Subarachnoid hemorrhage (SAH) accounts for 10-15% of strokes with an incidence of 5-20 in 100,000 people. Most cases of SAH occur intracranially; however, spinal SAH has also been reported, usually due to intramedullary tumors or spinal arteriovenous malformations (AVMs). Spinal SAH secondary to isolated aneurysm rupture or dissection is exceptionally rare.Reference Iihoshi, Miyata, Murakami, Kaneko and Yoyanagi 1 There have been eight previous cases of artery of Adamkiewicz (AA) aneurysm rupture. One patient was treated surgically and the rest conservatively.Reference Iihoshi, Miyata, Murakami, Kaneko and Yoyanagi 1 – Reference Aguilar-Salinas, Lima, Brasiliense, Hanel and Sauvageau 7 We report a unique case of spinal SAH secondary to AA dissection suspected to be caused by amphetamine use, as well as the first endovascular management of AA dissection in the setting of active extravasation.
An otherwise healthy 42-year-old Asian male walked into the emergency department and collapsed. He admitted to consuming five 3,4-methylenedioxymethamphetamine (MDMA) tablets, complained of nausea, but had no emesis, headache, vision changes, chest pain, or dyspnea. He was profoundly diaphoretic but afebrile, with an initial blood pressure of 165/120 mmHg, and heart rate of 140 beats per minute. He was noted to have developed paraplegia.
Although the white blood cell count was elevated, the patient had no other infectious symptomatology. He had an elevated troponin T of 31 ng/L but an unremarkable ECG. International normalized ratio and activated partial thromboplastin time were normal at 1.0 and 36 seconds, respectively.
The patient had two generalized tonic-clonic seizures and was treated with benzodiazepines. His level of consciousness declined and he was intubated before CT. The scan showed marked SAH, intraventricular blood, and hydrocephalus (Figure 1A). He was immediately transferred to a tertiary care hospital for neurosurgical assessment.
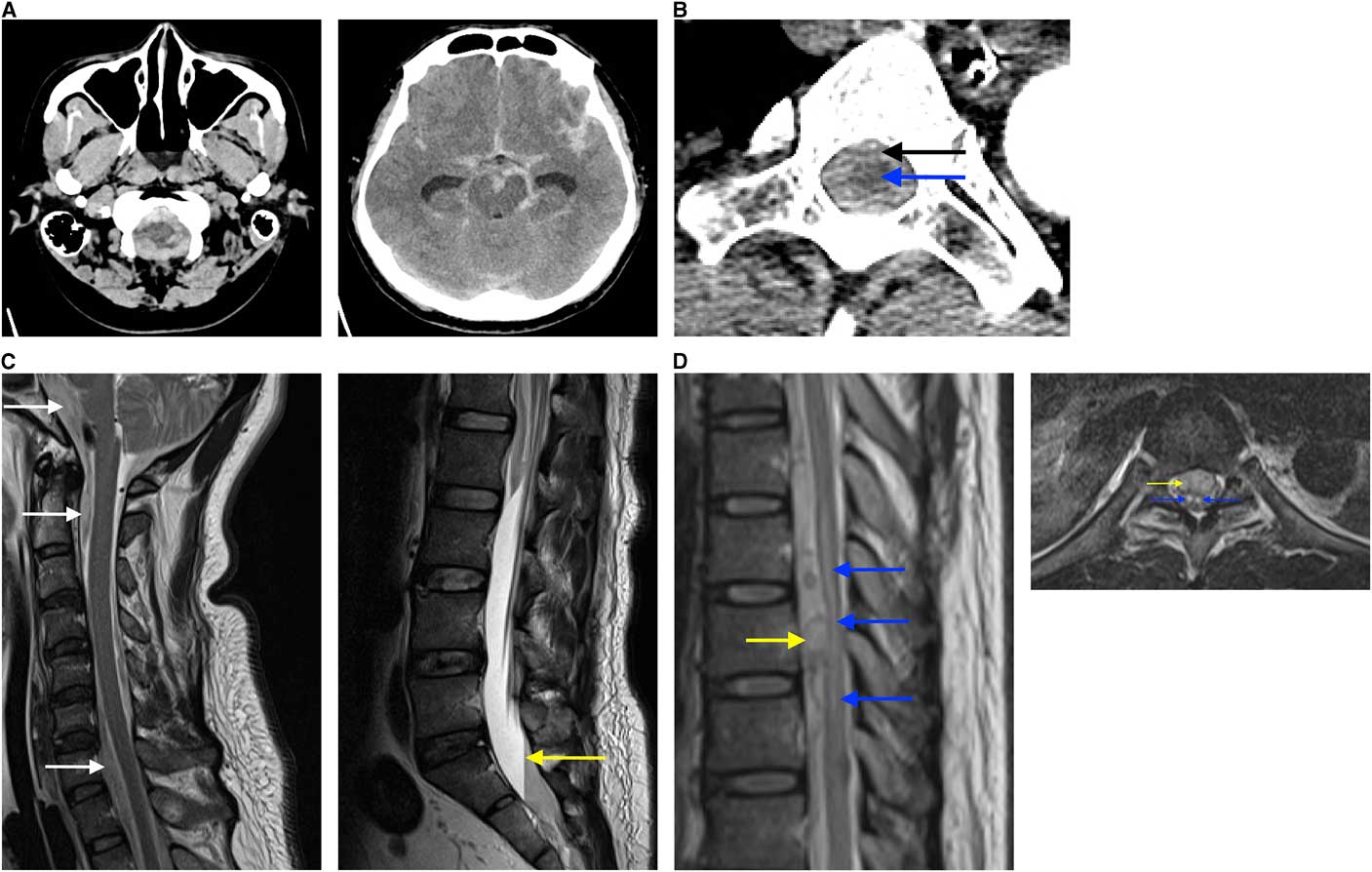
Figure 1 Findings on diagnostic imaging workup (A) The initial non-contrast CT head demonstrates extensive subarachnoid hemorrhage bilaterally extending into the foramen magnum and peri-mesencephalic cisterns (A1) with prominence of temporal horns (A2), reflecting hydrocephalus. (B) CTA from arch to vertex demonstrating extension of subarachnoid hemorrhage (black arrow) within the spinal canal surrounding the thoracic cord (blue arrow) (B1). (C) Sagittal MRI of the spine demonstrates hyperintense foci (white arrows) anterior to the spinal cord and brainstem in a T2 sequence (C1) and a plasma-CSF to red blood cell level (yellow arrow) present within the lower thecal sac when the patient is supine on the T2 sequence (C2). (D) A mass (yellow arrow) anterior to the spinal cord and a hypointense lesion in the spinal cord from T7 to T10 (blue arrows) on a sagittal T2 sequence (D1) and owl’s eyes sign of bilateral symmetric hyperintense signal (blue arrows) on axial T2 sequence at the level of T8, suggestive of anterior horn cell infarct secondary to mass effect (yellow arrow) (D2). This cord infarct suggested sacrifice of the anterior spinal artery at this level may not cause clinical worsening, as he already had an established infarct.
Upon arrival, an arch-to-vertex CT angiogram (CTA) was performed, demonstrating unchanged SAH and being the greatest in the cervical and thoracic spine (Figure 1B). The CTA also demonstrated a 4 mm left vertebral artery aneurysm/pseudoaneurysm at the C7 level (V1 segment), and acute left V3 segment dissection, from C1 to C3. The V4 segment was normal. No other intracranial source for bleeding was found. An external ventricular drain was placed with blood-tinged CSF encountered under some pressure.
Eight hours after presentation, the patient’s ECG showed T-wave inversion and his troponin was 564 ng/L, consistent with Takotsubo cardiomyopathy. Chest X-ray demonstrated neurogenic pulmonary edema. MRI of the brain and spine showed focal enhancement at the T9 level, suspicious for an underlying vascular abnormality, intrathecal hematoma at the T9 level with associated cord compression (Figures 1C and 1D), and acute infarcts in the left cerebellar hemisphere and right occipital lobe (images not shown).
Spinal angiography was performed, with segmental arteriogram at the T10 level demonstrating dissection of AA extending from mid-vessel into the origin of the anterior spinal artery (ASA), with associated fusiform aneurysmal enlargement (Figure 2A). The distal end of the pseudoaneurysm demonstrated active contrast extravasation.
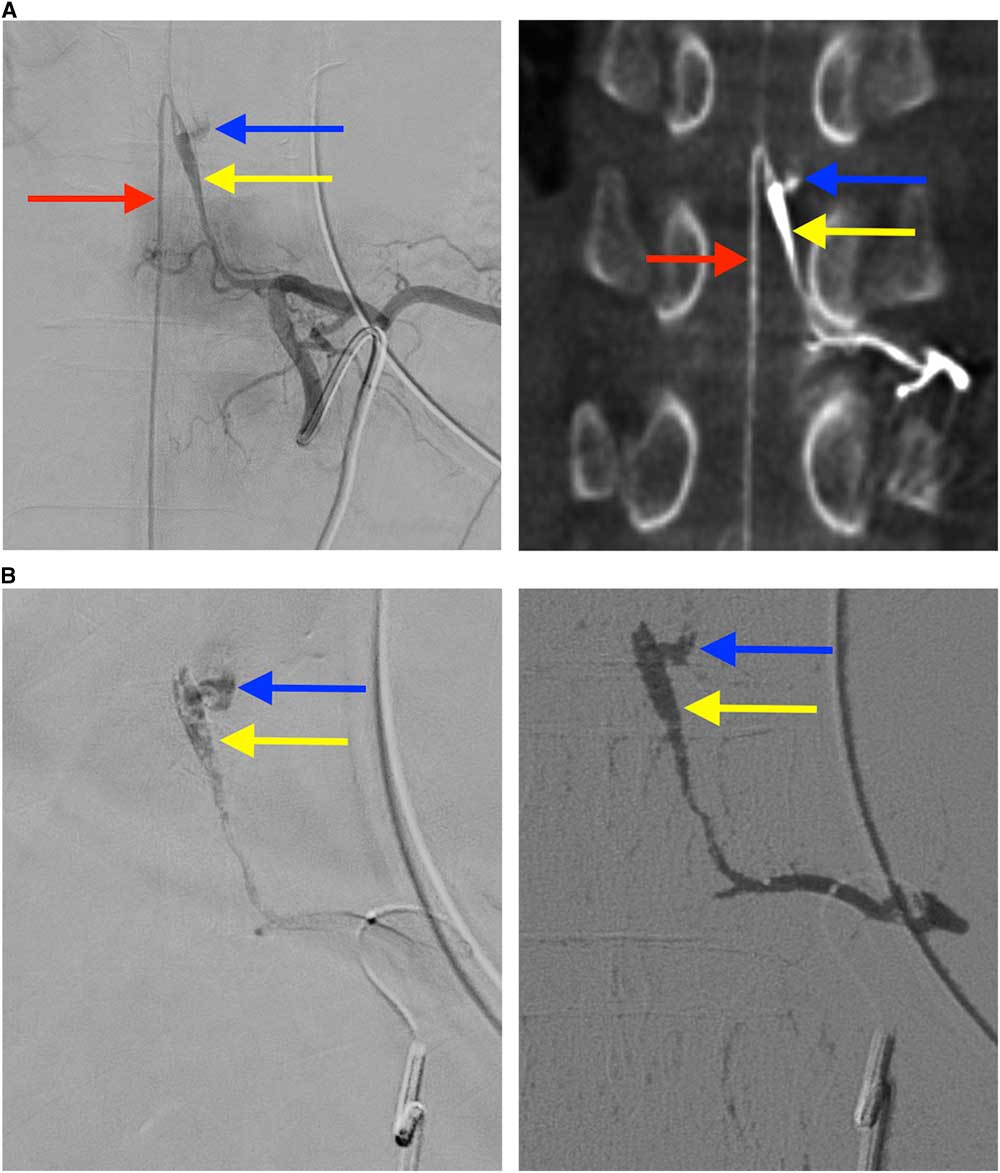
Figure 2 Findings on spinal angiography and glue embolization (A) The catheter is within the left T10 radicular artery and angiography shows localization of a fusiform pseudoaneurysm (yellow arrow) of the Adamkiewicz artery with extravascular contrast blush reflecting active extravasation (blue arrow) (A1), and connection to ascending and descending limbs of the anterior spinal artery (red arrow). Corroborating findings on rotational CT angiography were observed (A2). (B) Entry of glue into the dissecting aneurysm and occlusion of the site of active extravasation (B1). Digital subtraction angiography demonstrates glue has occluded the artery of Adamkiewicz and the culprit dissecting fusiform pseudoaneurysm, as well as the posterior branch of the T10 radicular artery (B2).
Owing to active extravasation and pre-existing paraplegia, it was decided that an endovascular approach was necessary to achieve hemostasis, and potentially avoid further neurologic worsening. Artery of Adamkiewicz was successfully cannulized using an Excelsior® SL-10® microcatheter and a Synchro 14R® guide wire, with embolization of the pseudoaneurysm performed using a 1:2.5 mixture of N-butyl-2-cyanoacrylate (Histoacryl®) to ethiodized oil (Lipiodol®). A small amount of liquid embolic passed through and excluded the site of active extravasation (Figure 2B).
Twenty-six hours after presentation, the patient opened his eyes spontaneously and nodded appropriately to questions. His upper extremity strength was 4/5 in the shoulders and upper arms bilaterally, and 5/5 in his fingers bilaterally. He had 0/5 strength in both lower extremities, no voluntary anal contraction, and 3+ reflexes in his left knee and ankle. Bulbocavernosus reflex and rectal tone were preserved.
The patient had no further complications and he was discharged to spine rehabilitation after two weeks, with mild cognitive deficits and a T10 American Spinal Injury Association Impairment Scale (AISA) B spinal injury. At 3 months, he was walking with hiking poles and independent with activities of daily living. At six months, extremity strength was 5/5 bilaterally throughout L2 to S1 myotomes (AISA-D conus and cauda mixed syndrome). The patient had relatively stable gait without assistance and was able to drive his car. The patient did not receive follow-up spine angiography.
Most spinal aneurysms occur in the intradural segment of the artery and are fusiform.Reference Son, Lee and Park 5 Spinal arteries have a much smaller caliber than most intracranial arteries, and tend to be less affected by atherosclerosis.Reference Son, Lee and Park 5 The majority of spinal aneurysms are flow-related, typically seen in association with spinal AVMs.
The eight reported cases of isolated AA aneurysm/pseudoaneurysm rupture are summarized along with the present case in Table 1. The mean age±SD was 49.0±12.7 years, with four males and five females. Lower extremity paresis occurred in eight (89%); also common was back pain (78%), headache (56%), and nausea or emesis (67%).
Table 1 Summary of reported cases of isolated artery of Adamkiewicz aneurysm ruptures and pseudoaneurysms
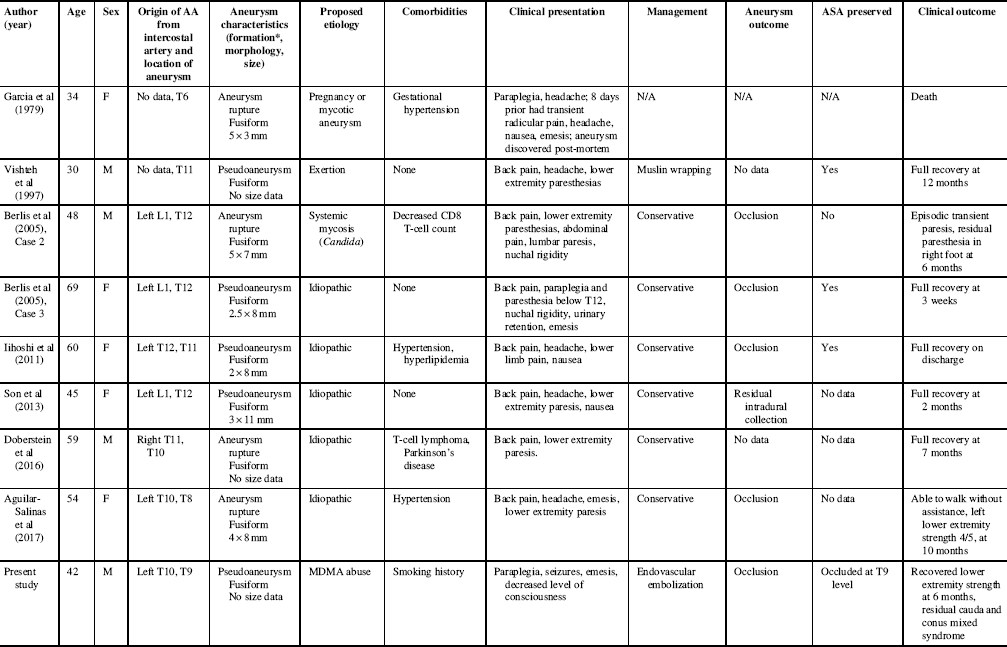
AA, Adamkiewicz artery; M, male; F, female; ASA, anterior spinal artery; N/A, not applicable.
* Without pathological confirmation, it is difficult to determine from angiography if fusiform morphology is the result of artery dissection or rupture of a fusiform aneurysm.
The amphetamine derivative 3,4-MDMA may be implicated in de novo aneurysm formation.Reference Johnson, Patel, Saraf-Lavi, Aziz-Sultan and Yavagal 8 Our patient had previous episodic non-chronic use of MDMA and cannabis, and smoked one-third of a pack of cigarettes per day. Given that he was otherwise healthy with no known infection or vascular abnormalities, it is possible that MDMA consumption caused intimal injury with consequent dissection of AA and left vertebral artery.
Artery of Adamkiewicz aneurysm/pseudoaneurysm rupture may be managed conservatively, surgically, or endovascularly. All cases in the past decade were managed conservatively, as surgical and endovascular methods were thought to be too risky.Reference Iihoshi, Miyata, Murakami, Kaneko and Yoyanagi 1 , Reference Son, Lee and Park 5 , Reference Doberstein, Bouley, Silver, Morrison and Jayaraman 6 Standard surgical clip placement is not usually possible with fusiform aneurysms, leaving trapping or wrapping of the aneurysm as possible surgical options.Reference Berlis, Scheufler, Schmahl, Rauer, Götz and Schumacher 4 Endovascular techniques risk occluding the ASA.Reference Iihoshi, Miyata, Murakami, Kaneko and Yoyanagi 1 , Reference Son, Lee and Park 5 In some cases, endovascular therapy was not feasible as the ASA was inaccessible and at risk of occlusion.Reference Son, Lee and Park 5
Our patient recovered lower extremity strength despite loss of AA and occlusion of ASA at the T9 level, suggesting that he had collateral supply to the distal ASA. Our case highlights the feasibility and utility of endovascular treatment in these cases, and the potential for recovery, even with short segment embolization of the ASA. The success of this therapy suggests that, if active bleeding is suspected, interventional approaches should be considered.
Statement of Authorship
All authors made significant contributions to this manuscript. MH was the most responsible physician for the procedure discussed in this case report, and supervised the design of this manuscript and reviews. JN wrote the initial draft of this manuscript and coordinated revisions. MF was the resident neurosurgeon involved in this case report and helped obtain patient information, clarify case information, and planned the first draft of this manuscript. CH was consulted during the operation described in this case report and reviewed all drafts of this manuscript.
Disclosures
MH, JN, MF, and CH have nothing to disclose. Portions of this work were presented in abstract form at the American Society of Neuroradiology 56th Annual Meeting, Vancouver, Canada, June 5, 2018.




