Background
Subthalamic nucleus deep brain stimulator (STN–DBS) insertion has become one of the standard forms of treatment for patients with advanced Parkinson’s disease (PD).Reference Limousin, Krack and Pollak 1 Clinical outcome after DBS insertion depends on careful patient selection and precise target localization. Common methods for target localization include brain imaging with a stereotactic headframe and intraoperative neurophysiological mapping with microelectrode recording (MER) and macrostimulation.Reference Houeto, Welter and Bejjani 2 Due to the effects of anesthetic agents on MER and the need for an awake patient for stimulation testing, local anesthesia (LA) with or without sedation has been the standard anesthetic technique for DBS insertion at many centers.Reference Houeto, Welter and Bejjani 2 , Reference Maltête, Navarro and Welter 3 However, some patients with claustrophobia or those with severe symptoms may not be able to tolerate this procedure under LA with sedation and often need general anesthesia (GA).Reference Lin, Chen and Lin 4 In addition, with advances in imaging, both target localization and placement of DBS electrodes have also been performed under GA using intraoperative magnetic resonance imaging (iMRI) guidance without the need for neurophysiological testing.Reference Starr, Martin, Ostrem, Talke, Levesque and Larson 5 - Reference Chabardes, Isnard and Castrioto 7 Though many studies have reported successful clinical outcomes with DBS insertion under GA, the reported outcome measures are quite heterogeneous and difficult to compare with the procedures done under LA.Reference Lin, Chen and Lin 4 , Reference Hertel, Züchner and Weimar 8 - Reference Fluchere, Witjas and Eusebio 11 Currently, there are limited data in the literature comparing clinical outcomes after DBS insertion under GA and LA.
The objective of this systematic review and metaanalysis was to determine whether or not the clinical outcomes following STN–DBS insertion under GA are comparable to those under LA in patients with Parkinson’s disease. The outcome measures included postoperative improvement in symptoms, accuracy of target placement, and procedure-related adverse events.
Methods
This systematic review and metaanalysis was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses—the PRISMA Statement.Reference Moher, Liberati, Tetzlaff and Altman 12 The PRISMA checklist is available as electronic supplementary material (presented as a table that illustrates the PRISMA checklist; see Supplemental Digital Content 1).
Search Strategy
The databases of Medline (Ovid) (1946 to week 2 of January of 2016), Medline In-Process, and other nonindexed citations (Ovid) (22 January 2016), Embase (Ovid) (1947 to 22 January 2016), the Cochrane Central Register of Controlled Trials (CENTRAL) (December of 2015), the Cochrane Database of Systematic Reviews (CDSR) in the Cochrane Library (2005 to 2nd quarter of 2015), and PubMed (not Medline) (1945 to 24 January 2016) were searched by a professional librarian, and the procedure was reviewed by two independent authors (VS and JM). Searches were conducted using two different components: (1) deep brain stimulation and anesthesia, and (2) Parkinson’s disease, effectiveness, or prognosis. The keywords used in the Medline and other searches included deep brain stimulation, electrical stimulation therapy, implantable neurostimulators, prostheses and implants, electrodes, anesthesia, conscious sedation, analog sedation, anesthetics or sedatives, isoflurane, sevoflurane, desflurane, propofol, fentanyl, midazolam, dexmedetomidine, Lewy body disease, essential tremors, Parkinson’s disease, paralysis agitans, experimental epidemiological studies, mortality, morbidity, randomized controlled trials, cohort studies, longitudinal studies, double blind, effectiveness, and prognosis. The more detailed search strategy is available as electronic supplementary material (presented as a table that illustrates the search strategy; see Supplemental Digital Content 2).
A search of trial registries and a manual search of the reference lists from the selected articles were conducted to identify additional trials. The authors of eligible studies were contacted by email for missing data. The search was restricted to the English language and only human studies.
Study Eligibility
The search results were evaluated by two independent reviewers (VS, JM), and studies eligible for probable inclusion were identified using predefined selection criteria. Any disagreements between authors were resolved via discussion or in consultation with the senior author (LV). Our inclusion criteria included studies comparing the clinical outcome of bilateral STN–DBS insertion under GA and LA with or without sedation in patients with PD. Our exclusion criteria included: (1) DBS insertion for movement disorders other than PD; (2) studies where reported outcomes were not separated by type of anesthesia; (3) case reports and review articles; (4) noncomparative studies (i.e., studies with only one group—GA or LA); and (5) studies comparing sedation versus no sedation.
Study Selection and Methodological Quality Assessment
In the first phase of the review, discernibly irrelevant articles were excluded from the search results by reviewing the title and abstracts. In the next phase, the full-text articles were evaluated to determine whether they met the eligibility criteria. Studies eligible for the qualitative review were selected and further evaluated for quantitative analysis. The studies eligible for quantitative analysis with similar outcome measures were pooled for the metaanalysis. The follow-up periods for the primary outcome measure ranged from 3 to 12 months.
Two independent reviewers (VS, NCR) assessed the quality of the studies, and any disagreements between them were resolved by discussion with the senior author (LV). Quality assessment of the studies was performed using the Newcastle–Ottawa Scale.Reference Wells, Shea and O’Connell 13 This tool has been used for assessing the quality of cohort or nonrandomized studies included in systematic reviews and/or metaanalyses. Each study is judged on eight items categorized into three groups: (1) selection of study groups, (2) comparability of study groups, and (3) ascertainment of the outcome of interest. One star is awarded for each quality item, four stars constitute the maximum for selection of groups, two stars the maximum for comparability, and three stars the maximum for ascertainment of outcome. The highest-quality studies are awarded a maximum of nine stars.
Data Extraction and Synthesis
Data extraction was performed by two reviewers and validated by the senior author. The extracted data included study design, sample size, characteristics of the study population, preoperative condition of the patient, dose of levodopa, method used for target planning, intraoperative localization and final electrode position, microelectrode recording (MER), stimulation intensity, details of anesthetic management, operative time, and, finally, outcome measures from individual studies.
Outcome Measures
The primary outcome of our study was to assess postoperative improvement in terms of symptoms, evaluated using either Unified Parkinson’s Disease Rating Scale (UPDRS) scores or levodopa equivalent daily dosage (LEDD) requirements. The UPDRS is a widely used four-part measure of impairment and disability associated with PD: Part I—nonmotor experiences of daily living; Part II—motor experiences of daily living; Part III—motor examination; and Part IV—motor complications.Reference Goetz, Tilley and Shaftman 14 It has 65 items, with a possible total of 260 points. Higher scores indicate greater disability (260 represents worst disability and 0 no disability). The postoperative UPDRS part III scores with DBS on/off and medication on/off states were compared with the baseline medication-off scores from the preoperative period.Reference Vitek, Lyons and Bakay 15 The LEDD in mg/day was calculated for each antiparkinsonian medication by multiplying the total daily dosage of each drug by its potency relative to a standard levodopa/decarboxylase inhibitor (DCI) preparation (has a value of 1).Reference Yamada, Gotoa and Kuratsua 16 The reduction in postoperative requirements suggested a good clinical outcome. The other outcome measures were accuracy in target placement of the DBS electrode and adverse events associated with the procedure.
Data Analysis
The effect sizes were expressed either as estimates of the odds ratio (OR) for dichotomous variables or as the standard mean difference (SMD) for continuous variables. When necessary, the standard deviation (SD) was estimated based on the reported 95% confidence interval (CI 95%) limits, standard error, or range values. Data from the selected studies were combined to estimate the pooled effect of GA and LA. For each outcome measure, pooled estimates and 95% confidence intervals were calculated by using an inverse variance random-effects approach. A random-effects model was utilized in all analyses because of the few eligible studies and the smaller sample sizes.Reference DerSimonian and Laird 17 Heterogeneity was measured using I 2 statistics.Reference Higgins, Thompson, Deeks and Altman 18 Sensitivity analyses were performed if deemed necessary to explain the heterogeneity. We did not test for publication bias or small-study effects due to the small number of studies included in this analysis. A value of p<0.05 was considered statistically significant. Metaanalyses were performed using Review Manager (RevMan, v. 5.3, The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, 2014).
Results
Literature Search and Baseline Characteristics of Included Studies
The initial search strategy yielded 395 citations (Figure 1). After screening the titles and abstracts, 365 studies that did not meet the eligibility criteria were excluded. The remaining 30 studies were included for full-text review. From this, 24 studies were excluded for the following reasons: only one group (GA or LA) with no control (14), compared sedation with no sedation (7), and nonrelevant outcome measures (3). In the end, six studies were considered for qualitative review and metaanalysis. All the included studies were retrospective cohort studies. Study demographics and baseline disease characteristics are given in Table 1. The total number of subjects in our study was 455 (194 in the GA group and 261 in the LA group).
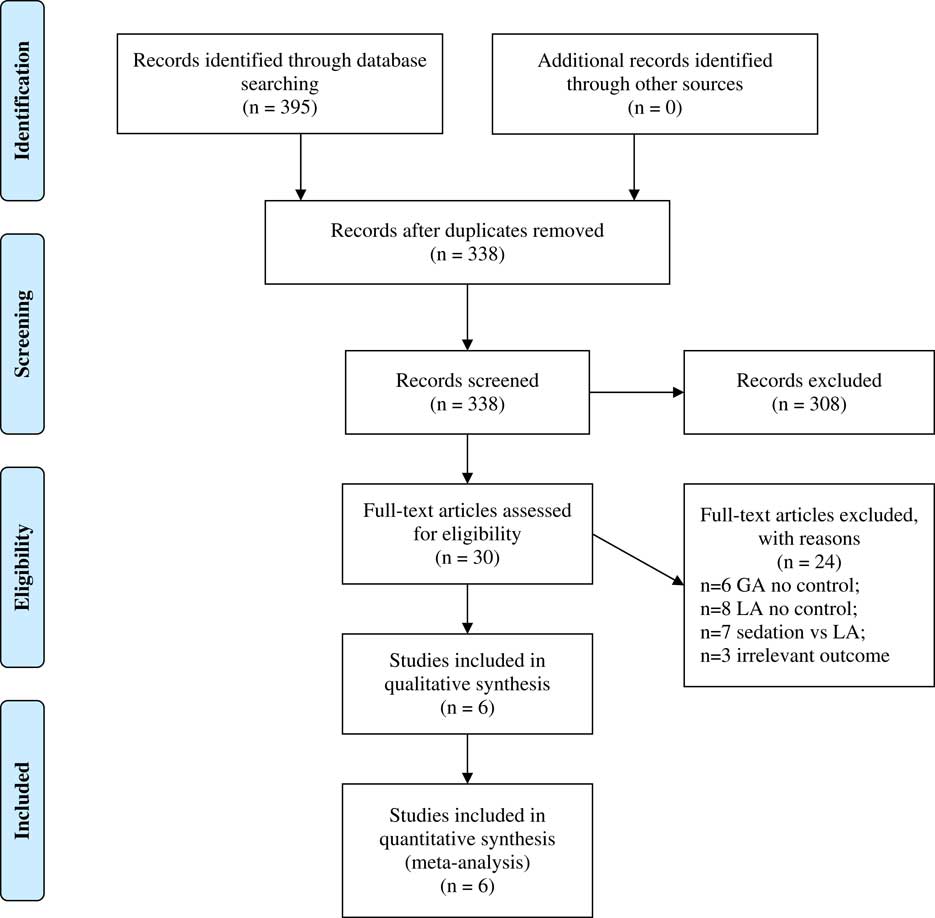
Figure 1 PRISMA 2009 flow diagram.
Table 1 Study demographics and baseline disease characteristics
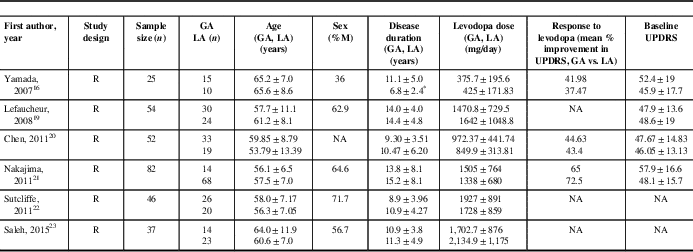
Values are presented as means±standard deviations.
* p<0.05.
NA=not available; R=retrospective; UPDRS=Unified Parkinson’s Disease Rating Scale.
Quality Assessment
All the included studies had at least seven stars on the Newcastle–Ottawa Scale. A summary of the included studies is presented in Table 2. Detailed quality-assessment analysis is available as electronic supplementary material (in the form of a table that illustrates detailed assessment using the Newcastle–Ottawa Scale; see Supplementary Digital Content 3).
Table 2 Assessment of the quality of the included studies using the Newcastle–Ottawa Scale
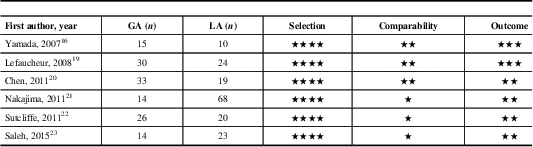
One star is awarded for each quality item. Four stars are the maximum for selection of groups, two stars the maximum for comparability, and three stars the maximum for ascertainment of the outcome.
Anesthesia
The details of the anesthetic regimen and the drugs used are given in Table 3. In the GA group, the details on anesthetics were reported in four studies, consisting of intravenous induction and endotracheal intubation followed by maintenance of anesthesia with an inhalational agent. In the LA group, two studies reported procedural sedation, in one study propofol was used for brief period, and moderate sedation was employed during most of the other studies.
Table 3 Details of anesthesia

ETT=endotracheal tube; MAC=minimum alveolar concentration; NA=not available.
Target Planning and Localization
MRI was used for preoperative target planning in all of the studies, and, in addition, ventriculography was utilized in two studies.Reference Yamada, Gotoa and Kuratsua 16 , Reference Lefaucheur, Gurruchaga and Pollin 19 Intraoperative target localization was either by an image-guided technique, as performed by Nakajima et al.Reference Nakajima, Zrinzo and Foltynie 21 and Saleh et al.Reference Saleh, Swanson, Lake and Sillay 23 (GA group), or combined imaging (either based on preoperative MRI or direct intraoperative imaging) and MER technique. General anesthesia did not affect the MER and localization of STN, and the MER was successful in all patients in four studies.Reference Yamada, Gotoa and Kuratsua 16 , Reference Lefaucheur, Gurruchaga and Pollin 19 , Reference Chen, Tsai and Lin 20 , Reference Sutcliffe, Mitchell, Gan, Mocroft and Nightingale 22 Intraoperative macrostimulation was performed in all studies from the LA group. Three studies reported postoperative confirmation of electrode location with either computerized tomography (CT) or MRI.Reference Chen, Tsai and Lin 20 , Reference Nakajima, Zrinzo and Foltynie 21 , Reference Saleh, Swanson, Lake and Sillay 23 Postoperative stimulation parameters were not significantly different between groups in four studies.Reference Yamada, Gotoa and Kuratsua 16 , Reference Lefaucheur, Gurruchaga and Pollin 19 - Reference Nakajima, Zrinzo and Foltynie 21
Clinical Outcomes
The primary outcome measures included in this metaanalysis were UPDRS scoreReference Yamada, Gotoa and Kuratsua 16 , Reference Chen, Tsai and Lin 20 , Reference Nakajima, Zrinzo and Foltynie 21 and LEDD requirement.Reference Yamada, Gotoa and Kuratsua 16 , Reference Chen, Tsai and Lin 20 - Reference Saleh, Swanson, Lake and Sillay 23 Though another studyReference Lefaucheur, Gurruchaga and Pollin 19 also reported UDPRS score and LEDD requirement as outcome measures, the data were reported as percentage reduction instead of absolute values, and it was thus not possible to include this study in the pooled analysis. Adverse events included in the metaanalysis were those related to general surgical and neurological events, as well as stimulation-induced and hardware-related side effects.
Postoperative UDPRS III scores after DBS insertion under GA were not statistically different compared to the procedure done under LA (p=0.09, SMD=0.32, CI 95%=–0.05 to 0.68). There was no heterogeneity among the studies (I 2=0%, τ2=0, χ2=0.41) (Figure 2).
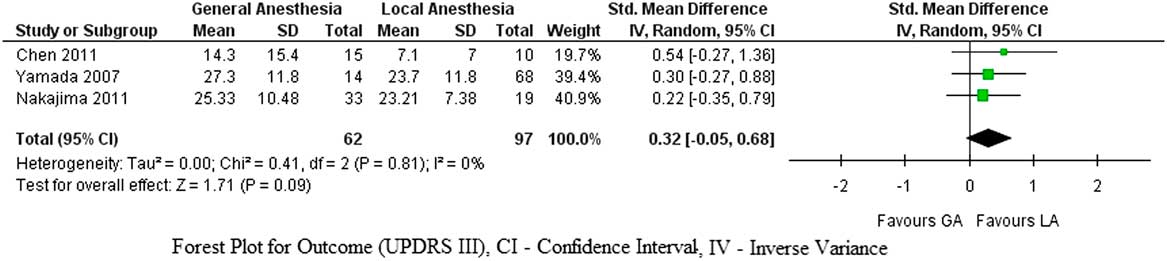
Figure 2 Forest plot for UPDRS III score improvement.
Similarly, no significant differences were found in postoperative LEDD requirements between DBS insertion under GA and LA (p=0.84, SMD=0.03, CI 95%=–0.26 to 0.32), and there was no significant heterogeneity among the studies (I 2=5%, τ2=0.01, χ2=4.20) (Figure 3).
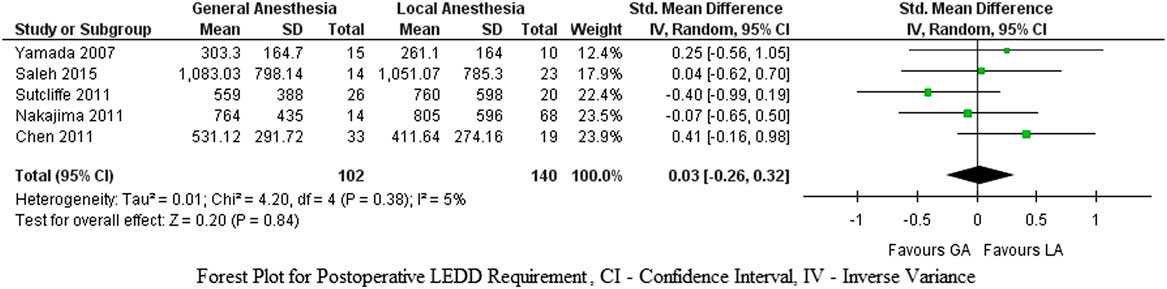
Figure 3 Forest plot for postoperative LEDD requirement.
With regards to the incidence of adverse events, there were no differences between the groups (pooled OR=1.12, CI 95%=0.66 to 1.91, p=0.67, Z=0.43). Figure 4 depicts the metaanalysis of the adverse events subgroups (stimulation-induced, general surgical, neurological complications, and hardware-related). Accuracy of target electrode placement was reported in four studies, where it was similar in both groups.Reference Yamada, Gotoa and Kuratsua 16 , Reference Lefaucheur, Gurruchaga and Pollin 19 - Reference Nakajima, Zrinzo and Foltynie 21
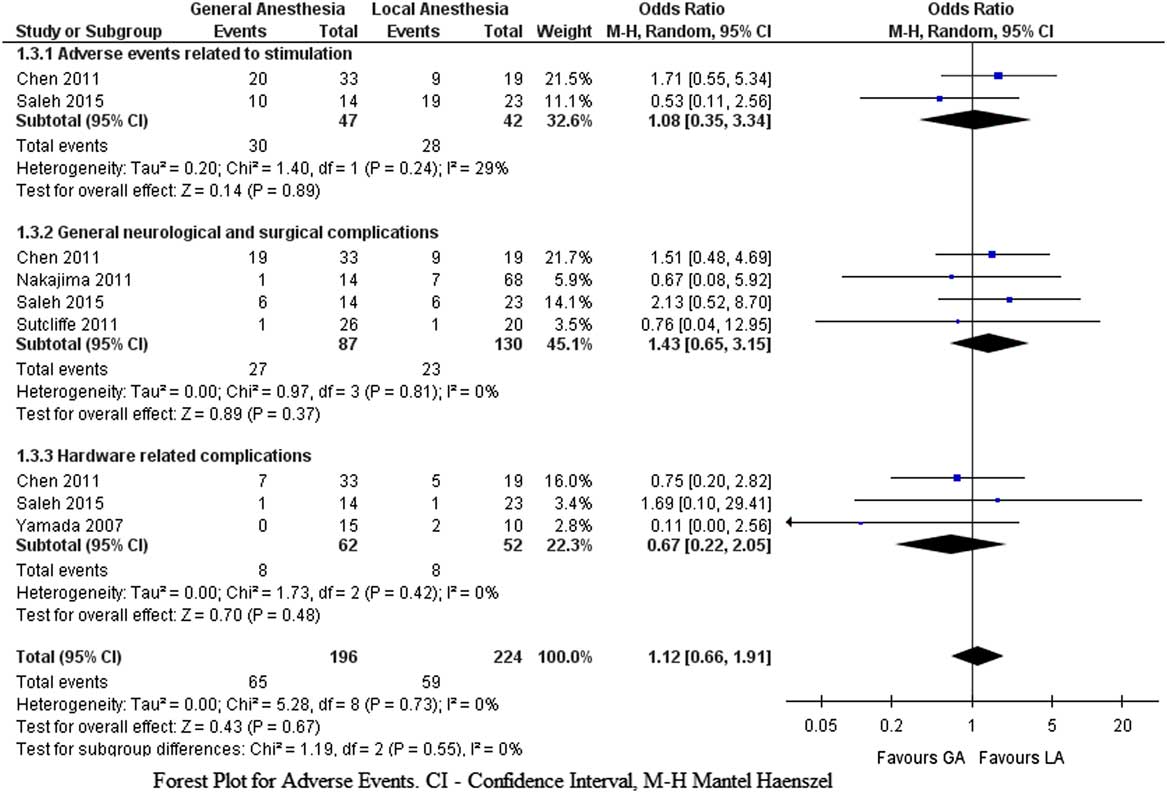
Figure 4 Forest plot for adverse events.
Discussion
This systematic review and metaanalysis has shown that in patients with PD the clinical outcomes and adverse effects after STN–DBS insertions performed under GA were similar to those performed under LA. There was no significant difference in targeting accuracy between groups. In addition, successful localization of the target nuclei was feasible under GA using intraoperative MER. However, all the studies included in the metaanalysis were retrospective cohort studies with small numbers in each group. Currently, there are only poor-quality data suggesting a trend toward improved postoperative motor outcomes with UPDRS scoring with LA, though this was not statistically significant. However, the small sample size in the included studies might have affected the statistical significance.
Insertion of DBS electrodes is commonly performed under LA with or without sedation to facilitate intraoperative neurophysiological testing. Intraoperative localization of the STN is considered indispensable despite preoperative image-based target planning. This could be due to brain displacement occurring as a result of cerebrospinal fluid leakage during surgery or accumulation of subdural air with burr holes, as well as the use of stiff microelectrode needles. In addition to the use of MER for target localization, the advantages of surgery performed while the patient is awake are that it allows for evaluation of the intraoperative stimulation-induced improvement in symptoms and also helps to detect stimulation-induced adverse effects.Reference Houeto, Welter and Bejjani 2 However, the disadvantages of the LA technique with an awake patient include restlessness, fatigue, anxiety, and agitation due to the long-duration surgery and probable exacerbation of symptoms in the “off-medication” state.
In contrast to the conventional practice of LA for DBS insertion, the procedure under GA allows patients to continue their preoperative antiparkinsonian medications and offers increased patient comfort and acceptance during surgery. Nevertheless, it can interfere with MER by suppressing or abolishing spontaneous neuronal firing and impede evaluation of the clinical benefits of intraoperative stimulation. In addition, the patient cannot report subjective adverse effects, such as paresthesia or abnormal motor activity due to stimulation of adjacent structures.Reference Lettieri, Rinaldo and Devigili 24
Improved understanding of neuronal activity during STN has enabled patients to undergo STN–DBS under GA with intraoperative MER guidance. Though few studies reported reduced baseline background noise from STN–MER, many studiesReference Moll, Payer and Gulberti 10 , Reference Lefaucheur, Gurruchaga and Pollin 19 , Reference Sutcliffe, Mitchell, Gan, Mocroft and Nightingale 22 have shown that localization of STN using intraoperative electrophysiological recordings is possible with a variety of inhalational and intravenous anesthetic agents. In addition, they also reported good clinical outcomes. Studies comparing GA and LA groupsReference Yamada, Gotoa and Kuratsua 16 , Reference Lefaucheur, Gurruchaga and Pollin 19 , Reference Chen, Tsai and Lin 20 , Reference Sutcliffe, Mitchell, Gan, Mocroft and Nightingale 22 reported successful target localization using MER with no significant differences. However, it was difficult to compare the quality of MER between groups because the details of neuronal activity, number of electrode passes, depth of STN, and changes in target tracts and coordinates were not reported uniformly across these studies.
Though there are ongoing studies to address the best anesthetic technique for MER, many clinicians are doing DBS insertion under GA without MER. Recent advances in imaging techniques, especially the intraoperative MRI–CT techniques, have enabled DBS insertions to be performed under GA without MER. In some cases, the whole procedure—including targeting, lead placement, and confirmation of electrode location—is now performed under GA with iMRI. The advantages of this technique include real-time imaging, reduced duration, reduced number of penetrations for electrode placement, and early detection of such complications as intracerebral hemorrhage. Recent studiesReference Ostrem, Ziman and Galifianakis 6 , Reference Chabardes, Isnard and Castrioto 7 have shown that iMRI DBS implantation has comparable outcomes to those with frame-based MER-guided surgery. However, these are small case series, and prospective studies are needed to accurately compare study outcomes.
Though there are many studies with DBS insertion under GA, it is difficult to translate the data regarding clinical outcomes from these studies due to the absence of a control group. The reported outcomes evaluated in the literature include either clinical measures such as UPDRS I to IV, Hoehn and Yahr staging, Schwab and England scores, reduction in LEDD, or those related to successful target localization (i.e., image guidance or MER). Among these, UPDRS III has been the most commonly used clinical outcome measure and has been shown to correlate well with the accuracy of target placement using either image or MER guidance. Thus, we utilized UPDRS III and LEDD reduction in our study for comparison between the GA and LA groups.
Clinical Implications and Future directions
In view of the retrospective nature of the available studies and the heterogeneity among the reported clinical outcomes, this metaanalysis could not definitively answer the question of whether DBS under GA is comparable to that with LA. However, our study has laid down a platform for further research in this field. The use of GA will provide higher levels of acceptance to subgroups of patients where LA may not be a realistic option. Hence, there is a need for a prospective randomized controlled study with adequate power to provide the evidence for comparable clinical outcomes with GA for STN–DBS insertion in PD patients. The outcome reporting methodology should also be improved to ensure comparability among studies. Recent guidelines published by the Movement Disorder Society have provided a framework for data presentation in order to facilitate the comparison and interpretation of results across clinical DBS studies in PD (Guide4DBS–PD).Reference Vitek, Lyons and Bakay 15 The preferred reporting of postoperative details include time of assessment relative to implantation, levodopa requirements, stimulation settings, UPDRS scores (at least III), and adverse events.
Limitations
We acknowledge several limitations of our study, mainly due to the retrospective nature of the included studies. The type of anesthesia was not randomized in any of them. Patient assignments to the GA or LA group depended primarily on patient comorbidities, and in most of the studies patients who could not tolerate an awake procedure had GA. Hence, there was a huge selection bias, a major confounding factor for our study. Target accuracy was not reported in all the studies. The postoperative follow-up times might have been different, as these studies were retrospective in nature. In addition, the results from many of the reported studies had large confidence intervals, indicating possible small sample sizes in the primary studies. This would have affected the final results of our metaanalysis. Finally, we did not assess publication bias, as more than 10 studies are usually required to detect funnel plot asymmetry.
Conclusions
This systematic review and metaanalysis demonstrates that at present there are no good-quality data to suggest equivalence of GA to LA in terms of safety and efficacy during STN–DBS insertion in patients with PD, with some factors trending toward LA. A large prospective randomized controlled trial is required for adequate validation of GA during STN–DBS insertion in patients with PD.
Disclosures
The authors hereby declare that they have no conflicts of interest to disclose.
Statement of Authorship
VS helped with study design, literature review, data entry, data analysis, and writing the manuscript. NCR helped with literature review, data entry, and writing the manuscript. JM helped with literature search, data collection, and writing the manuscript. ME helped with literature search and writing the manuscript. PM helped to write the manuscript. LV helped with study design, data analysis, and writing the manuscript. All the authors approved the final manuscript.
Supplementary materials
To view the supplementary materials for this article, please visit https://doi.org/10.1017/cjn.2017.224.








