Introduction
Traumatic brain injury (TBI) is the leading cause of death and disability among individuals younger than 45 years old in the USA. Reference Nguyen, Fiest and McChesney1 A recent meta-analysis found a worldwide TBI incidence of 349 cases per 100,000 across all ages. Reference Nguyen, Fiest and McChesney1 This number is likely to be an underestimate given that mild TBI is often unreported by both patients and physicians. Reference Rusnak2 However, even mild TBI can lead to neuropsychiatric illness, disability, and significant economic burden. Reference Selassie, Zaloshnja, Langlois, Miller, Jones and Steiner3
In TBI, a primary injury, including direct damage to the tissues and blood vessels, occurs immediately following trauma. TBI is classified as mild, moderate, or severe depending mostly on the clinical status and Glasgow Coma Scale following the primary injury and on the presence or absence of structural abnormalities on clinical brain imaging. While severe TBI causes direct damage to brain tissue, the precise neurobiology of mild to moderate TBI is still poorly understood. A long-standing hypothesis is that multiple neurotransmission systems including serotonergic, dopaminergic, noradrenergic, and cholinergic systems are perturbed after an event. Reference Osier and Dixon4 – Reference Kosari-Nasab, Shokouhi, Azarfarin, BannazadehAmirkhiz, Mesgari Abbasi and Salari9 The evidence supporting this hypothesis mostly comes from mild to moderate TBI models in rodents Reference Saija, Hayes, Lyeth, Edward Dixon, Yamamoto and Robinson10 – Reference Dunn-Meynell, Hassanain and Levin13 ; however, multiple other lines of evidence link neurotransmission system disturbances with mild to moderate TBI. For example, patients with Chronic Traumatic Encephalopathy (CTE), a neurodegenerative disease that has been associated with repetitive mild TBI, often present with similar neuropsychiatric and psychiatric symptoms to those with TBI. Reference Iverson, Keene, Perry and Castellani14 Interestingly, CTE neuropathology was recently shown to involve, in addition to cortical areas, isodendritic core nuclei such as the locus ceruleus, dorsal raphe nuclei, nucleus basalis of meynert, and substantia nigra, which are, respectively, the major outputs of adrenergic, serotonergic, cholinergic, and dopaminergic neurotransmitters in the brain. Reference Schwab and Hazrati15 , Reference McKee, Cairns and Dickson16 This suggests that these centers are also probably altered after a mild TBI episode and that the ensuing neuroinflammation leads to degeneration after repeated insults. These same perturbations in neurotransmission are themselves known to be central to the neurobiology of many neuropsychiatric and psychiatric disorders which often arise after TBI, suggesting that they may underlie neuropsychiatric and psychiatric symptoms generally, whether in the context of TBI or not. Indeed, the pharmacotherapy for psychiatric illness post-TBI is almost identical to that of psychiatric illness without TBI and has a similar efficacy, especially in the case of selective serotonin reuptake inhibitors for depression, lithium and valproic acid for bipolar illness, venlafaxine for Generalized Anxiety Disorder, and olanzapine for psychosis. Reference Warden and Gordon17 Moreover, multiple cellular and molecular pathological mechanisms appear to be shared between these disorders and TBI. A combination of inflammation, mitochondrial dysfunction, free radical release, and glutamate excitotoxicity have been shown to induce neuronal damage and are suggested to explain patient clinical deterioration following the initial disruption in neurotransmission systems. Reference Park, Bell and Baker18 These processes are hypothesized to contribute not only to the immediate post-TBI symptoms in the first 12 weeks but also to post-concussion syndromes. Reference Broshek, De Marco and Freeman19 , Reference Blennow, Brody and Kochanek20 Thus, there is a need for pharmacotherapies that can halt the neuropathological cascades leading to neurodegeneration in addition to alleviating symptoms. Microglial-mediated neuroinflammation in particular was shown in the last decade to be an interesting common pathological mechanism implicated in TBI, as well as neuropsychiatric and psychiatric disorders. Reference Simon, McGeachy, Bayir, Clark, Loane and Kochanek21 – Reference Hong, Kim and Im24 This has led researchers to test anti-inflammatory molecules in the treatment of TBI symptoms and associated illnesses Reference Bergold25 and to re-visit the anti-inflammatory properties of known treatments such as cannabis. Reference Zurier and Burstein26 , Reference Katz, Sulzer, Impastato, Teng, Rogers and Molina27
Current pharmacotherapies to mitigate the various symptoms of TBI include autonomic system modulators (clonidine and propranolol for sympathetic hyperactivity and agitation in moderate to severe TBI), antidepressants, opioids, and agents to alleviate neuropathic pain. Reference De Guzman and Ament28 , Reference Abou El Fadl and O’Phelan29 The numerous unwanted side effects, and the complexity of this pharmacotherapy in neurocognitively impaired patients, can in turn lead to reduced compliance and severe dependence issues (especially with opioids and benzodiazepines). Thus, there is a need for a milder, more comprehensive pharmacotherapy to alleviate TBI symptoms. Given its analgesic, anxiolytic, antiemetic, and anti-inflammatory properties, cannabis may be an effective treatment for TBI. However, there are to this date no studies assessing the pharmacotherapeutic potential of cannabis in TBI.
Cannabis Neurobiology
The psychoactive compound of exogenous cannabinoid, tetrahydrocannabinol (THC) Reference Devane, Hanus and Breuer30 and the main non-psychoactive compound cannabidiol (CBD), in addition to endocannabinoids such as N-arachidonoylethanolamine (anandamide) and 2-arachidonoylglycerol, bind to receptors CB1 and CB2 in the central nervous system (CNS). Reference Reggio31 CB1 receptors are predominantly found in areas of the brain such as the cerebral cortex, hippocampus, and basal ganglia. Reference Herkenham, Lynn, Johnson, Melvin, de Costa and Rice32 While the activation of CB1 receptors in these areas produces the psychomimetic effects of cannabis in the brain, Reference Schurman and Lichtman33 the regional and molecular specificity of these compounds is still poorly understood. Cannabis has been shown to reduce inflammation and neuronal damage following TBI Reference Mechoulam, Panikashvili and Shohami34 by inhibiting tumor necrosis factor-α (TNF-α)-mediated neurotoxicity in the brain. Reference Shohami, Gallily, Mechoulam, Bass and Ben-Hur35 More precisely, CBD was shown to both inhibit TNF-α and increase the production of anandamide, another anti-inflammatory endocannabinoid. In addition, some studies have shown that THC is up to 80 times more potent than aspirin in reducing acute inflammation in specific rodent models of acute inflammation and that CBD is also effective in murine models of arthritis. Reference Zurier and Burstein26 While many more studies will be required to truly understand the effects of the myriad of pharmacologically active cannabinoid compounds in the brain, the recent legalization of cannabis in Canada will surely accelerate research on cannabis and shed light on both its benefits and side effects in the context of TBI.
Cannabis and Psychiatric Disorders
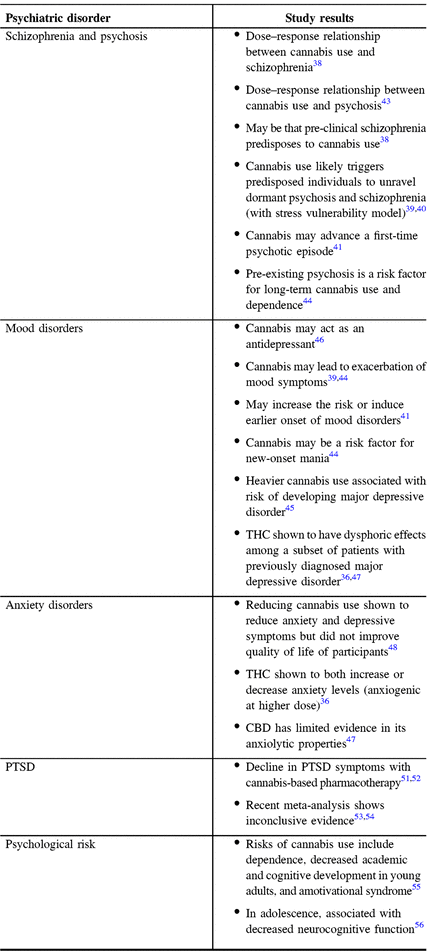
A large part of the concern with cannabis use lies in the associated risk of drug-induced psychosis and schizophrenia. Reference Turna, Patterson and Van Ameringen36 , Reference Degenhardt and Hall37 The first robust critical study to suggest an association between mental health disorder and cannabis use was the famous 15-year cohort study by Andreasson et al., which followed 45,570 Swedish male conscripts from the time of conscription to follow-up. Reference Andreasson, Allebeck and Rydberg38 They found a dose–response relationship between cannabis use at the time of conscription (9.4% of men based on questionnaire) and subsequent development of schizophrenia. While the authors controlled for multiple confounders including alcohol and tobacco use, as well as psychiatric diagnoses at conscription, they acknowledged that their study is only correlative. Thus, they could not exclude that pre-clinical schizophrenia might increase cannabis use, a matter that is still debated today. They concluded that cannabis use probably triggers predisposed individuals to unravel dormant psychosis and schizophrenia in accordance with the stress vulnerability model, which was confirmed in later studies. Reference Hamilton39 , Reference Arseneault, Cannon, Poulton, Murray, Caspi and Moffitt40 Indeed, cannabis use seems to advance time to a first psychotic episode by 2–6 years. Reference Di Forti, Sallis and Allegri41 While Andreasson’s study prompted many questions pertaining to the adverse effects of cannabis use, most experts advise cautious interpretation of this study, as the results may be confounded by biases, including over/under-reporting, and the compounding effects of other drugs. Reference Zammit, Allebeck, Andreasson, Lundberg and Lewis42
Otherwise, high-quality studies of cannabis-related pharmacotherapy in psychiatric disorders have been sparse, but highlight potential adverse outcomes. A recent meta-analysis demonstrated a dose–response relationship between level of cannabis use and psychosis, where higher risk of developing psychiatric illness was seen with heavy cannabis use. Reference Marconi, Di Forti, Lewis, Murray and Vassos43 Given that pre-existing psychosis was also shown to be a risk factor for long-term cannabis use and dependence, Reference Gibbs, Winsper, Marwaha, Gilbert, Broome and Singh44 cannabis pharmacotherapy poses a risk for users to enter a vicious cycle of cannabis use and mental health degradation, at least in susceptible individuals. Other concerns include exacerbation of current psychiatric symptoms and increased risk for or earlier onset of mood disorders. Reference Hamilton39 , Reference Di Forti, Sallis and Allegri41 , Reference Gibbs, Winsper, Marwaha, Gilbert, Broome and Singh44 Some studies suggested that cannabis use may be a risk factor for new onset of manic symptoms and that it may exacerbate manic symptoms in known bipolar disorder. Reference Gibbs, Winsper, Marwaha, Gilbert, Broome and Singh44 A systematic review and meta-analysis also found that heavier cannabis use is associated with a higher risk of developing depressive disorder. Reference Lev-Ran, Roerecke, Le Foll, George, McKenzie and Rehm45 Indeed, despite cannabis’ reported euphoric effect and antidepressant properties, Reference Gruber, Pope and Brown46 one of its active compounds, THC, lead to dysphoric effects among a subset of patients with a previously diagnosed depression. Reference Turna, Patterson and Van Ameringen36 , Reference Karniol, Shirakawa, Kasinski, Pfeferman and Carlini47 This emphasizes the need to further study the various active compounds of cannabis in the setting of psychiatric disease, as they might have opposing effects. Finally, according to a study by Hser and colleagues, a decrease in cannabis use was shown to reduce anxiety and depression symptoms within a trial period of 12 weeks. Reference Hser, Mooney and Huang48 However, these changes had no measurable effect on patient quality of life.
Currently, there is a paucity of high-quality evidence for cannabis use in treating anxiety disorders both in the general population and in patients with TBI. However, the current available literature suggests that THC and CBD, again, seem to have differential effects. On one hand, THC was shown to potentially both increase and decrease anxiety levels depending on dosage (anxiogenic at higher doses) in otherwise healthy adults without any other psychiatric comorbidities, Reference Turna, Patterson and Van Ameringen36 whereas CBD has some limited evidence supporting anxiolytic properties in attenuating the anxiogenic effects of THC. Reference Ablon and Goodwin49 , Reference Zuardi, Shirakawa, Finkelfarb and Karniol50 At this time, we can only speculate that this effect on healthy populations may also apply to patients with TBI.
As for cannabis use in post-traumatic stress disorder (PTSD), a retrospective study by Greer and colleagues showed a decline in PTSD symptoms with cannabis-based pharmacotherapy in a group of patients from the New Mexico Medical Cannabis Program. Reference Greer, Grob and Halberstadt51 This with similar studies led to hopeful claims that cannabis may be beneficial in the context of PTSD. Reference Mizrachi Zer-Aviv, Segev and Akirav52 A recent meta-analysis and literature review showed that the evidence is still insufficient to make such conclusions, but that ongoing studies should soon answer this question. Reference O’Neil, Nugent and Morasco53 , Reference Steenkamp, Blessing, Galatzer-Levy, Hollahan and Anderson54
Aside from the previously mentioned association with psychiatric disorders, the psychological risks of cannabis use include dependence, decreased academic, and cognitive development in young adults, as well as amotivational syndrome. 55 These are not to be taken lightly and are particularly important considerations for teenagers and young adults, in whom the brain is still developing. Indeed, earlier introduction of cannabis during adolescent years has been associated with decreased neurocognitive function. Reference Fontes, Bolla and Cunha56 Taken together, current evidence has amounted to a consensus, where the risk of harm seems to outweigh the potential benefits of cannabis use for psychiatric disorders. Reference Volkow, Baler, Compton and Weiss57 These risks are important enough to caution against cannabis use for other indications. According to Canadian Medical Association, cannabis has some potential benefit in patients with end-stage or chronic disease when other medical interventions have failed, but the psychiatric risks associated with the medical use of cannabis require further study. 55 Importantly, interactions with other medications, side effects, potency with regards to levels of THC and CBD, and safe therapeutic dosages need to be clarified. Thus, public health-based education campaigns and patient education will be paramount to limit the potential adverse impacts of cannabis use and guide its appropriate use in the setting of psychiatric disorders, particularly until additional research is conducted. More stringent pharmaceutical regulations will also have to be implemented in order to provide optimal medical care to these patients; the recent legalization and control of cannabis products by governmental entities should facilitate this safeguarding. 55
Cannabis, Psychiatric Disorders and TBI
As mentioned earlier, the imbalance of neurotransmitters seen in animal models of mild to moderate TBI, and the subcortical nuclei neurodegeneration seen in post-mortem studies in patients with a history of mild to moderate TBI, together suggests that neurotransmission systems are dysfunctional following mild to moderate TBI. Reference Osier and Dixon4 – Reference Massucci, Kline, Ma, Zafonte and Dixon11 , Reference Iverson, Keene, Perry and Castellani14 – Reference McKee, Cairns and Dickson16 This in part might explain the increased risk of developing psychiatric sequelae such as depression, anxiety, and suicidal ideation after TBI. Reference Anstey, Butterworth, Jorm, Christensen, Rodgers and Windsor58 – Reference Guskiewicz, Mihalik and Shankar60 Silver and colleagues showed that in addition to exacerbated depression, patients that have experienced mild TBI also undergo a decline in cognitive function. Reference Silver, McAllister and Arciniegas61 Moreover, a survey study of 30 years follow-up post-TBI showed an approximately two-fold increase in life-time prevalence of psychiatric disorders in TBI patients compared to the general Finnish population. Reference Koponen, Taiminen and Portin62 These included major depression, panic disorder, specific phobia, psychotic disorders; and avoidant, paranoid, and schizoid personality disorders. Reference Koponen, Taiminen and Portin62
To our knowledge, there have been no case–controls of cohort studies specifically exploring the relationship between cannabis use and psychiatric disorders in the post-TBI setting. However, a cross-sectional study and a few case reports have indicated associations, as follows. Multiple studies have suggested that both adolescents and adults have an increased risk of future substance use, including cannabis, post-TBI. Reference Kennedy, Heron and Munafo63 – Reference Ilie, Mann and Hamilton67 Studies have suggested that this may be partly related to impairments in executive function resulting from a TBI. Reference Walker, Cole, Logan and Corrigan64 – Reference Cicerone, Levin, Malec, Stuss and Whyte68 Indeed, all four domains of executive dysfunction (executive cognitive function, behavioral self-regulatory function, activating regulating functions, and metacognitive process) can be directly disrupted by a focal lesion in severe TBI but also by secondary neuronal damage in all types of TBI. Reference Cicerone, Levin, Malec, Stuss and Whyte68 An interesting cross-sectional study by Walker and colleagues analyzed the clinical records and questionnaires of 7784 patients with a history of substance abuse treatment, which included history of psychiatric illnesses and TBI. Reference Walker, Cole, Logan and Corrigan64 The authors found that the frequency of TBI correlated with subsequent length of drug abuse and incidence of psychiatric illnesses. It is however unclear which of TBI or substance abuse would have been more contributive to executive dysfunction in these scenarios. Moreover, variables such as the accuracy of self-reported results and the temporal relation with use must be considered when interpreting such studies.
More anecdotally, a case report by Payne and colleagues indicated that moderate use of cannabis in a post-TBI patient with co-morbid depression worsened the depressive symptoms. The patient’s mood initially improved with cannabis discontinuation post-TBI and subsequently deteriorated after the re-introduction of cannabis. Reference Payne69 Additional evidence of four separate young adults with a history of TBI suggests that cannabis use might increase the risk of developing psychosis in this setting, even in the absence of a positive family history for schizophrenia. Reference Rabner, Gottlieb, Lazdowsky and LeBel70 – Reference Jain72 Jain and colleagues discussed the case of an 18-year-old male who had experienced TBI at the age of three and began using cannabis for a period of 3 years starting in his adolescent years, after which he developed symptoms of psychosis. Reference Kennedy, Heron and Munafo63 Rabner et al. evaluated two adolescents with a prior history of TBI and cannabis use who self-reported developing psychosis later on. Reference Rabner, Gottlieb, Lazdowsky and LeBel70 Gonzalez and colleagues carried out a 3-year follow-up study of an adolescent who was diagnosed with TBI with psychotic symptoms as well as dependence on cannabis. It was noted that the use of cannabis during that period had worsened the patient’s symptoms of psychosis. Reference Soler, Muller, Trautlein and Donovan71 While these studies remain anecdotal, they portray a clinical picture where a clinician can hardly ignore the risks that cannabis use poses to the patient’s psychiatric condition and highlight how unlikely it is that cannabis could be safely proposed as a therapeutic option for teenagers with TBI history.
Another specific population of interest for the study of cannabis use post-TBI is war veterans, who are also at increased risk of developing trauma and stress-related disorders such as PTSD secondary to blast and explosions. Reference Wilson73 This might represent an ideal population in which to test cannabis for post-TBI psychiatric disorders as the individuals are generally older and less prone to develop psychosis and new-onset schizophrenia, one of the most feared side effects of cannabis use. As previously mentioned, while no clear consensus can be made on the utility of cannabis use in PTSD as of now, Reference Steenkamp, Blessing, Galatzer-Levy, Hollahan and Anderson54 multiple studies with veterans are ongoing and their secondary analysis might represent the first robust clinical investigations for cannabis use post-TBI. Reference O’Neil, Nugent and Morasco53
Taken together, these studies indicate that cannabis use seems to have similar associations with psychiatric disorders in TBI and non-TBI settings. Therefore, once clear guidelines for cannabis use can be devised for non-TBI-associated psychiatric illnesses, they should also be applicable to TBI patients. Moreover, given that pharmacotherapy for psychiatric disorders post-TBI is parallel to the treatment of psychiatric illnesses following TBI, Reference Warden and Gordon17 it would be hard to argue a completely differential practice for cannabis.
Our Experience
The head injury clinic at St. Michael’s Hospital in Toronto, Canada sees approximately 2000 patients per year. Our clinical impression is that a large percentage of these patients utilize cannabis for a multitude of reasons including neurosensory (pain, headache) and neuropsychiatric (depression, anxiety, sleep) issues post-TBI. Although this remains anecdotal evidence, some patients self-report that cannabis has helped them to become more focused when faced with multitasking situations. An ongoing study at our brain injury clinic (prior to cannabis legalization in Canada) also surveyed 1145 post-concussion patients who were experiencing persistent symptoms such as disruptions in cognition, behavior, and physical and emotional health. The questionnaire inquired about self-medication with cannabis for persistent post-concussion symptoms, as well as adverse childhood events such as psychological or physical abuse. The particular form of cannabis was not indicated in the questionnaire and therefore included all forms (smoked/vaped or medication). It was noted that patients with a history of negative childhood experiences were more likely to use cannabis for symptom management. This suggests that cannabis use in post-TBI patients might be more a testament to prior psychiatric history and anxiety self-medication than a means to alleviate TBI symptoms per se. Also taken into consideration was that these adverse childhood events may have influenced greater risk-taking behavior, drug abuse, and risk of concussion as an adult. Reference Hunt, Guo and Michalak74
There are no clinical studies found in the literature proposing cannabis as a treatment for attention deficit and hyperactivity disorder (ADHD). A recent study suggested that ADHD patients seem prone to cannabis use and those who use it are in turn more susceptible to other psychiatric disorders associated with cannabis use. This was determined using the Alcohol Use Disorder and Associated Disabilities Interview Schedule which included items assessing depression, psychosis, and schizophrenia. Reference Brandt, Rehm and Lev-Ran75 However, as with other association studies, this must be interpreted with caution as external factors such as other substance use and genetic predisposition to developing psychiatric disorders could influence the results. Reference Fergusson and Boden76 Altogether, the associations between cannabis and attentional disorders with or without TBI should be further investigated to clarify the risks and benefits of cannabis use in this setting, which we aim to do in future studies.
Additionally, we have found that the stigma surrounding cannabis use is a psychosocial issue commonly discussed at office visits. Because of this stigma, many patients hesitate to inquire about using cannabis as a potential treatment for psychiatric issues post-TBI. With the recent legalization of cannabis for recreational use in Canada, the most common question asked by patients in our practice concerns the existence of evidence-based cannabis treatment for both acute and chronic symptoms of TBI. Thus, we are providing a review and discussion of the current literature to help clinicians guide TBI patients with psychoeducation about cannabis use. In our opinions, clinicians should advise their patients of both the risks and benefits of cannabis use for treating psychiatric disorders as summarized in Table 1, but be careful in suggesting even modest use.
Table 1: Summary of effects of cannabis on psychiatric disorders based on the studies referred above
Post-TBI patient might also require advice and information on cannabis use for chronic pain. This review is not directly aimed at this question, but we reference here numerous reviews and a meta-analysis on the subject that can help guide clinicians. Reference Nugent, Morasco and O’Neil77 – Reference Mucke, Phillips, Radbruch, Petzke and Hauser79 Overall, the conclusion of these studies is that, presently, harms might outweigh benefits in the setting of chronic pain as well. Moreover, while there has been much recent excitement over the use of cannabis for chronic and neuropathic pain, further studies are required to elucidate its precise role, and clinicians should remain alert for psychiatric side effects. This is not to detract from the great promise cannabis might hold in treating chronic and neuropathic pain, especially given the dire consequences of opioid abuse and its inefficacy in treating neuropathic pain. We however recommend that patients willing to use cannabis to alleviate chronic or neuropathic pain be referred to a specialized chronic pain clinic. Importantly, the primary clinician or psychiatrist should continue to follow and inquire about possible psychiatric side effects secondary to cannabis use.
Conclusion
By providing our experience and a review on the topic, this paper aims to establish a guide that clinicians can use to more accurately aid patients in understanding the role of cannabis in managing the neurocognitive and neuropsychiatric sequelae of their brain injury. Evidence suggests that TBI is associated with psychiatric sequelae, which can present many years post-TBI. The post-TBI changes in neurobiology of the CNS parallel those found in psychiatric disorders. Due to the paucity of literature evaluating cannabis use in TBI, we have also summarized evidence of cannabis use among non-brain injured patients, which shows that cannabis can play a role in psychosis, depression, PTSD, and substance use disorders. However, our clinical experience and the literature suggest that cannabis use might have the same benefits and harms with TBI patients as with non-TBI patients. Limited data on the use of cannabis in TBI leaves the clinician with minimal guidance on providing high level evidence to patients with TBI, but should not deter clinicians from discussing the subject. We hope this manuscript can provide a foundation to guide these discussions. We feel that with the legalization of cannabis in Canada, patients will move away from their concerns of the stigma of “substance” use and begin to inquire more about cannabis use.
In concluding, we hope that clinicians can utilize this paper to advise TBI patients with the following information: (A) One useful mechanism of cannabis can be attributed to its anti-inflammatory properties, which are important for both neuropsychiatric and neurosensory TBI sequelae. (B) Cannabis, beyond becoming a substance that can be misused, can increase your risk of developing depression, psychosis, less possibly mania, and can both increase or decrease anxiety among patients without TBI. These risks are especially important in young adults and teenagers. (C) While it is not currently possible to apply to TBI patients’ non-TBI clinical data on cannabis use for psychiatric disorders, we feel that the benefits and harms are likely similar in both groups. In post-TBI patients, cannabis use may result in increased substance use, or psychosis, while withdrawal from cannabis may lead to improved mood in depressed patients. (D) Cannabis use in the treatment of chronic or neuropathic pain post-TBI should be discussed by a multidisciplinary team, ideally in a specialized chronic pain clinic. (E) The best ratio of benefits to harms for cannabis use for post-TBI psychiatric disorders might be in the setting of PTSD. However, further evidence will be required to solidify these claims given the high likelihood of other psychiatric co-morbidities in this context.
Acknowledgements
The authors would like to thank St. Michael’s Hospital Head Injury Clinic.
Conflicts of Interest
No conflicts of interest.
Statement of Authorship
The authors KG, FP, DL, SB, and RL were all involved reviewing literature, writing parts of the manuscript, and editing the final version of the manuscript for submission.



