Introduction: Current Concepts of Neurodegenerative Diseases
Demographic changes have led to an increased prevalence of age-related brain disorders; however, no drugs are approved for clinical use that halt or even slow the disease process. Furthermore, there are no reliable markers of the earliest stages of disease, which would be essential to provide a window for preventive or neuroprotective interventions before the onset of clinical symptoms. A major group of age-related brain disorders are neurodegenerative diseases that are characterised by selective regional neuronal loss and dysfunction of neuronal and glial networks, which correlate to the diverse clinical phenotypes observed in patients. Discoveries over the past decades have recognised that a crucial feature of neurodegenerative diseases is the deposition of abnormal protein aggregates in neurons and/or glia, which are considered sufficient to cause disease. Recognition that biochemical modification/s of these proteins are central to the pathogenesis of neurodegenerative diseases has served as the foundation for developing disease-specific biomarkers that capture and differentiate the main type/s of protein abnormality responsible for neurodegenerative diseases, some of which are used in clinical practice. Further advances in biomarker development will provide much needed opportunities to confirm the clinical diagnosis in patients, track disease progression, better stratify patients for clinical trials and monitor the effects of therapeutic intervention. Reference Teunissen, Verberk and Thijssen1,Reference Hansson2 However, despite these advances, there are no biomarkers to preclinical disease, which would allow predication of neurodegenerative disease conditions or combined neurodegenerative diseases.
Major concepts to understand neurodegenerative disease can be summarised as follows:
-
1. To emphasise the role of abnormally deposited proteins in the pathogenesis, neurodegenerative diseases are also referred to as proteinopathies. Recent advances in deciphering the molecular pathological, biochemical, genetic and structural properties of protein aggregates have led to the reclassification of these conditions. This allows more accurate diagnosis and classification of neurodegenerative diseases, and the potential development of sensitive biomarkers and therapeutic strategies not previously considered for these disorders.
-
2. Significant advances in disease classification and understanding the pathogenesis of neurodegenerative diseases have emerged from key studies investigating the role/s of proteinaceous infectious particles (prion), Reference Prusiner3 cell-to-cell propagation and disease-specific protein strains that are currently discussed for various neurodegenerative diseases. This implies that a conformational change of a physiological protein leads to abnormal filaments that can convert further physiological protein molecules into an abnormal structure. This concept is the basis of the protein misfolding cyclic amplification and has been used to develop a diagnostic procedure called real-time quaking induced conversion (RT-QuIC). Reference Atarashi, Moore and Sim4–Reference Saborio, Permanne and Soto7 The structure of pathological filaments and the seeding behaviour of the same protein can be associated with distinct clinicopathological phenotypes.
Experimental observations have revealed different mechanisms leading to cell-to-cell propagation of pathological forms of proteins. Reference Guo and Lee8 This has been complemented by the recognition of sequential involvement of brain regions in neuropathology leading to the definition of stages and phases of diseases. Reference Brettschneider, Del Tredici, Lee and Trojanowski9 As a translation to the clinical practice, the concept of preclinical or pre-symptomatic disease has been introduced. Reference Dubois, Hampel and Feldman10,Reference Michell, Lewis, Foltynie and Barker11 Further observations show that these proteins may deposit in peripheral organs and provide the opportunity for peripheral tissue-based diagnostics. Reference Orimo, Ghebremedhin and Gelpi12
The concept of strains of disease has been discussed for various neurodegenerative proteinopathies. Reference Woerman13 Accordingly, various protein folds show distinct propagation patterns in the brain (and in experimental models) leading to heterogenous clinicopathological phenotypes associated with the same proteins and subsequent subtyping of neurodegenerative diseases. Subclassification might differ for the distinct proteins and include genetic polymorphisms or biochemical alterations of the specific protein, the structure of protein filaments or be solely based on morphological differences in the deposition of pathologically altered proteins in the brain. Reference Kovacs14
-
3. Over the past decade, it is increasingly recognised that one or more proteinopathies and/or cerebrovascular disease (CVD) occur in individuals with neurodegenerative diseases, rather than occurring in isolation. Reference Kovacs, Milenkovic and Wöhrer15,Reference Rahimi and Kovacs16 Large autopsy studies have demonstrated that the prevalence of age-related proteinopathies and CVD increase in prevalence with advancing age and are commonly found in individuals aged >65 years and in a range of neurodegenerative diseases, and careful discrimination is required to differentiate these from neurodegenerative proteinopathies.
This review provides an overview of the current classification of neurodegenerative diseases and age-related pathologies and presents the spectrum and complexity of mixed pathologies and their clinicopathological relevance. To describe cases with the presence of multiple neurodegenerative proteinopathies or cases with mixed neurodegenerative pathologies and another type of pathology including age-related pathologies and/or CVD, collectively, the term "mixed pathology" is used in this review. Mixed pathologies are generally considered to lower the threshold for cognitive impairment and dementia. In addition, mixed pathologies add to the complexity of how neurodegenerative diseases are classified and have obvious implications for concepts surrounding disease pathogenesis and determining the clinicopathological relationship, biomarkers and neuroimaging studies.
Classification of Neurodegenerative Diseases
Neurodegenerative diseases are characterised histologically by neuronal and synaptic loss, associated with activated microglia- and reactive astrogliosis, with the severity and distribution of selective neurodegeneration correlating with the diverse clinical phenotypes observed in patients. Early studies established the presence of a neuroinflammatory response in Alzheimer's disease (AD), Reference Terry17 which is also observed in Lewy body diseases (LBD), frontotemporal dementia (FTD) and other neurodegenerative diseases. Reference Bright, Werry and Dobson-Stone18 However, limited studies have focused on the neuroinflammatory response in cases with mixed pathologies and whether combined pathologies are likely to accelerate this response in affected brain regions remains unknown. These are likely to be areas of future interest given the prevalence of mixed pathologies observed in longitudinal aging and neurodegenerative cohorts.
The classification of neurodegenerative and age-related proteinopathies is based on the brain regions and cell type/s affected, the main abnormally deposited protein/s, and disease aetiology if known, e.g., the presence of a genetic abnormality. Reference Kovacs14 Together, the protein abnormalities and cell types affected determine the specific regional and cellular vulnerabilities in these disorders. The molecular neuropathological classification and diagnosis of neurodegenerative diseases are based on the following: Reference Kovacs14
-
A. The anatomical distribution and severity of neuronal loss and gliosis. Additional features including spongiform change and vascular lesions as observed with histological stains are also considered. A peculiar pattern of neuronal degeneration is called frontotemporal lobar degeneration (FTLD) that is associated with various clinical presentations summarised under the clinical umbrella term FTD. Importantly, neurodegenerative proteinopathies associated with FTLD are further classified as FTLD-tau, -TDP, FET or UPS. Reference Mackenzie, Neumann and Bigio19,Reference Neumann and Mackenzie20 In FTLD, these proteins converge on the same anatomical brain regions and cellular populations to produce similar clinical phenotypes. Furthermore, multiple pathological subtypes within each proteinopathy are recognised that are associated with heterogeneous biochemical properties and cell type/s and/or cellular compartment affected. This complex diversity in the main proteinopathy deposited with the same clinical phenotype is not observed to same extent in other neurodegenerative diseases.
-
B. The presence of intracellular and/or extracellular protein deposits in disease-specific anatomical regions and cell type/s, which are evaluated by immunohistochemical stains. The following are the major proteins associated with almost all adult-onset sporadic and genetic neurodegenerative diseases and are summarised in Figures 1 and 2:
-
1. Tau, a microtubule-associated protein encoded by the microtubule-associated protein tau (MAPT) gene. Tau is normally expressed in neurons (and only expressed in trace amounts in astrocytes and oligodendroglia (http://www.brainrnaseq.org/)) and has critical functions in the assembly and stabilisation of microtubules, regulating transport and is known to interact with other cellular structures. Reference Buée, Bussière, Buée-Scherrer, Delacourte and Hof21,Reference Ittner and Ittner22 Six isoforms of tau are expressed in the adult human brain from alternate splicing of exons 2, 3 and 10 of the MAPT gene. Exclusion of exon 10 generates three isoforms with three microtubule repeat domains (3-repeat tau), and inclusion of exon 10 generates three isoforms with four microtubule repeat domains (4-repeat tau). Reference Arendt, Stieler and Holzer23 Currently, Tau-proteinopathies are further classified based on their biochemical properties: Reference Kovacs24
-
i. 3-repeat tau-immunopositive inclusions predominantly in neurons,
-
ii. 4-repeat tau-immunopositive inclusions in neurons and glia and
-
iii. 3-repeat and 4-repeat tau-immunopositive inclusions predominantly in neurons.

Figure 1: Classification of the major neurodegenerative diseases and subtypes, and ageing-related pathologies by the main protein deposited. aFTLD-U = atypical frontotemporal lobar degeneration with ubiquitin inclusions; AD = Alzheimer’s disease; AGD = argyrophilic grain disease; ARTAG = ageing-related tau astrogliopathy; BIBD = basophilic inclusion body disease; CAA = cerebral amyloid angiopathy; CBD = corticobasal degeneration; DLB = dementia with Lewy bodies; DRPLA = dentatorubral-pallidoluysian atrophy; FET/FUS = FET family of protein, including fused in sarcoma; FTLD = frontotemporal lobar degeneration; FTLDNOS = FTLD not otherwise specified; FTLDUPS = FTLD with ubiquitin-proteasome system; FXTAS = fragile X-associated tremor/ataxia syndrome; GGT = globular glial tauopathy; HD = Huntington’s disease; iLBD = incidental Lewy body disease; LATE-NC = limbic-predominant age-related TDP-43 encephalopathy-neuropathological change; MND = motor neuron disease; MSA = multiple system atrophy; NFerr = hereditary ferritinopathy; NSerp = neuroserpinopathy; NIFID = neuronal intermediate filament inclusion disease; PART = primary age-related tauopathy; PD = Parkinson’s disease; PiD = Pick’s disease; PSP = progressive supranuclear palsy; PrP = prion protein; SBMA = spinal-bulbar muscular atrophy; SCA = spinocerebellar ataxia; TDP-43 = TAR DNA-binding protein 43; TO-CI = Tangle-only associated cognitive impairment. Ageing-related conditions are indicated by lighter coloured boxes with dashed borders. The most frequent mixed pathologies include AD-neuropathological change (tau and Aβ), PART, AGD and ARTAG (tau), LATE-NC (TDP-43) and Lewy body disorders (α-synuclein).

Figure 2: Neuropathological features of the major neurodegenerative diseases and ageing-related tau astrogliopathy. Panels a–d show neuronal tau morphologies including a neurofibrillary tangle in Alzheimer’s disease (a), globose tangle in progressive supranuclear palsy (b), Pick body in Pick’s disease (c), tau-immunopositive grains found in dendrites in argyrophilic grain disease (d). Panels e–h show characteristic glial tau-immunopositive features of FTLD-tau including astrocytic plaques in corticobasal degeneration (e), tufted astrocyte in progressive supranuclear palsy (f), globular astrocytic inclusions (g) and globular oligodendroglial inclusions (h) in globular glial tauopathy. Panels i–l show α-synuclein-immunopositive pathological features characteristic of Lewy body disorders and multiple system atrophy including Lewy bodies and Lewy neurites (i–k) and glial cytoplasmic inclusions (l). Panels m–p show characteristic TDP-43-immunopositive inclusions in MND (m) and FTLD-TDP (n-p) including skein-like inclusions in the hypoglossal nucleus (m), neuronal cytoplasmic inclusions and short dystrophic neurites (n), long dystrophic neurites (o), and neuronal intranuclear inclusions (p). FUS-immunopositive inclusions in the hippocampal dentate gyrus in FTLD-FUS (q) and the anterior horn of the spinal cord in FUS-gene mutation related motor neuron disease (r). Diffuse synaptic (s) and patchy/perivacuolar (t) immunoreactivity for disease-associated PrP in sporadic Creutzfeldt-Jakob disease. Aβ diffuse (u) and neuritic (v) plaques, and Aβ deposition in vessel walls (w, x), characteristic of cerebral amyloid angiopathy. Panels y–b’ show grey matter (y), white matter (z), perivascular (a’) and subpial (b’) ageing-related tau astrogliopathy (ARTAG). Scale bar in a = 20 μm (applies to b, c, g, h, p), in d = 20 μm (applies to l), in e = 50 μm (applies to i, o), in f = 30 μm (applies to j, k, m, n), in q = 30 μm (applies to v), w = 500 μm, r = 50 μm (applies to s, t, x–b’).
-
-
2. α-Synuclein, encoded by the α-synuclein (SCNA) gene, is a 140 amino acid protein abundantly expressed in the brain. α-Synuclein is the main component of filamentous neuronal and glial inclusions characteristic of LBD and multiple system atrophy (MSA), respectively. Reference Spillantini, Crowther, Jakes, Cairns, Lantos and Goedert25,Reference Spillantini, Schmidt, Lee, Trojanowski, Jakes and Goedert26
-
3. TAR DNA-binding protein 43 (TDP-43), a highly conserved 494 amino acid nuclear protein encoded by the TDP (TARDBP) gene. TDP-43 is normally expressed in neuronal and glial nuclei and has important functions in transcription and splicing regulation and a number of cellular processes including mRNA stability. Reference Buratti and Baralle27 Under pathological conditions, TDP-43 abnormally redistributes from the nucleus to the cytoplasm and forms aggregates. Further genetic abnormalities are also associated with TDP-43 pathology; the most frequent are associated with the progranulin (GRN) gene and the chromosome 9 open reading frame 72 (C9orf72) gene. Reference Neumann and Mackenzie20
-
4. FET proteins, a family of RNA-binding proteins with critical functions in regulating gene expression and mRNA/microRNA processing, which include fused in sarcoma (FUS), Ewing’s sarcoma RNA-binding protein 1 (EWSR1) and TATA-binding protein-associated factor 15 (TAF15). Reference Mackenzie, Munoz and Kusaka28 FET/FUS-proteinopathies comprise a small proportion of cases, Reference Mackenzie, Munoz and Kusaka28 and currently, mutations in FET genes are not considered to be a leading aetiology. However, mutations in the FUS gene have been reported in familial amyotrophic lateral sclerosis (ALS). Reference Neumann and Mackenzie20,Reference Vance, Rogelj and Hortobagyi29
-
5. Amyloid-β (Aβ) derives from the amyloid precursor protein (APP) with the C-terminus ending at amino acids 40 (Aβ40) or 42 (Aβ42). Both isoforms are found in Aβ plaques. Other genes associated with Aβ deposition and familial Alzheimer’s disease (AD) include the presenilin-1 (PSEN1) and presenilin-2 (PSEN2) genes. Reference Cacace, Sleegers and Van Broeckhoven30 Aβ can also accumulate in vessel walls in cerebral amyloid angiopathy (CAA). Reference Attems, Jellinger, Thal and Van Nostrand31
-
6. Prion protein (PrP) is a 253 amino acid protein encoded by the PRNP gene. Prion diseases are classified primarily based on their sporadic, genetic or acquired aetiology and further based on biochemical/genetic features and morphologically as transmissible spongiform encephalopathies (i.e., various aetiological forms of Creutzfeldt-Jakob disease, kuru), thalamic degeneration (i.e., fatal familial insomnia), or PrP cerebral amyloidoses (i.e., Gerstmann-Sträussler-Scheinker disease and PrP cerebral amyloid angiopathy). Reference Quadrio, Perret-Liaudet and Kovacs32
-
In addition to these major neurodegenerative proteinopathies, there are further neurodegenerative diseases where the proteinaceous aggregates responsible for disease are yet to be identified, however, these comprise a small proportion of cases. Reference Kovacs14 Furthermore, there are many hereditary neurodegenerative diseases that are characterised by other abnormal proteinaceous aggregates. For example, the trinucleotide repeat disorders (e.g. Huntington’s disease, some spinocerebellar ataxias and spinal and bulbar muscular atrophy, atrophin-1), and rare, inherited disorders including neuroserpinopathy, ferritin-related neurodegenerative diseases, and familial cerebral amyloidosis. Reference Kovacs14,Reference Kovacs33
Recent Advances on the Stratification of Neurodegenerative Diseases
In the near future, a further stratification level incorporating unique self-seeding behaviour and structure of pathological protein filaments will add further layers to the classification of neurodegenerative diseases. Indeed, recent studies have identified unique structures of protein filaments by cryo-electron microscopy (cryo-EM) (for review see), Reference Creekmore, Chang and Lee34 as described for Aβ, Reference Kollmer, Close and Funk35,Reference Yang, Arseni and Zhang36 and allows further subtyping for example of major tauopathies, Reference Shi, Zhang and Yang37 or better understanding the differences in disease course between cases within the same neuropathology group, as exemplified by observations in α-synucleinopathies. Reference Schweighauser, Shi and Tarutani38 Despite less cryo-EM data for human tissue-derived TDP-43, Reference Arseni, Hasegawa and Murzin39 or only currently initial structural studies provide a basis for future attempts. Reference Creekmore, Chang and Lee34 The concept of strains is also increasingly considered for human disease subtyping. Reference Vaquer-Alicea, Diamond and Joachimiak40,Reference Holec and Woerman41 As a provocative concept, combined proteinopathies might represent distinct "strain-like" features from those associated with conditions accumulating only one protein, Reference Robinson, Lee and Xie42 which might open further aspects for classification.
Disease Propagation and Pathological Staging
Disease progression is characterised by the spreading of pathological aggregates and neuronal degeneration to an increasing number of brain regions over time, from one vulnerable region to the next. For most neurodegenerative diseases, this is well-described and occurs in a stereotypical and hierarchical pattern of selective regional involvement, summarised in Table 1. These studies underpin the concept that neurodegenerative proteins propagate throughout the nervous system, where disease-associated protein seeds are responsible for the initiation and spreading of protein aggregates. To date, while the majority of pathological staging studies have focused on neuron-to-neuron propagation, emerging evidence suggests that glia (astrocytes and oligodendroglia) are likely to have an important role in pathological spread throughout the brain. Defining cell-specific stages of pathological staging/disease progression, as recently described in progressive supranuclear palsy (PSP), Reference Kovacs, Lukic and Irwin43 have important implications for understanding the earliest stages of disease, propagation and progression.
Table 1: Summary of sequential distribution patterns of protein pathology in neurodegenerative diseases and brain ageing

* Note that PART pathology does not extend beyond the medial temporal lobe, therefore remains Braak NFT stage I-IV. Braak NFT stages V and VI are generally associated with the presence of Aβ plaques, compatible with the diagnosis of AD neuropathological change.
Pathological staging has relevance for cases with mixed pathology, which might show a high or end stage or phase of one proteinopathy and an early stage or phase of another neurodegenerative or age-related proteinopathy. The earliest pathological stages of various neurodegenerative proteinopathies are frequently observed and reported in large autopsy studies and are considered incidental pathologies. For example, amygdala-predominant or early Braak Lewy body stages, early Braak neurofibrillary degeneration stages, and early Thal Aβ phases (Table 1). The clinical relevance of incidental pathologies is largely unknown and whether these would have progressed to the neurodegenerative disease characterised by that proteinopathy is difficult to determine. In addition, the presence of ageing-related pathologies is commonly observed in neurodegenerative diseases and in the brains of elderly individuals, particularly over 65 years of age. Hierarchical pathology distribution patterns and staging schemes have recently been proposed for two common ageing-related proteinopathies, ageing-related tau astrogliopathy (ARTAG) Reference Kovacs, Xie and Robinson44 and limbic-predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC) (Table 1). Reference Nelson, Dickson and Trojanowski45
Importantly, the progression and pathological spread of these neurodegenerative and age-related pathologies occurs through synaptic and functional connectivity, rather than spatial proximity, Reference Ahmed, Cooper and Murray46 and appears to be more selective than observed in prion diseases. Unlike PrP, despite numerous studies that demonstrate that tau, Aβ and α-synuclein aggregates can propagate from human tissue to preclinical models, there is no epidemiological evidence to suggest they have similar transmissibility or pose a risk of infectivity of these diseases. Reference Asher, Belay and Bigio47
Common Age-Related and Incidental Pathologies
In addition to the neurodegenerative disease entities, in the ageing brain peculiar neurodegenerative pathologies, associated with tau, TDP-43, Aβ, α-synuclein deposition can be seen. In isolation, these do not necessarily associate with clinical symptoms but may contribute to lowering of threshold for the development of cognitive decline.
Tau
-
Primary ageing-related tauopathy (PART): a common age-related tauopathy in individuals > 60 years Reference Crary, Trojanowski and Schneider48 that is characterised by Braak neurofibrillary tangle (NFT) stage ≤ IV (severe hippocampal involvement) with few or an absence of Aβ plaques and does not fulfill AD neuropathological criteria. In addition, the regional distribution pattern of NFTs in PART and AD differ, where a higher density of NFTs are observed in the hippocampal CA2 region in PART. Reference Jellinger49 PART is found in neurologically normal individuals and individuals with cognitive impairment, and also includes Tangle-only associated cognitive impairment (TO-CI), which is also considered a subtype of FTLD-tau. PART is one of the most common age-related pathologies, and there is debate surrounding whether PART progresses to AD or TO-CI. Reference Duyckaerts, Braak and Brion50,Reference Jellinger, Alafuzoff and Attems51 Large autopsy studies have shown that by the third decade of life, almost all individuals will show NFT development. Reference Braak, Thal, Ghebremedhin and Del Tredici52
-
Argyrophilic grain disease (AGD): a common, predominantly age-related tauopathy characterised by argyrophilic and tau-immunopositive grains (in dendrites) and oligodendroglial coiled bodies in the medial temporal lobe. Reference Braak and Braak53,Reference Togo, Sahara and Yen54 Similar to PART, while AGD is a well-recognised and common age-related pathology, it is also a rare pathological subtype of FTLD-tau. Reference Cairns, Bigio and Mackenzie55
-
Ageing-related tau astrogliopathy (ARTAG): consensus recommendations described in 2016 standardised the classification and nomenclature of tau-immunopositive astrocytes commonly found in the brains of individuals >60 years. Reference Kovacs, Ferrer and Grinberg56 Identification of five ARTAG types including grey and white matter, perivascular, subpial (Figure 2) and subependymal, characterised by two astrocytic morphologies: 1) thorn-shaped and/or 2) granular fuzzy astrocytes. Classification is based on regional and sub-regional involvement, and extent of astrogliopathy. ARTAG can be differentiated from other tau-depositing disorders and occur in isolation, but is more commonly observed as an additional co-existing pathology in a range of neurodegenerative diseases. Reference Kovacs, Ferrer and Grinberg56,Reference Kovacs, Robinson and Xie57 The medial temporal lobe (particularly the amygdala) and basal forebrain are predilection sites for ARTAG pathology, however, the regional distribution and type of ARTAG varies between neurodegenerative diseases and normal aging. Reference Kovacs, Robinson and Xie57 For example, the basal forebrain is a predilection site for ARTAG in aged individuals, lobar white matter ARTAG is frequent in AD and neocortical grey matter ARTAG is common in FTLD-tau. Reference Kovacs, Robinson and Xie57 In addition, different types of ARTAG are associated with different clinicopathological entities, and age at death, brain atrophy, ventricular enlargement, Braak neurofibrillary stage and CERAD score. Reference Kovacs, Robinson and Xie57,Reference Nolan, De Paula Franca Resende and Petersen58 Despite these studies, further investigation is required to determine why different types of ARTAG and why the anatomical distribution of ARTAG varies between neurodegenerative diseases. This might be associated with the stage of sequential involvement of brain regions that reflect distinct pathogenic mechanisms, such as barrier dysfunction or mechanical impact. Reference Kovacs, Xie and Robinson44 The clinical relevance of ARTAG still requires further clarification. Reference Kovacs59
TDP-43
-
Limbic-predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC): a recently described nomenclature and common TDP-43 proteinopathy typically associated with advanced age (>80 years) and is also found in individuals with cognitive impairment, in the absence of a motor neuron or FTD clinical phenotype. Reference Nelson, Dickson and Trojanowski45 Limbic TDP-43 pathology has been a recognised feature of aging and a range of neurodegenerative diseases in earlier studies. Reference Amador-Ortiz, Lin and Ahmed60 It is characterised by a TDP-43-immunopositive neuronal cytoplasmic inclusions and/or dystrophic neurites in the medial temporal lobe. Additional hippocampal sclerosis may be observed in cases with LATE-NC. Although it is still debated whether LATE-NC represents an independent entity, recent studies support the notion that LATE-NC can be differentiated from FTLD-TDP Reference Robinson, Porta and Garrett61 and may occur in isolation, but is commonly observed in AD. Reference Nelson, Dickson and Trojanowski45 Hippocampal sclerosis is reported to occur in up to 20% of individuals >85 years. Reference Leverenz, Agustin and Tsuang62,Reference Nag, Yu and Capuano63 It is characterised by neuronal loss and gliosis in the hippocampal CA1 region and subiculum that is out of proportion to the extent of AD neuropathologic change Reference Montine, Phelps and Beach64 and is associated with the presence of TDP-43 pathology in almost all cases. Reference Nelson, Schmitt and Lin65 However, the definition of hippocampal sclerosis is often subjective, which has led to inconsistent interpretation and reporting of hippocampal sclerosis in the literature.
Aβ
-
Aβ plaques, most commonly observed in early phases, begin to develop in individuals in their 5th-6th decade of life and are observed in approximately 10% of individuals in this age group. The incidence and Thal Aβ phase increase in prevalence with advancing age. Large autopsy studies have demonstrated that cases with Aβ plaques also have NFT development. Reference Braak, Thal, Ghebremedhin and Del Tredici52 Aβ is also frequently observed in the walls of capillaries and arteries, referred to as CAA. CAA is a common age-related and incidental pathology found in asymptomatic individuals, but also occurs in a variety of hereditary forms of CAA. Reference Attems, Jellinger, Thal and Van Nostrand31,Reference Revesz, Holton and Lashley66
α-synuclein
-
Lewy bodies confined to the brainstem (earliest Braak LB stages) and/or amygdala are commonly observed even without specific clinical symptoms.
In addition to these common ageing-related and incidental pathologies, unusual constellations of pathologies have been described in the elderly Reference Kovacs, Molnár and László67 that do not meet criteria for the above ageing-related and incidental pathologies. Rare cases of neuropathologically confirmed corticobasal degeneration (CBD), Reference Martínez-Maldonado, Luna-Muñoz and Ferrer68,Reference Milenkovic and Kovacs69 PSP, Reference Evidente, Adler and Sabbagh70,Reference Yoshida, Hata, Kinoshita, Takashima, Tanaka and Nishida71 MSA Reference Fujishiro, Ahn and Frigerio72 have also been reported in the absence of clinical symptoms. These cases are considered incidental and suggest a pathological burden needs to be reached before the development of a clinical phenotype.
CVD is another common incidental pathology that has been described in large autopsy series and increases in prevalence with advancing age. The incidence and contribution of CVD to clinical symptoms has proved to be difficult to reconcile largely due to inconsistent terminologies used. In 2016, the Vascular Cognitive Impairment Neuropathology Guidelines (VCING) were published in order to assess the contribution of CVD to cognitive impairment. Reference Skrobot, Attems and Esiri73 According to this, seven pathologies, including leptomeningeal CAA, large infarcts, lacunar infarcts, microinfarcts, arteriolosclerosis, dilation of the perivascular space and myelin loss predicted cognitive impairment. Reference Skrobot, Attems and Esiri73 The VCING model proposed to predict the likelihood that CVD contributed to cognitive impairment based on the combinations of three main determinants, including at least one large (>10 mm diameter) infarct, moderate/severe occipital leptomeningeal CAA, and moderate/severe arteriolosclerosis in the occipital white matter. These are used to assign a low, intermediate or high likelihood that CVD contributed to cognitive impairment in an individual. Reference Skrobot, Attems and Esiri73 Vascular lesions are interpreted as strategic for cognitive decline or not and have been designated as multi-infarct dementia, strategic infarct or subcortical vascular encephalopathy. Reference McAleese, Alafuzoff and Charidimou74 The frequency of vascular lesions in community-based neuropathology cohorts vary between 28 and 70% without clear differences between individuals with or without cognitive decline. Reference Rahimi and Kovacs16 The lack of consensus regarding assessment of vascular pathology has led to a wide range of reported prevalence rates of vascular dementia or vascular cognitive impairment. However, these discrepancies may reflect regional and ethnic differences or distinct management of cardiovascular risk factors. Reference McAleese, Alafuzoff and Charidimou74 Finally, there is a lack of systematic and comparative data on the prevalence of combined non-Alzheimer neurodegenerative proteinopathies and vascular lesions.
Nomenclature and Definitions Associated with Mixed Pathology
A combination of pathological alterations and the deposition of multiple protein deposits is commonly observed in the brains of elderly individuals and in neurodegenerative diseases. However, there are inconsistencies in the literature used to describe these cases (Table 2), and current nomenclature is often difficult to decipher, which has hindered a standardised classification for cases with mixed pathologies. In addition, the terms "proteinopathy" and "pathology" are frequently used interchangeably when describing cases with mixed pathology and it is important to distinguish the two entities. Here, the term "mixed pathology" is used to describe cases with a) mixed neurodegenerative pathologies or b) cases with mixed neurodegenerative pathologies and another type of pathology e.g., CVD, age-related pathology.
Table 2: Nomenclature and definitions used in the literature to describe cases with multiple proteinopathies and/or vascular abnormalities
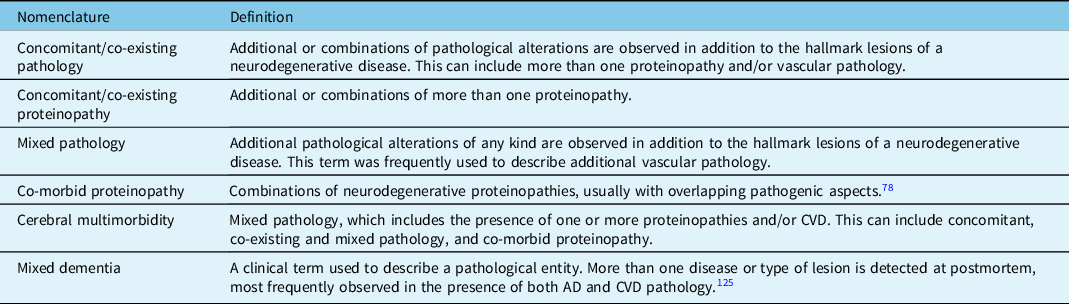
Based on the literature, mixed pathology usually refers to three underlying conditions:
-
1. Low-level concomitant pathologies: different proteinopathies and/or CVD with a restricted anatomical distribution and early pathological stage or phase. These pathologies are not typically associated with a specific clinical phenotype. For example, cortical ARTAG and brainstem-predominant Lewy bodies.
-
2. One severe pathology and further low-level concomitant pathologies: this includes one main proteinopathy, which is considered sufficient to cause the clinical phenotype alone, and at least one additional low-level concomitant proteinopathy and/or CVD, which in isolation is not typically associated with a clinical phenotype. For example, AD and low stage LATE-NC, AD and white matter ARTAG, FTLD-tau and ARTAG.
-
3. Two (or more) severe pathologies: each is anatomically widespread with a severe density of pathology. Either pathology in isolation would be considered sufficient to cause a clinical phenotype. Additional low-level concomitant pathologies may be observed. For example, concurrent FTLD-tau and FTLD-TDP, or late stages of AD and DLB and severe forms of CVD.
Prevalence of Mixed Pathologies with Ageing and in Neurodegenerative Diseases
Mixed pathologies are commonly reported in large autopsy studies and while the majority of studies have focused on individuals >65 years and the elderly >80 years with and without cognitive impairment, it is increasing recognised that mixed pathologies are observed in younger individuals (<65 years). Despite the high number of reports describing mixed pathologies in ageing and neurodegenerative diseases, only recent studies use a combination of histological, immunohistochemical, biochemical and genetic methods to evaluate the spectrum of mixed pathologies. These studies have revealed a previously underappreciated and complex constellation of mixed pathologies. The prevalence of mixed pathologies in individuals with and without cognitive impairment indicates that deposition of these proteins inevitably occur with ageing, independent of neurodegenerative disease. Reference Karanth, Nelson and Katsumata75 These age-related pathologies are likely to be present by the time late-onset neurodegenerative diseases develop and the number of mixed pathologies increasing with advanced age and longer survival. Reference Rahimi and Kovacs16,Reference Spina, La Joie and Petersen76 However, it is recognised that some neurodegenerative diseases more commonly have mixed pathologies than others, and some neurodegenerative disorders are rarely mixed. Studies reporting the prevalence of mixed pathologies are summarised as follows:
-
1. Mixed pathologies in community-based, longitudinal ageing studies
The prevalence of tau, Aβ, α-synuclein and TDP-43 pathologies, hippocampal sclerosis and vascular pathology in unselected longitudinal ageing studies has highlighted the complexity, and diverse range and extent of proteinopathies and mixed pathologies in the ageing brain. Reference Kovacs, Milenkovic and Wöhrer15,Reference Rahimi and Kovacs16,Reference Karanth, Nelson and Katsumata75 These studies report prevalence rates of up to 68% for AD-related pathology, 38% for ARTAG, 13% for AGD, 75% for TDP-43 pathology, 39% for LB pathology, 29% for hippocampal sclerosis and up to 70% for vascular pathology (Table 3).
Table 3: Prevalence (%) of mixed pathologies in community-based, longitudinal ageing studies and major neurodegenerative diseases

AD = Alzheimer’s disease; AGD = argyrophilic grain disease; ARTAG = ageing-related tau astrogliopathy; CBD = corticobasal degeneration; CJD = Creutzfeldt-Jakob disease; CVD = cerebrovascular disease; FTLD = frontotemporal lobar degeneration with tau or TDP-43 inclusions; GGT = globular glial tauopathy; HS = hippocampal sclerosis; LATE-NC = limbic-predominant age-related TDP-43 encephalopathy neuropathological change; LB = Lewy body; MND = motor neuron disease; MSA = multiple system atrophy; n/d: not determined; PART = primary age-related tauopathy; PD = Parkinson’s disease; PiD = Pick’s disease; PSP = progressive supranuclear palsy.
1 Lewy body disorders (LBD) here include Parkinson’s disease (PD), Parkinson’s disease with dementia (PDD), and dementia with Lewy bodies (DLB)
2 Depending on the definitions used (AD with DLB or DLB)
3 Depending on the stage of Lewy body pathology.
4 Determined for Lewy body disorders and not stratified for PD/DLB.
A recent longitudinal community-based cohort of 375 individuals (mean = 87 years) reported that approximately 12% had four concomitant proteinopathies (tau, Aβ, α-synuclein and TDP-43), and 38% had three concomitant proteinopathies. Reference Karanth, Nelson and Katsumata75 Another recent study provides reference data on the frequency of distinct tau-immunopositive pathologies in the depths of cortical sulci. Reference Forrest, Kril and Wagner77 Since TDP-43 was not discovered until 2006, the true prevalence of concomitant TDP-43 pathology is likely to be underestimated in cases collected before this time, which may not have been re-evaluated and screened for the presence of TDP-43. However, LATE-NC has been reported to occur in ∼20% of individuals in a community-based cohort aged 75 years, and its prevalence increases with advancing age to ∼75% in the >100-year age group. Reference Nelson, Dickson and Trojanowski45
-
2. Mixed pathologies in major proteinopathies
A range of mixed pathologies have been reported (for detailed review see: Kovacs Reference Kovacs78 ) for the major proteinopathies (Table 3), which further highlight the complexity of these disorders. For example, AD is frequently associated with ARTAG, TDP-43/LATE-NC, LB pathology, hippocampal sclerosis and CVD in almost all cases. Depending on the FTLD-tau subtype, approximately 60% have Aβ plaques, almost all have ARTAG or AGD and LB and TDP-43 pathology has been reported in up 20% and 45% of all FTLD-tau cases, respectively. Interestingly, two recent studies report that early-onset FTLD and AD can also associate with mixed pathologies. Reference Spina, La Joie and Petersen76,Reference Tan, Yang and Halliday79 Approximately 40% of FTLD-TDP and motor neuron disease (MND) cases have Aβ plaques, one-third have ARTAG or AGD and LB pathology is reported in up to 15% of all FTLD-TDP and MND cases. The Lewy body diseases (i.e., Parkinson’s disease, Parkinson’s disease dementia and Dementia with Lewy bodies) are frequently reported with Aβ plaques (80%), ARTAG (37%) and TDP-43 pathology (33%). Approximately 40% of MSA cases have Aβ plaques, 10% have LBs and a small proportion have TDP-43 pathology. Depending on the age and molecular subtypes examined, up to 23% of sporadic CJD cases have LB pathology, 69% have NFTs and less than 15% have ARTAG or AGD. TDP-43 pathology is rare in CJD.
-
3. Mixed pathologies in genetic conditions
Emerging studies have highlighted the presence of mixed pathologies in a range of genetic neurodegenerative diseases, which are not associated with the protein encoded by the mutated gene. Historically, genetic neurodegenerative diseases were not previously considered to have mixed pathologies and in addition are likely to show the diverse range and complexity of age-related and incidental pathologies described above. As summarised in Yoshida et al., Reference Yoshida, Hata, Kinoshita, Takashima, Tanaka and Nishida71 for example, 1) mutations in the APP, PSEN-1 and PSEN-2 genes causing AD have been reported with LB pathology, and TDP-43 pathology has been reported in cases with mutations in PSEN-1 and PSEN-2 genes; 2) PRNP mutations have been reported with a diverse range of mixed pathologies including AD-related pathology, FTLD-tau pathologies, ARTAG and LB pathology, however, TDP-43 pathology has not been observed; 3) genetic abnormalities in the TARDBP, GRN and C9orf72 genes associated with TDP-43 pathology have been reported with AD pathology (NFTs and Aβ), LB pathology and ARTAG and 4) mutations in the HTT gene have been reported with AD and TDP-43 pathologies; AD and TDP-43 pathology, and α-synuclein glial cytoplasmic inclusions have been reported in cases with mutations in LRRK2 and SNCA. Reference Kovacs78 Few mixed pathologies have been reported in cases with a mutation in MAPT, with TDP-43 pathology reported in a small number of cases. Reference Kovacs78,Reference Forrest, Shepherd, McCann, Kwok, Halliday and Kril80 The APOE ϵ4 allele is well established to be the greatest genetic risk factor for AD pathology Reference Li, Wetten and Li81,Reference Saunders, Strittmatter and Schmechel82 and is associated with the largest number of concomitant pathologies in both early- and late-onset AD. Reference Robinson, Richardson and Xie83
Clinicopathological Correlations in Cases with Mixed Pathology
The regional distribution and severity of neurodegeneration underlie the diverse clinical phenotypes associated with neurodegenerative diseases. In many of these disorders, neurodegenerative changes and different proteinopathies converge on the same anatomical regions as the disease progresses to produce similar and overlapping cognitive, behavioural, linguistic and motor deficits, rather than the type of protein abnormality present. Throughout the disease course, additional anatomical regions become involved, with the development of subsequent diverse and complex clinical symptoms.
These concepts need to be carefully considered in cases with the presence of mixed pathology to determine whether concomitant pathology contributes to the clinical phenotype observed. Similar to the neurodegenerative proteinopathies, this will be influenced by the anatomical distribution, severity and nature of the concomitant pathology. While severe concomitant pathologies are considered sufficient to cause a clinical phenotype, e.g., one severe proteinopathy with clear links to the clinical phenotype, the extent to which low-level concomitant pathologies are likely to contribute to the patient’s cognitive and/or motor impairment is difficult to determine. Low-level concomitant pathologies are not typically associated with a clinical phenotype and are considered insufficient to cause clinical symptoms in isolation. However, while there is a paucity of data on the impact of ageing-related and mixed pathologies, and the number of mixed pathologies present, on clinical phenotype/s, it is generally assumed that they lower an individual’s threshold for developing neurodegenerative proteinopathies and/or cognitive decline. Reference Kapasi, DeCarli and Schneider84 In addition, the severity of mixed pathologies increase with advancing age and correlate with the severity of clinical symptoms. Reference Robinson, Lee and Xie42 Large autopsy studies in individuals >80 years of age have shown that concomitant pathologies increase the risk of cognitive impairment Reference Power, Mormino and Soldan85,Reference Boyle, Yang and Yu86 and dementia, and are associated with a more rapid disease course. Reference Karanth, Nelson and Katsumata75 Importantly, a recent study has found that the presence of a single concomitant pathology increases the odds of transitioning from mild cognitive impairment to dementia by 20-fold. Reference McAleese, Colloby and Thomas87 Other studies have reported that each concomitant pathology contributes differently to specific cognitive domains and cognitive decline, and cognitive trajectories over time Reference Robinson, Lee and Xie42,Reference Nag, Yu and Capuano63,Reference Kapasi, DeCarli and Schneider84,Reference Boyle, Yang and Yu86,Reference Arvanitakis, Capuano, Leurgans, Bennett and Schneider88–Reference Boyle, Yu, Wilson, Schneider and Bennett91 and the number of mixed non-AD pathologies present have a cumulative effect on clinical progression rate of early- and late-onset AD. Reference Spina, La Joie and Petersen76
In addition to prognostic relevance, there are also examples that additional pathologies modify the clinical phenotype. The presence of AD-neuropathologic change and LATE-NC continue to rise with advancing age, and recent studies have reported that the presence of both LATE-NC and AD-neuropathologic change is associated with a clinical phenotype that is more severe than observed when either pathology is observed in isolation. Reference Karanth, Nelson and Katsumata75 Moreover, individuals with LATE-NC are more likely to have AD neuropathology, and are clinically distinct from FTLD-TDP with the presence of visuospatial impairments, delusions, and/or visual hallucinations. Reference Teylan, Mock and Gauthreaux92 Additional TDP-43 pathology can alter the clinicopathological phenotype as exemplified by CBD with TDP-43 pathology presenting with PSP syndrome as a distinct clinicopathological subtype. Reference Koga, Kouri and Walton93 A few studies have reported that some ARTAG types correlate with clinical symptoms, but further studies are required to clarify the clinical relevance of ARTAG. For example, while white matter ARTAG is associated with AD, the presence of any type of ARTAG is equally prevalent in typical and atypical presentations of AD. Reference Nolan, De Paula Franca Resende and Petersen58 However, a negative impact of white matter ARTAG to language and possibly visuospatial networks has been discussed, Reference Resende, Nolan and Petersen94 expanding observations on widespread grey matter ARTAG associated with dementia of the elderly. Reference Kovacs, Milenkovic and Wöhrer15,Reference Kovacs, Molnár and László67 Indeed, cortical grey matter ARTAG has been associated with dementia in individuals aged over 90 years, although ARTAG in other regions (limbic and brainstem) were not. Reference Robinson, Corrada and Kovacs95 Concomitant CVD and/or other vascular pathology is challenging to measure and standardise neuropathologically, and how this correlates to the clinical phenotype and disease course is debated. The presence of LBD as an additional pathology can lead to complex clinical phenotypes, including sleep disorder and parkinsonism in otherwise dementing illnesses. Indeed, a recent study showed that association of Lewy body pathology in FTLD leads to clinical parkinsonism, therefore impacting the clinical course and therapy strategies. Reference Forrest, Crockford and Sizemova96 Co-existing Lewy body pathology can cause Creutzfeldt-Jakob disease-like rapidly progressive neurological symptoms in sporadic and APP mutation-related AD. Reference Sieczkowski, Milenkovic and Venkataramani97 Further interesting aspects to mention is when a genetic prion disease begins with long-standing parkinsonism due to LBD but associates with rapidly progressive dementia in the terminal phase due to additional idiopathic or genetic prion disease. Reference Kovacs, Seguin and Quadrio98 On the other hand, additional pathologies are not thought to have a significant effect on the clinical course in larger cohorts of PSP cases, Reference Jecmenica Lukic, Kurz and Respondek99,Reference Robinson, Yan and Caswell100 although on an individual level subtle differences might be noted. Reference Videira, Damasio, Pinto, Melo-Pires and Taipa101 Since protein biomarkers are already available (Aβ, tau, α-synuclein, prion protein), together with their biochemical modifications, markers of neuronal degeneration (neurofilament light chain, tau, MRI atrophy), neuroinflammation and genetic mutations or polymorphisms, in vivo follow-up studies will translate current molecular pathology observations into the clinical practice and will allow better understanding of the effect of mixed pathologies during the course of disease and not in the terminal phase as in postmortem studies. Reference Kovacs, Botond and Budka102
Molecular Pathogenic Mechanisms in Cases with Mixed Pathologies
As recently reviewed, four main pathogenic mechanisms have been proposed to explain why mixed pathologies occur in the ageing human brain, Reference Kovacs78 which might be capable of lowering the threshold for cognitive decline and/or accelerate the disease course in neurodegenerative diseases.
Age is considered to be the most important risk factor for developing sporadic neurodegenerative diseases and mixed pathologies, but the reason for this has not been fully elucidated and is an area of great research interest. In addition, there is a correlation with the number and extent of mixed pathologies with advancing age. Large longitudinal community ageing studies have demonstrated that the prevalence of NFT, Aβ plaques and Lewy bodies increase in prevalence with advancing age, as well as the number of brain regions affected and the density of pathological inlcusions. Reference Nelson, Dickson and Trojanowski45,Reference Braak, Thal, Ghebremedhin and Del Tredici52,Reference Spires-Jones, Attems and Thal103 For example, almost all individuals by their third decade of life will have some degree of NFT development, and the severity and regional involvement increases with advancing age. Similarly, Aβ plaques are observed in a small proportion of individuals in their third decade of life, which increases to approximately 60 and 80% of individuals in their 9th and 10th decade of life, respectively Reference Braak, Thal, Ghebremedhin and Del Tredici52 . More recently, this has been demonstrated with the increased frequency of LATE-NC and AGD as mixed pathologies in aged individuals. Reference Nelson, Dickson and Trojanowski45,Reference Rodriguez, Suemoto and Molina104 The prevalence of these pathologies suggests that they inevitably occur with aging, and by the time an individual reaches advanced age, it is likely that multiple mixed pathologies will have developed in the brain, whereas younger individuals might show less mixed pathologies. However, fewer studies have investigated the range and extent of mixed pathologies in younger cohorts. Recent studies highlight that some young-onset neurodegenerative diseases Reference Spina, La Joie and Petersen76,Reference Tan, Yang and Halliday79 show mixed pathologies, which suggests additional pathogenic mechanisms are likely to be involved including disturbances in protein processing systems, cell clearance etc.
A complex synergistic interaction of proteins has also been discussed, Reference Robinson, Richardson and Xie83,Reference Spires-Jones, Attems and Thal103,Reference Nonaka, Masuda-Suzukake and Hasegawa105 which is intriguing when the same cell type/s and brain region/s are affected. In the human brain, fibrillar structures of different proteins have been reported to occur in the same cell and show partial co-localisation. Reference Kovacs78 These studies suggest interactions of tau, Aβ, α-synuclein and TDP-43, and indicate the deposition of one protein can lead to fibrillization of other proteins. For example, α-synuclein can induce tau fibrillization Reference Nonaka, Masuda-Suzukake and Hasegawa105 and Aβ may aggravate the spread of TDP-43 pathology. Reference Spires-Jones, Attems and Thal103 However, many mixed pathologies do not occur in the same cell type/s and/or anatomical brain regions, for example FTLD with Lewy bodies, suggesting that an interaction of proteins is less unlikely. Reference Forrest, Crockford and Sizemova96,Reference Forrest, Kim and De Sousa106 These pathologies are likely to develop independently in vulnerable brain regions and cell types, in anatomically distinct regions.
A single pathogenic event in a vulnerable brain region and cellular system has also been suggested to induce aggregation of multiple proteins, indicating common pathways are involved in disease pathogenesis. This includes insufficient clearance by the autophagic-lysosomal network and other clearance systems such as the ubiquitin-proteosome system, or clearance through the blood-brain barrier and glymphatic system. Reference Kovacs78,Reference Boland, Yu and Corti107 Building on this concept, combined proteinopathies might represent distinct “strain-like” properties from those associated with conditions that deposit only one abnormal protein, or the “behaviour” of protein strains is different when multiple proteins are deposited. Reference Robinson, Lee and Xie42
Finally, a common gene variation might contribute to the development of additional proteinopathies. A recent review has outlined the role of APOEe4 in modulating the clearance and aggregation of Aβ and exacerbate neurodegeneration, tau pathology and inflammation. Reference Tzioras, Davies, Newman, Jackson and Spires-Jones108 In addition, the APOEe4 allele has been reported to increase the burden of TDP-43 pathology Reference Yang, Yu and White109 and severity of Lewy body pathology. Reference Dickson, Heckman and Murray110
Conclusions and Future Considerations
Mixed pathologies in the ageing brain and neurodegenerative disease are more common than previously assumed, and combinations of mixed pathologies are now also recognised in hereditary neurodegenerative diseases. The most frequent concomitant neurodegenerative pathologies observed are AD, Lewy body pathology and ageing-related pathologies including ARTAG, AGD and LATE-NC. Recent studies suggest that age is not a major risk factor for mixed pathologies in early- and late-onset FTLD and AD, Reference Spina, La Joie and Petersen76,Reference Tan, Yang and Halliday79 although mixed pathologies are likely to be already present with late-onset neurodegenerative diseases.
Mixed and age-related pathologies reported in large community-based, longitudinal ageing studies and various studies in neurodegenerative cohorts are associated with lowering the threshold for developing cognitive decline and cognitive impairment. Patients with mixed pathologies are likely to show a different clinical course, compared to those with pure proteinopathies. This has implications to develop panels of biomarkers that are able to screen various neurodegeneration proteins and markers of disease progression, which, together with genetic markers, will facilitate better stratification of patients according to their underlying pure or combined proteinopathy. Reference Kovacs, Botond and Budka102 Analogously, therapeutic development might need to consider combined strategies, eventually based on the atomic level on structures of disease-associated protein filaments.
Regarding neuropathology, standardisation in the nomenclature used to describe multiple proteinopathies is needed. Careful discrimination is required to distinguish neurodegenerative proteinopathies versus ageing-related pathologies, and in particular what pathological burden is considered sufficient to cause clinical symptoms. With multiple proteinopathies, discriminating age-related and co-pathologies from the main proteinopathy causing disease might be more challenging and may require a more personalised approach to therapeutic strategies. Finally, defining cell-specific stages of pathological staging/disease progression will have important implications for understanding the earliest stages of disease.
A number of studies have suggested common pathologic mechanisms and/or interaction of pathological proteins in neurodegenerative diseases. Reference Spires-Jones, Attems and Thal103 Currently, preclinical models have proved informative for understanding many molecular mechanisms underlying various neurodegenerative diseases, but these have largely focused on modelling one type of protein abnormality with limited consideration of mixed pathologies in the ageing human brain. More focused preclinical models will be critical to better capture the spectrum of mixed pathologies observed in ageing and in neurodegenerative diseases and further explore disease mechanisms.
Acknowledgements
The authors wish to thank staff at the Sydney Brain Bank (supported by the University of New South Wales and Neuroscience Research Australia) and the Institute of Neurology, Medical university of Vienna for the initial characterisation of the cases used to prepare the sections for figurework. GGK is supported by the Edmond J. Safra and Rossy philanthropic funds.
Disclosures
SF declares no conflicts of interest. GK receives funding from the Rossy Foundation and Edmond Safra Philanthropic fund; receives royalties from Cambridge University Press, Elsevier and Wiley, and previously received consulting fees from Biogen; and declares a shared patent for a 5G4 antibody with Roboscreen GmbH.
Statement of Authorship
SLF and GGK drafted and edited the manuscript.










