MS patients receiving or considering treatment with natalizumab.Reference Gorelik, Lerner and Bixler 5 - Reference Warnke, Ramanujam and Plavina 12 Across these reports, anti-JCV antibodies were detected in approximately 50% to 60% of patients using two-step ELISA. Several other findings from the Canadian MS patients were similar to other studies. The prevalence of anti-JCV antibodies was significantly affected by age and sex, with higher prevalence rates in older patients and males. Seroprevalence rates were significantly different across the four regions of Canada, with the lowest in the Western provinces and the highest in Quebec; however, all rates fell within the reported range of approximately 50% to 60%. Each of these three factors (age, sex, and region) was found to be significantly associated with anti-JCV antibody prevalence in multivariate analyses adjusting for the other factors. The JEMS study included patients with all types of MS irrespective of treatment, but no significant independent associations were observed between anti-JCV antibody status and MS disease duration, MS type, number of prior and current MS therapies, or MS treatment duration in Canadian MS patients.
Anti-JCV antibodies were detected at rates that were similar to the results of studies that included only MS patients receiving natalizumab.Reference Trampe, Hemmelmann and Stroet 8 - Reference Warnke, Ramanujam and Plavina 12 These findings suggest that natalizumab exposure does not influence seroprevalence rates of anti-JCV antibodies, but controlled studies are needed to confirm these data. The effect of immunosuppressant therapy is less clear. In this report, the prevalence of anti-JCV antibodies was significantly lower in patients who had not received immunosuppressant therapy compared with patients who had received immunosuppressants after adjusting for age, sex, and region. The overall population of JEMS (n=7724), which included MS patients from Europe, Australia, and Canada, showed an absolute difference in anti-JCV antibody prevalence based on immunosuppressant use of 7.1%, but it was not statistically significant (p=0.091).Reference Bozic, Subramanyam, Richman, Plavina, Zhang and Ticho 14 In a recent study, which enrolled 10,280 patients from eight European countries and Israel, anti-JCV antibody prevalence was consistently lower in patients who reported no immunosuppressant use, but the difference for the overall population as well as individual countries, except Israel (p=0.0129), was not significant.Reference Olsson, Achiron and Alfredsson 11 In a report by Trampe et al., 2253 German MS patients who received natalizumab were tested for anti-JCV antibodies, and no effect of prior immunomodulatory or immunosuppressive treatment was observed on the rate of anti-JCV antibody seropositivity.Reference Trampe, Hemmelmann and Stroet 8
Assessment of anti-JCV antibody status is an important part of benefit-risk discussions with MS patients. The risk of PML is low in patients who are negative for anti-JCV antibodies compared with those who are positive,Reference Bloomgren, Richman and Hotermans 13 and as of November 2013, 189 of 191 PML patients (99%) tested anti-JCV antibody–positive using the two-step ELISA at least 6 months before diagnosis. 15 Additional data have indicated that the level (or index) of anti-JCV antibodies may also contribute to risk stratification, suggesting that even among anti-JCV antibody–positive patients the risk of PML is not the same.Reference Plavina, Subramanyam and Bloomgren 16
The Canadian cohort of JEMS was representative of the general MS population.Reference Goodin 17 , Reference Young 18 However, this study was limited by the cross-sectional design and the small sample size from some regions, resulting in its inability to detect ethnic differences in anti-JCV prevalence. To demonstrate this would require larger trials, and prospective studies are under way to characterize the longitudinal stability of anti-JCV antibody seroprevalence.
In conclusion, anti-JCV antibodies were detected in 56.3% of MS patients from Canada. The prevalence of anti-JCV antibodies was significantly affected by age, sex, and region. These findings are consistent with previous reports using the two-step anti-JCV antibody ELISA in MS patients. The use of immunosuppressant therapy was associated with a significantly higher rate of seropositivity, whereas MS disease characteristics did not significantly influence anti-JCV antibody prevalence.
Acknowledgments and Funding
This study was funded by Biogen Idec Inc. Editorial support (funded by Biogen Idec Inc.) was provided by Michelle McDermott, PharmD, a freelance medical writer, and Ed Parr, PhD, of Excel Scientific Solutions (Southport, CT, USA); these contributions did not influence the content of the manuscript.
Disclosures
VB has the following disclosures: Biogen Idec, advisor, honoraria; EMD Serono, advisor, honoraria; Genzyme, advisor, honoraria; Novartis, advisor, honoraria; Teva, advisor, honoraria.
PD has the following disclosures: Biogen Idec, research support, consultant, honoraria; EMD Serono, research support, consultant, honoraria; Novartis, research support, consultant; Genzyme, research support, consultant, honoraria; Teva Neuroscience, consultant, honoraria; MS Society of Canada, grants (2), research support; CIHR, grant, research support.
MSF has the following disclosures: Bayer HealthCare, research/educational support; Genzyme, research/educational support; Biogen Idec, advisor, honoraria; EMD Serono, advisor, honoraria; Novartis, advisor, honoraria; Teva, advisor, honoraria.
YL has the following disclosures: EMD Serono, advisor, speaker, honoraria; Novartis, advisor, speaker, honoraria; Biogen Idec, speaker; Teva, editorial board member, honoraria.
VM has the following disclosures: Biogen Idec Canada, employee; MigMed Clinical Research Services, consultant.
DS has the following disclosures: Teva, advisor, honoraria, speaker fees; Merck Serono, advisor, honoraria, speaker fees; Genzyme, advisor, honoraria, speaker fees; Novartis, advisor, honoraria, speaker fees; Biogen Idec, advisor, honoraria, speaker fees; Bristol-Myers Squibb, advisor, honoraria; Boehringer Ingelheim, advisor, honoraria.
LW has the following disclosures: Biogen Idec Canada, employee.
AZ has the following disclosures: Biogen Idec, employee.








