Botulism was first described in victims of food poisoning who developed a rapidly progressive descending paralysis, and has since been developed into a therapeutic agent. Reference Ney and Joseph1 The mechanism of action stems from an anaerobic bacterium, Clostridium botulinum, that secretes various serotypes of botulinum neurotoxin. Reference Ney and Joseph1 Botulinum Toxin A (BoNT-A) has many clinical indications including movement disorders, spasticity, migraines, pain syndromes, hyperhidrosis, and cosmetics. Reference Ney and Joseph1 BoNT-A is considered to be generally safe with reported side effects including injection site focal weakness, cough, fever, malaise, headache, and injection site reactions. Reference Ney and Joseph1 A review of the literature reveals rare reports of generalized weakness and iatrogenic botulism following therapeutic use of BoNT-A for dystonia, Reference Bhatia, Münchau and Thompson2,Reference Crowner, Torres-Russotto and Carter3 hyperhidrosis, Reference Rouientan, Otaghvar, Mahmoudvand and Tizmaghz4 spasticity, Reference Crowner, Torres-Russotto and Carter3,Reference Bakheit, Ward and McLellan5 and movement disorders. Reference Coban, Matur and Hanagasi6 We present a case of probable iatrogenic botulism following administration of BoNT-A for hyperhidrosis that is uniquely confounded by recent fluoroquinolone antibiotic use (ciprofloxacin), which also has documented action on the neuromuscular junction. Reference Jones, Sorbello and Boucher7
A 31-year-old female was referred for assessment of hand weakness after her second trial of BoNT-A intradermal injection for hyperhidrosis. Her only comorbidities were a remote lumbar spine fusion and a recently treated urinary tract infection with ciprofloxacin. She did not have any personal or family history of neuromuscular disease. She first had a Botulinum A (Onabotulinum) injection 2 months prior to presentation, where she received 50U in each axilla (total of 100U). Two weeks prior to presentation, she had a second treatment that involved 100U of Onabotulinum A into each of her palms (2:1 dilution) and 50U into each axilla (1:1 dilution). The injections were administered in a grid pattern by a physician with extensive experience and skill using BoNT-A. She noticed symptoms 5 days following her second round of injections, which included fatigue, dry mouth, blurred vision, mild headache, and bilateral hand weakness. Her initial exam was significant for mild neck flexion weakness, fatigable deltoid weakness, and bilateral hand weakness (finger extensors 4+/5, first dorsal interossei (FDI) 4/5, abductor pollicis brevis (APB) 4−/5). Her electrophysiologic studies demonstrated decreased amplitude of the right median and ulnar nerves on motor studies (Table 1). Repetitive nerve stimulation at 2Hz (right median to APB) showed maximal decrement following maximal voluntary contraction (MVC), with maximal decrement 4 min post-MVC. Her repetitive nerve stimulation at 50Hz (right median to APB) showed facilitation of 181% (Figure 1). Electromyography (EMG) demonstrated small motor (MUAPs) suggestive of myopathic units in FDI, APB, and the deltoid. These studies are suggestive of a presynaptic neuromuscular junction localization and given her medical history, iatrogenic botulism was considered. At her 3-month follow-up, her exam had improved as she now had only mild FDI and APB weakness. Her electrophysiologic studies improved as well, with only a mildly low ulnar motor amplitude, no facilitation on rapid RNS, less decrement on slow RNS, and improved appearance of myopathic units on EMG in the hand intrinsic muscles. By 9 months, her physical exam and electrophysiology were completely normal. No other adverse events were reported among other patients who used this particular batch of Onabotulinum A.
Table 1: Motor nerve conduction studies demonstrating low amplitudes in the right median and ulnar nerves without evidence of facilitation at 10 s due to lack of strength for significant maximal contraction
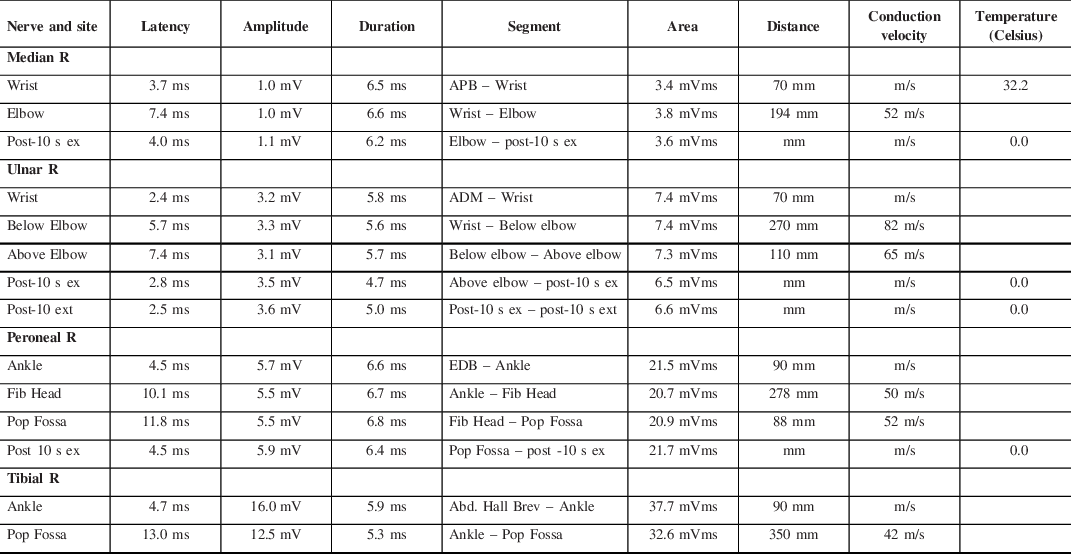

Figure 1: 50Hz repetitive stimulation waveforms demonstrating significant facilitation (181%).
Botulism is clinically characterized by symmetric descending paralysis that is often associated with dilated pupils, dry mouth, diplopia, ptosis, dysphagia, dysarthria, and respiratory failure. Reference Rouientan, Otaghvar, Mahmoudvand and Tizmaghz4 Our patient demonstrated symptoms consistent with this syndrome, including visual disturbances, weakness, and dry mouth. Electrophysiology in botulism classically shows a reduced compound muscle action potential (CMAP) amplitude that increases with high-frequency repetitive nerve stimulation demonstrating facilitation, and decrement at low-frequency repetitive nerve stimulation, which was seen in our case. Reference Bhatia, Münchau and Thompson2 Botulinum neurotoxin binds to the SV2 receptor on the presynaptic membrane of the neuromuscular junction, and then interferes with exocytosis of acetylcholine, which results in reduced acetylcholine released into the junction and therefore reduced muscle activation. Reference Ney and Joseph1 Fluoroquinolone antibiotics also act on the neuromuscular junction by chelating ionized calcium, and therefore inhibiting acetylcholine release presynaptically and may also act directly on acetylcholine receptors as well. Reference Jones, Sorbello and Boucher7 To our knowledge, this combination of drugs has not been previously reported to cause neuromuscular junction issues, however, it is likely that both contributed to our patient’s presentation.
The mechanism for the involvement of sites distant from the injection of BoNT-A is controversial, with main theories including hematogenous spread, retrograde axonal transport of the toxin, or an immune-mediated phenomenon. Reference Bakheit, Ward and McLellan5 Single-fiber EMG done at sites distant from the injection site have been shown to have jitter and blocking, demonstrating a presynaptic process, even in the absence of clinical weakness or abnormal routine electrophysiologic testing. Reference Lange, Brin and Warner8 It seems that higher doses of BoNT-A and repeated use may be risk factors for the dissemination of BoNT-A after local injection. Reference Bhatia, Münchau and Thompson2–Reference Coban, Matur and Hanagasi6 It is important for clinicians to be aware of the neuromuscular complications of commonly used medications including antibiotics and botulinum toxin.
Acknowledgments
The authors would like to thank Dr. Donna Jubin MD CCFP for her expertise and assistance with this project.
Conflicts of Interest
None of the authors have any conflicts of interest to disclose.
Statement of Authorship
RV was responsible for manuscript writing and literature review.
KS was responsible for project oversight, clinical guidance and discussion, and manuscript editing.




